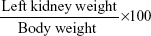Back to Journals » Drug Design, Development and Therapy » Volume 10
Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model
Authors Dkhil M , Al-Khalifa M, Al-Quraishy S, Zrieq R, Moneim A
Received 1 February 2016
Accepted for publication 24 March 2016
Published 2 June 2016 Volume 2016:10 Pages 1847—1856
DOI https://doi.org/10.2147/DDDT.S105511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Mohamed A Dkhil,1,2 Mohamed S Al-Khalifa,1 Saleh Al-Quraishy,1 Rafat Zrieq,3 Ahmed Esmat Abdel Moneim2
1Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia; 2Department of Zoology and Entomology, Faculty of Science, Helwan University, Cairo, Egypt; 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, University of Hail, Hail, Saudi Arabia
Abstract: This study was conducted to appraise the protective effect of Indigofera oblongifolia leaf extract on lead acetate (PbAc)-induced nephrotoxicity in rats. PbAc was intraperitoneally injected at a dose of 20 mg/kg body weight for 5 days, either alone or together with the methanol extract of I. oblongifolia (100 mg/kg). Kidney lead (Pb) concentration; oxidative stress markers including lipid peroxidation, nitrite/nitrate, and glutathione (GSH); and antioxidant enzyme activities, namely superoxide dismutase, catalase, GSH peroxidase, and GSH reductase were all determined. The PbAc injection elicited a marked elevation in Pb concentration, lipid peroxidation, and nitrite/nitrate, with a concomitant depletion in GSH content compared with the control and a remarkable decrease in antioxidant enzymes. Oxidant/antioxidant imbalance, Pb accumulation, and histological changes in the kidneys were successfully prevented by the pre-administration of I. oblongifolia. In addition, the elevated expression of proapoptotic protein, Bax, in the kidneys of the PbAc-injected rats was reduced as a result of I. oblongifolia pre-administration, while the hitherto reduced expression of the anti-apoptotic protein Bcl-2 was elevated. Based on the current findings, it can be concluded that I. oblongifolia successfully minimizes the deleterious effects in kidney function and histological coherence associated with nephrotoxicity by strengthening the antioxidant defense system, suppressing oxidative stress, and mitigating apoptosis.
Keywords: lead acetate, Indigofera oblongifolia, oxidative stress, apoptosis, kidney
Introduction
Lead (Pb) is a very widespread environmental pollutant that has produced a wide range of devastating toxic effects that are known to have a negative influence on some organs and to induce a wide range of biochemical, physiological, and behavioral dysfunctions.1,2 The kidney is especially susceptible to the damaging effects of Pb due to its major role in the excretion of Pb from the body.2,3 Lead is removed readily by proximal tubule cells, where it damages mitochondria, suppresses mitochondrial function, and alters the physical absorptive activities of the cell, causing renal tubular damage and renal failure. Furthermore, the long-term Pb exposure can result in the development of chronic Pb nephropathy.4 Like other heavy metals, Pb has been reported to generate reactive oxygen species (ROS), thereby inducing oxidative injury to the kidney.5
Exposure to Pb2+ via different routes has been found to enhance lipid peroxidation (LPO) in kidney and deplete glutathione (GSH). Consistently, Pb has been found to inhibit sulfhydryl-dependent enzymes, interfere with metals that are important for antioxidant enzyme activities, and/or increase the vulnerability of cells to oxidative attack by impairing the integrity and fatty acid composition of renal cell membranes.6,7
Herbal and natural products possess antioxidant properties, and antioxidant molecules of plant origin have been widely investigated as scavengers of free radicals and suppressors of LPO. In this regard, numerous studies have exhibited the antioxidant activities of several natural products against many toxic metals.5,8 Indigofera oblongifolia is a perennial shrub belonging to the Fabaceae family and has a large geographical distribution, including Saudi Arabia. This plant contains a novel alkylated xanthene called indigin in addition to indigoferic acid, the fatty acid ester of p-hydroxy (E)-cinnamic acid, β-sitosterol, and 3-hydroxybenzoic acid.9 I. oblongifolia has been shown to protect hepatocytes from carbon tetrachloride-induced hepatotoxicity through its strong capacity to inhibit oxidative stress-induced membrane lipids, nuclear DNA, and protein oxidation.10
To our knowledge, no other studies are available on the protective effect of I. oblongifolia leaf extract (IOLE) on Pb-induced nephrotoxicity in rats. In view of this, the current study was conducted to elucidate whether IOLE, when pre-administered to lead acetate (PbAc), can ameliorate oxidative stress-induced nephrotoxicity.
