Back to Journals » Drug Design, Development and Therapy » Volume 14
In vivo Screening of Natural Products Against Angiogenesis and Mechanisms of Anti-Angiogenic Activity of Deoxysappanone B 7,4ʹ-Dimethyl Ether
Authors Chen K, Fan Y, Gu J, Han Z, Zeng H, Mao C, Wang C
Received 5 March 2020
Accepted for publication 26 June 2020
Published 30 July 2020 Volume 2020:14 Pages 3069—3078
DOI https://doi.org/10.2147/DDDT.S252681
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Kan Chen, Yuqi Fan, Jun Gu, Zhihua Han, Huasu Zeng, Chengyu Mao, Changqian Wang
Department of Cardiology, Shanghai Ninth People’s Hospital Affiliated Shanghai Jiaotong University School of Medicine, Shanghai 200011, People’s Republic of China
Correspondence: Changqian Wang Tel +86-21-23271699-5836
Email [email protected]
Introduction: The aim of this study was to screen the leading compounds of natural origin with anti-angiogenic potential and to investigate their anti-angiogenic mechanism preliminarily.
Materials and Methods: An initial screening of 240 compounds from the Natural Products Collection of MicroSource was performed using the transgenic zebrafish strain Tg [fli1a: enhanced green fluorescent protein (EGFP)]y1. The zebrafish embryos at 24 h post-fertilization were exposed to the natural compounds for an additional 24 h; then, morphological changes in the intersegmental vessels (ISVs) were observed and quantified under a fluorescence microscope. The expression profiles of angiogenesis-related genes in the zebrafish embryos were detected using quantitative real-time PCR.
Results: Five compounds were identified with potential anti-angiogenic activity on the zebrafish embryogenesis. Among them, deoxysappanone B 7.4ʹ-dimethyl ether (Deox B 7,4) showed anti-angiogenic activity on the formation of ISVs in a dose-dependent manner. The inhibition of ISV formation reached up to 99.64% at 5 μM Deox B 7,4. The expression of delta-like ligand 4 (dll4), hes-related family basic helix-loop-helix transcription factor with YRPW motif 2, ephrin B2, fibroblast growth factor receptor (fgfr) 3, cyclooxygenase-2, protein tyrosine phosphatase, receptor type B (ptp-rb), phosphoinositide-3-kinase regulatory subunit 2, slit guidance ligand (slit) 2, slit3, roundabout guidance receptor (robo) 1, robo2, and robo4 were down-regulated, while vascular endothelial growth factor receptor-2, fgfr 1, and matrix metallopeptidase 9 were up-regulated in the zebrafish embryos treated with Deox B 7,4.
Conclusion: Deox B 7,4 has a therapeutic potential for the treatment of angiogenesis-dependent diseases and may exert anti-angiogenic activities by suppressing the slit2/robo1/2, slit3/robo4, cox2/ptp-rb/pik3r2, and dll4/hey2/efnb2a signaling pathways as well as activation of vegfr-2/fgfr1/mmp9.
Keywords: angiogenesis, natural products, deoxysappanone B 7, 4ʹ-dimethyl ether, delta-like ligand 4, slit guidance ligand/roundabout guidance receptor, protein tyrosine phosphatase, receptor type B
Introduction
Angiogenesis is the physiological process by which new blood vessels generate from pre-existing vessels.1,2 Physiological angiogenesis is a normal and a vital process in embryo development and wound healing. However, abnormally accelerated angiogenesis or pathological angiogenesis is associated with several diseases, including cancer.3 The proliferation and the metastatic spread of cancer cells depend on an adequate supply of nutrients and oxygen, which are key components of blood.4 Both activators and inhibitors regulate angiogenesis. Keeping a balance between activators and inhibitors is crucial for vascular homeostasis.3 Angiogenesis inhibitors may systematically disrupt blood vessel formation or support removal of existing vessels by acting on several proteins that have been identified as angiogenic activators, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), angiogenin, transforming growth factor (TGF)-α, TGF-β, tumor necrosis factor-α, platelet-derived endothelial growth factor, granulocyte colony-stimulating factor, placental growth factor, interleukin-8, hepatocyte growth factor, and epidermal growth factor.5 Therefore, the discovery of novel angiogenic inhibitors may help to reduce both morbidity and mortality related to carcinomas.