Materials and methods
Chemicals and animals
Lead(II) acetate trihydrate (Pb(CH3CO2)2·3H2O; CAS Number 6080-56-4), nitro blue tetrazolium, N-(1-naphthyl) ethylenediamine dihydrochloride, and Tris–HCl were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). 2-Thiobarbituric acid and trichloroacetic acid were obtained from Merck Millipore (Billerica, MA, USA). The chemicals and reagents used were all of analytical grade.
In this study, we used adult male Wistar albino rats, which were 150–180 g in weight. The animals were purchased from the animal facility of the Holding Company for Biological Products and Vaccines (Vacsera, Cairo, Egypt). The rats were maintained on a standard commercial pelleted diet in an air-conditioned animal house at 22°C–25°C. All experiments were conducted according to the ethical standards approved by the Institutional Animal Ethics Committee guidelines for animal care and use, Helwan University, Egypt.
Preparation of I. oblongifolia extract
I. oblongifolia leaves were obtained from Jazan city located in the southwest of Saudi Arabia. The plant material was authenticated by Doctor Pandalayil (Botany Department, College of Science, King Saud University). The plant leaves were air dried at a room temperature and ground into powder using a pulveriser. One hundred grams of powdered leaves were extracted with 70% methanol at 4°C for 24 hours by occasional mixing. The leaf extract was filtered and then evaporated until it was dry in a vacuum evaporator (Heidolph, Schwabach, Germany). Residues were dissolved in water before use in the experimental study.
Chromatography analysis
Analysis was performed using a high-performance liquid chromatography (HPLC) system (Waters Corporation, Milford, MA, USA). The HPLC system was equipped with a 717 automatic injector and provided with a column oven, two pumps (model 510), a diode array detector (model 2996), and Millennium software v.3.1 data module (Waters Corporation, Milford, MA, USA). The separation was executed on a reversed-phase Nucleosil 120 C18 (25 cm ×4.6 mm, 3 μm) column obtained from Teknokroma (Barcelona, Spain). The mobile phase composed of water and methanol with the gradient elution system at a flow rate of 0.8 mL/min. The injection volume was 20 μL after filtration through a 0.22 μm polyvinylidene difluoride membrane. The detection of ultraviolet (UV) wavelength was set at 280 nm. The column temperature was set at 25°C.
Experimental design
Rats were randomly allocated into four groups of seven animals per group. Rats in the first group (group I) were orally gavaged with 0.3 mL saline, then, after 1 hour, 100 μL of saline was injected intraperitoneally (IP). Groups II (PbAc group) and IV (IOLE + PbAc group) received a daily IP injection of PbAc (20 mg/kg body weight [bwt]), and groups III (IOLE group) and IV were orally treated with IOLE (100 mg/kg bwt). Animals were inoculated with their respective doses daily for 5 days. In the IOLE + PbAc group, the treatment of IOLE was given before, about an hour, PbAc. IOLE was orally administered at a dose of 100 mg/kg bwt according to Lubbad et al,11 while PbAc was IP injected with an acute toxic dose of 20 mg/kg bwt, according to Abdel Moneim.12
Twenty-four hours after administering the last dose, blood was collected from all the animals by cardiac puncture. Blood serum was separated by centrifugation at 1,000× g for 15 minutes and used for the kidney function parameters, while the rats were sacrificed by means of mild ether anesthesia. Rat kidneys were removed, weighed, and washed twice in ice-cold 50 mM Tris–HCl, pH 7.4. The kidneys were homogenized in ten volumes of ice-cold medium of 50 mM Tris–HCl (pH 7.4). Kidney homogenates were centrifuged at 1,000× g for 10 minutes at 4°C. The supernatants were used for various biochemical studies. The protein level of the homogenates was assessed using Lowry et al’s method.13
Pb concentration in the kidney
The Pb concentration in the kidney was determined following the method set out by Abdel Moneim.12 Briefly, kidney tissue was dried and then combusted for 24 hours at 450°C. Digested samples were analyzed at 283.3 nm using a flame atomic absorption spectrophotometer (3100; PerkinElmer Inc., Waltham, MA, USA). Pb accumulation in the kidney tissue was determined as microgram per gram wet weight.
Changes in kidney index of rats
The relative kidney weight was calculated using the following formula:
|
|
Kidney oxidative damage
Kidney homogenates were subjected to a thiobarbituric acid reaction to determine LPO levels, which were expressed in terms of the amount of malondialdehyde (MDA) formed.14 Nitrite/nitrate (nitric oxide, NO) and GSH were assayed using the methods of Sun et al15 and Rahman et al,16 respectively.
Antioxidant status
Kidney homogenates were used for the determination of superoxide dismutase (SOD) according to Fisher et al,17 catalase (CAT) as described by Aebi,18 and GSH peroxidase (GPx) and GSH reductase (GR) following De Vega et al.19
Quantitative real-time polymerase chain reaction
After isolating the total kidney RNA, the RNA was purified using an RNeasy Plus Minikit (Qiagen NV, Venlo, the Netherlands). cDNA synthesis was carried out using the RevertAid™ H Minus Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA samples were run in triplicate for real-time polymerase chain reaction (PCR) analysis. Real-time PCR reactions were performed using Power SYBR® Green (Thermo Fisher Scientific) on the Applied Biosystems 7500 system (Thermo Fisher Scientific). Relative values of gene expression were normalized to β-actin. The PCR primers for the following genes were synthesized by Jena Bioscience GmbH (Jena, Germany): β-actin (accession number: NM_031144.3; forward: 5′-GGCATCCTGACCCTGAAGTA-3′; reverse: 5′-GGGGTGTTGAAGGTCTCAAA-3′), SOD2 (manganese-dependent SOD; accession number: NM_001270850.1; forward: 5′-AGCTGCACCACAGCAAGCAC-3′; reverse: 5′-TCCACCACCCTTAGGGCTCA-3′), CAT (accession number: NM_012520.2; forward: 5′-TCCGGGATCTTTTTAACGCCATTG-3′; reverse: 5′-TCGAGCACGGTAGGGACAGTTCAC-3′), GPx (accession number: NM_017006.2; forward: 5′-CGGTTTCCCGTGCAATCAGT-3′; reverse: 5′-ACACCGGGGACCAAATGATG-3′), B-cell lymphoma 2 (Bcl-2; accession number: NM_016993.1; forward: 5′-CTGGTGGACAACATCGCTCTG-3′; reverse: 5′-GGTCTGCTGACCTCACTTGTG-3′), and Bcl-2-like protein 4 (Bax; accession number: NM_017059.2; forward: 5′-GGCGAATTGGCGATGAACTG-3′; reverse: 5′-ATGGTTCTGATCAGCTCGGG-3′).
Histological examination
The right kidney was fixed in 10% neutral buffered formaldehyde for 24 hours, dehydrated in alcohol, cleared in xylene, and mounted in molten paraffin wax. The paraffin-embedded tissues were sectioned at 4–5 μm and stained with hematoxylin and eosin. Sections were examined using a light microscope (Eclipse E200-LED; Nikon Corporation, Tokyo, Japan).
Caspase-3 in kidney tissue
Kidney tissue samples were fixed in neutral buffered formalin (10%), embedded in paraffin, and cut into 4 μm thick sections. Immunocytochemical reactions were performed using the peroxidase/anti-peroxidase method. Nonspecific peroxidase reactions were blocked with methanol containing 0.1% H2O2, and the sections were also incubated with normal goat serum to avoid nonspecific reactions with the background once the samples were incubated with the specific antibody against caspase-3 (rabbit polyclonal, dilution, 1:2,000, category no: ab2302; Abcam, Cambridge, MA, USA). Tissue sections were then washed with phosphate buffer and incubated with a secondary antibody (1:2,000; Sigma-Aldrich Co.), before being washed in phosphate buffer again and, finally, incubated with the peroxidase/anti-peroxidase complex (dilution, 1:200). The peroxidase reaction was carried out using a solution of 3,3′-diaminobenzidine tetrahydrochloride containing 0.01% H2O2 in Tris–HCl buffer (0.05 M, pH 7.6). After being washed in phosphate-buffered saline, tissues were counterstained with hematoxylin and observed under a light microscope.
Statistical analysis
All results were expressed as mean ± standard error of the mean. Data for multiple variable comparisons were analyzed by one-way ANOVA. For the comparison of significance between groups, Duncan’s test was used as a post hoc test. The acceptable level of significance was established at P<0.05.
Results
Phenolic profiles evaluated at 280 nm for the IOLE evaluation are shown in Figure 1. The chromatogram shows the presence of many peaks with retention times between 4 minutes and 65 minutes. Based on the UV–visible spectral data and their retention time, the IOLE has UV band characteristic for phenolic compounds, possibly quercetin, kaempferol, cinnamic acid, vanillic acid, flavonol, or flavone derivatives.
At the end of the course, rats injected with PbAc alone had a nonsignificant decline in their final body weight with a significant (P<0.05) elevation in the relative kidney weight compared with the control animals. The pre-administration of IOLE (group IV), however, significantly ameliorated all these parameters to the control levels (Table 1).
The data illustrated in Figure 2 shows that Pb2+ concentration in kidney tissue was significantly raised in the PbAc group compared with the control rats (P<0.05). Pre-administration of IOLE, however, significantly decreased the Pb2+ concentration in the kidneys of the IOLE + PbAc group compared with the PbAc-treated group.
Rats subjected to Pb-induced oxidative stress exhibited severe kidney injury, as evidenced by obvious increases in the levels of serum uric acid, urea, and creatinine compared with the control rats (Figure 3). Treatment with 100 mg/kg of IOLE, however, significantly reversed this elevation (P<0.05).
PbAc injection significantly elevated the production of MDA and NO generation in the kidney (P<0.05). Meanwhile, an obvious decrease in the GSH content in the kidney was found when compared with the control group (P<0.05). The results also illustrated that PbAc injection inhibited the activities of SOD and CAT (P<0.05), and there was also an evident (although nonsignificant) inhibition in GR and GPx activities. Interestingly, treatment with 100 mg/kg IOLE significantly attenuated the production of MDA and NO generation, as well as reversed the depletion of GSH content induced by PbAc injection in the kidney (P<0.05). In addition, the current study reveals that treatment with 100 mg/kg IOLE elevated SOD, CAT, and GPx activities (P<0.05), although it failed to change the GR activity significantly compared with the control group (Table 2). Consistent with the biochemical analysis, the quantitative PCR results showed that the mRNA of SOD2, CAT, and GPx were downregulated after PbAc injection but that IOLE was able to upregulate these genes (Figure 4).
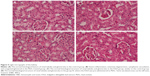
Rats intoxicated with PbAc revealed moderate-to-severe inflammation and extensive degeneration as indicated by the mononuclear cell infiltrations, cytoplasmic vacuolation, and congested glomeruli (Figure 5). In addition, serious apoptosis and necrosis were observed in the section as indicated by the presence of karyomegaly and hyperchromatic nuclei in the tubular epithelial cells of the renal tissue. Treatment of rats with IOLE largely improved these Pb-induced histopathological alternations in the kidney tissue (Figure 5).
To investigate whether the observed protective effects of IOLE were related to the antiapoptotic property of IOLE, the expression levels of Bcl-2 and Bax in the kidney were determined. The results indicated that the expression of Bcl-2 mRNA was downregulated significantly (Figure 6), and the expression of Bax mRNA was upregulated in the PbAc treated group (P<0.05). However, these effects were reversed in rats which received 100 mg/kg of IOLE prior to PbAc (P<0.05; Figure 6).
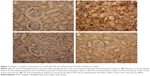
An immunohistochemical examination was performed to confirm the apoptosis induced in the kidney. In the PbAc-injected group, the expression of the apoptotic protein, caspase-3, was increased (Figure 7). The pre-administration of IOLE to rats exhibited improvement represented by the expression of caspase-3 in the cytoplasm was largely decreased compared with that in the PbAc rats (Figure 7).
Discussion
It is worth noting that the kidney is the first target organ of Pb toxicity because of its ability to reabsorb and accumulate Pb.20 Accordingly, it has been reported that both acute and chronic intoxication with Pb causes nephrotoxicity, In addition, a previous study revealed that Pb inhibits sulfhydryl-dependent enzymes by forming high-affinity multidentate complexes with thiol (–SH) and other functional groups.21 Our study revealed that even when Pb content in kidney tissue was highly concentrated, IOLE pretreatment reduced the level markedly. This suggests that IOLE removes Pb from the kidney by chelating and/or increasing the clearance of Pb from the kidney. IOLE is rich in flavonoids that act as metal chelators due to the presence of multiple hydroxyl groups forming a coordination bond with Pb2+.22 The mechanism governing the metal chelation property of IOLE is not yet clearly known; however, further investigation is needed.
Increases in kidney relative weight as observed in the current study reflected renal hypertrophy which is in accordance with previous studies.5 Furthermore, the decrease in body weight of PbAc-intoxicated rats is also consistent with the previous report and supports the increase in the kidney relative weight noted here. Pretreatment of rats with IOLE prevented these observations. This suggests that IOLE has the ability to protect renal tissue from PbAc-induced renal hypertrophy.
The deleterious effects produced by Pb might be attributed to its ability to generate ROS which induce oxidative injury in the kidney by two separate, although related, pathways.20 The first pathway is the ability of Pb to enhance LPO, while the second pathway operates through the depletion of antioxidant reserve, whereby Pb interacts with sulfhydryl groups or metal cofactors in different enzymatic and nonenzymatic antioxidant molecules so as to induce a decline in antioxidant enzyme activity and in GSH.23
LPO is a prominent marker of oxidative stress as it is known to increase the production of epoxides, hydroperoxides, and MDA, all of which may interact with proteins, DNA, and RNA in the cell causing renal tissue damage.24 Here, IOLE has been shown to augment Pb-induced nephrotoxicity through its considerable effect in reducing LPO production.
The increase in NO generation, evident in the current study, may contribute to the ability of Pb to enhance pro-inflammatory processes through the stimulation of NF-κB, which in turn stimulates inducible NO synthase in macrophages.25 NF-κB usually induces inflammation through inducible NO synthase/NO generation by controlling the transcriptional activation of the gene.26 The toxicity of NO increases greatly when it reacts with the superoxide radical, forming the highly reactive peroxynitrite anion (ONOO−). Flavonoids, however, have been shown to directly scavenge NO.27 The presence of flavonoids could explain why IOLE was so effective in reducing NO.
GSH is a major, nonenzymatic antioxidant molecule, existing both intracellularly and extracellularly in living organisms, acting against xenobiotics and neutralizing ROS. The level of GSH in the body is, therefore, considered to be an important indicator of the antioxidative strength, and any disturbance in GSH can lead to serious consequences for the biological system.28 Oxidative stress is known to lower GSH content,29 and in the current experiment GSH content was indeed decreased in PbAc-treated rats. This depletion in GSH content might have contributed to the enhanced LPO. Furthermore, Pb inactivates GSH synthesis from cysteine via the γ-glutamyl cycle which further depresses the GSH content.20 This study found, however, that the GSH content in the kidneys of rats pretreated with IOLE was increased significantly suggesting that IOLE protects GSH from being depleted in kidney and thus maintains the antioxidant reserves in the kidney in the face of oxidative stress.
Diminished activities of antioxidant enzyme are frequently implicated under oxidative stress, and here, rats intoxicated with PbAc were reported to have significantly decreased levels of renal antioxidant enzymes. Among these enzymes, SOD catalyzes the conversion of superoxide anion-free radicals to H2O2 through a dismutation reaction, but Pb, apart from targeting the sulfhydryl groups, appears also to interfere with the zinc ions that serve as cofactors for SOD, thereby inactivating it. CAT, meanwhile, is an important antioxidant enzyme having heme as the prosthetic group. Pb is known to decrease iron absorption and prevent heme biosynthesis.30 Similarly, Pb inactivates GPx and GR. Pretreatment with IOLE protected each of these enzymes from being altered, suggesting the regulatory role of IOLE on antioxidant enzymes.
In the current study, we have also examined the kidney function markers, finding PbAc-induced elevations in serum uric acid, urea, and creatinine that indicate kidney dysfunction. Pretreatment of rats with IOLE mitigated this elevation of negative kidney function markers. Overall, therefore, our results suggest that IOLE can prevent Pb-induced renal damage and can help to preserve the normal function of the kidney.
The findings of the histological study support the abovementioned results, whether for the effect of PbAc or the preventive effects of the IOLE. The deleterious effect of PbAc on renal tissue, due to the oxidation actions, is a result of the overproduction of ROS, which induces cell injury and apoptosis.5 In order to have this effect, Pb is freely passed into the glomerulus and by endocytosis, and is reabsorbed in the proximal tubular cells.25 Once passed into the renal cell, Pb causes mitochondrial damage, uncoupling of the respiratory chain and cell death.31 Pretreatment of rats with IOLE serves to protect the kidney from this disruption by means of the various mechanisms discussed earlier. Our results, therefore, further strengthen the contention that IOLE can protect the kidney from PbAc-induced oxidative damage and can thus prevent PbAc-induced pathogenesis of the kidney.
In the current study, both the caspase-3 protein level and Bax mRNA level were significantly elevated in the renal tissue, while the Bcl-2 mRNA level was decreased significantly. These findings support those of Rong et al32 who suggested that the nephrotoxicity of Pb in vitro may be due to the acceleration of apoptosis related to the stimulation of the NF-κB and p53 genes and the depression of the Bcl-2 gene. Furthermore, Agarwal et al33 have revealed that the activation of the caspase cascade and simultaneous extracellular signal-regulated kinase dephosphorylation is the most significant operative pathway directly associated with apoptotic signals triggered by PbAc in vitro. Moreover, Yedjou et al34 found that Pb-induced apoptosis occurs, at least in part, via induction of phosphatidyl serine externalization and caspase-3 stimulation. Conversely, pretreatment with IOLE prevented apoptosis induction in the kidney. The protective effect of IOLE on kidney tissue is related to the inhibition of apoptosis, based on the ability of different phytochemicals to protect against stress-induced apoptosis.
In the current experiment, PbAc markedly increased caspase-3 expression indicating that Pb provokes cell death in the rat kidney. Interestingly, the administration of IOLE improved the kidney by decreasing the expression of caspase-3. This agrees with the work of Yuan et al35 who showed that the expression of caspase-3 was distinctly elevated in rats treated with PbAc. Caspase-3 plays a major role in the downstream part of mitochondrial pathway-mediated cell death, after dysfunction of the mitochondria and the release of cytochrome c into the cytoplasm. It is well documented that caspase-3 activation occurs in Pb-induced apoptosis in the reproductive system36 and apoptosis in human leukemia (HL-60) cells.37 Caspase-3 is believed to be the final executor of apoptotic DNA damage.38
Conclusion
This study has shown that IOLE has significant antioxidant and anti-apoptotic activities and is able to effect a significant reversal of the Pb-induced oxidative injury in kidney. The observed results could be due to the different polyphenols and flavonoids present in the extract. The current findings elucidate for the first time that the leaves extract of I. oblongifolia has anti-apoptotic and antioxidant properties in kidney tissue.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project no PRG-1436-02.
Disclosure
The authors report no conflicts of interest in this work.
References
El-Tantawy WH. Antioxidant effects of spirulina supplement against lead acetate-induced hepatic injury in rats. J Tradit Complement Med. In press 2015. | ||
Jia Q, Ha X, Yang Z, Hui L, Yang X. Oxidative stress: a possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol Mech Methods. 2012;22(9):705–710. | ||
Jabeen R, Tahir M, Waqas S. Teratogenic effects of lead acetate on kidney. J Ayub Med Coll Abbottabad. 2010;22(1):76–79. | ||
El-Khishin IA, El-fakharany YM, Abdel Hamid OI. Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicol Rep. 2015;2:824–832. | ||
Abdel Moneim AE, Dkhil MA, Al-Quraishy S. The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater. 2011;194:250–255. | ||
Abdel-Moneim AE, Dkhil MA, Al-Quraishy S. The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res. 2011;143(1):457–467. | ||
Sadek KM. Barley phenolic compounds impedes oxidative stress in lead acetate intoxicated rabbits. Oxid Antioxid Med Sci. 2012;1(2):141–146. | ||
Abdou HM, Hassan MA. Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. Biomed Res Int. 2014;2014:435857. | ||
Sharif A, Ahmed E, Malik A, et al. Lipoxygenase inhibitory constituents from Indigofera oblongifolia. Arch Pharm Res. 2005;28(7):761–764. | ||
Shahjahan M, Vani G, Devi CS. Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. J Med Food. 2005;8(2):261–265. | ||
Lubbad MY, Al-Quraishy S, Dkhil MA. Antimalarial and antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Parasitol Res. 2015;114(9):3431–3438. | ||
Abdel Moneim AE. Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res. 2012;148(3):363–370. | ||
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. | ||
Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–540. | ||
Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors. 2003;3(8):276. | ||
Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2007;1(6):3159–3165. | ||
Fisher AEO, Maxwell SC, Naughton DP. Catalase and superoxide dismutase mimics for the treatment of inflammatory diseases. Inorg Chem Commun. 2003;6(9):1205–1208. | ||
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. | ||
De Vega L, Fernandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB. Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail. 2002;24(4):421–432. | ||
Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58. | ||
Sharma SK, Goloubinoff P, Christen P. Heavy metal ions are potent inhibitors of protein folding. Biochem Biophys Res Commun. 2008;372(2):341–345. | ||
Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Scientific World J. 2013;2013:162750. | ||
Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008;128(4):501–523. | ||
Kehrer JP, Klotz L-O. Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health. Crit Rev Toxicol. 2015;45(9):765–798. | ||
Reyes JL, Molina-Jijon E, Rodriguez-Munoz R, Bautista-Garcia P, Debray-Garcia Y, Namorado Mdel C. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed Res Int. 2013;2013:730789. | ||
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. | ||
Boora F, Chirisa E, Mukanganyama S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J Food Process. 2014;2014:918018. | ||
Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333(1):19–39. | ||
Bast A, Haenen GR. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta. 1988;963(3):558–561. | ||
Flora SJ, Flora G, Saxena G, Mishra M. Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol (Noisy-le-grand). 2007;53(1):26–47. | ||
Wang L, Wang H, Hu M, Cao J, Chen D, Liu Z. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 2009;83(5):417–427. | ||
Rong J, Chang W, Lv L, Chen J. [Study on the roles of nuclear factor-kappaB, p53 and Bcl-2 gene in lead acetate induced apoptosis in PC12 cells]. Wei Sheng Yan Jiu. 2008;37(3):262–263, 268. Chinese. | ||
Agarwal S, Roy S, Ray A, Mazumder S, Bhattacharya S. Arsenic trioxide and lead acetate induce apoptosis in adult rat hepatic stem cells. Cell Biol Toxicol. 2009;25(4):403–413. | ||
Yedjou CG, Milner JN, Howard CB, Tchounwou PB. Basic apoptotic mechanisms of lead toxicity in human leukemia (HL-60) cells. Int J Environ Res Public Health. 2010;7(5):2008–2017. | ||
Yuan G, Dai S, Yin Z, et al. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol. 2014;7(6):2905–2914. | ||
Elgawish RAR, Abdelrazek HMA. Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicol Rep. 2014;1:795–801. | ||
Yedjou CG, Tchounwou HM, Tchounwou PB. DNA Damage, cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int J Environ Res Public Health. 2015;13(1):ijerh13010056. | ||
Xu J, Ji LD, Xu LH. Lead-induced apoptosis in PC 12 cells: involvement of p53, Bcl-2 family and caspase-3. Toxicol Lett. 2006;166(2):160–167. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.