Due to a vast molecular, structural, and chemical diversity, natural products (NPs) have a higher likelihood to exhibit specific bioactivities than the ones exhibited by many synthetic small molecules.6 The use of NPs in drug discovery has significantly declined because of the phenomenal advances in high-throughput screening and combinatorial synthesis during the past two decades; however, they remain the best sources of drugs and drug leads.7 The vast, untapped, ecological biodiversity of NPs favor their consideration in drug discovery and drug development. The Zebrafish is an ideal in vivo model platform to systematically identify bioactive natural products with therapeutic potential. There is a strong conservation in genetics, development, and physiology between the zebrafish and humans.8 The zebrafish embryos are optically transparent, allowing for visual screening, and observation of the effects of drugs on internal organs during the embryogenesis process.9 The zebrafish embryos are small, only 1 mm to 5 mm, and consequently compatible with microtiter plates for screening, and require only microgram amounts of the compound to be tested.10 The ease of maintenance at high densities and high fecundity of the zebrafish provide representative significant statistical data for experimental analysis.9
In the present study, we screened 240 compounds from the Natural Products Collection of MicroSource Discovery Systems [NP150704]11 (http://www.msdiscovery.com) using a transgenic zebrafish line with fluorescent blood vessels.12 Among the screened compounds, five compounds, dihydro-munduletone, deoxysappanone B 7,4ʹ-Dimethyl Ether (Deox B 7,4), Mundoserone, ononetin, and pomiferin, were identified to have potential anti-angiogenic activity on the zebrafish embryogenesis. Since the anti‑angiogenic activity of dihydro-munduletone and mundoserone has been reported in previous studies,13,14 we focused on the anti-angiogenic activity of Deox B 7,4 on the zebrafish embryos in this study. qRT-PCR was used to examine the expression pattern of various genes that regulate angiogenesis in the zebrafish embryos treated with Deox B 7,4. Our data supported the potential utility of Deox B 7,4 in the treatment of angiogenic-dependent diseases.
Materials and Methods
Zebrafish Care and Maintenance
The zebrafish strain Tg (fli1a:EGFP)y1 with transgenic endothelial cells expressing EGFP was obtained from the Shanghai Research Center for Model Organisms (Shanghai, China). The adult zebrafishes were maintained at 28.5°C under a 14 h light/10 h dark cycle. The embryos were generated by natural pair-wise breeding. Five pairs to six pairs of the zebrafishes can produce up to 200 embryos–300 embryos per mating session. The embryos were raised at 28.5°C in deionized water containing 0.2% Instant Ocean Salt (Aquarium Systems, Inc., Mentor, OH, USA). The embryos were washed and staged, as described in the previous study.15 The establishment and characterization of the fli1a-EGFP transgenic lines were performed according to the methods described by a previous study.16 All protocols comply with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Drug Library and Drug Treatment
The 240 compounds used for the initial screening in this study were obtained from the Natural Products Collection Library (MicroSource Discovery Systems, Gaylordsville, CT, USA) (Supplementary Table 1). All compounds in the product were at 95% purity or greater and were provided in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA) solution at a concentration of 10 mM. The compounds were aliquoted into ten 96-well plates with one compound per well and 80 compounds per plate. The plates were stored at −80°C for the initial screening and re-testing procedure.
At 24 h post-fertilization (hpf), the Tg (fli1a:EGFP) embryos were distributed into 12-well plates (30 embryos/well) with 250 μL deionized water containing 0.2% Instant Ocean Salt in each well. The compounds were diluted in 0.1% DMSO and added to each well at a final concentration of 10 μM. PTK787 (5 μM, Selleck Chemicals, Houston, TX, USA), a vascular endothelial growth factor receptor (VEGFR) antagonist,17 was used as a positive control and 0.1% DMSO was used as a negative control. To observe the concentration-dependent anti-angiogenic effects of Deox B 7,4, the Tg (fli1a:EGFP) embryos were added to 12-well plates (30 embryos/well) and exposed to 1 μM, 2.5 μM, and 5 μM of Deox B 7,4 for 24 h.
Angiogenesis Assessment
After 48 hpf, ten embryos in each well were anesthetized with 0.016% MS-222 (tricaine methanesulfonate, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and the morphology of the intersegmental vessels (ISVs), which normally connect the dorsal longitudinal anastomotic vessels (DLAVs) to the dorsal aorta (DA), was visualized and assessed using the Nikon SMZ 1500 Fluorescence microscope (Nikon Corp., Tokyo, Japan) equipped with a digital camera. Quantitative image analyses were performed using image-based morphometric analysis software NIS-Elements D3.1 (Nikon Corp., Tokyo, Japan). To optimally visualize the morphology of ISVs, the Adobe Photoshop 7.0 software (Adobe, San Jose, CA, USA) was used to adjust the images for their levels of brightness, contrast, hue, and saturation. Lead compounds were defined as the compounds which inhibited the total number of ISVs formed and/or the number of complete ISVs (ie, the number of ISVs that connect DA to DLAV). Drug effects were calculated according to the following formula:
Inhibition (%) = (1 – ISVconcentration of compound/ISVvehicle) × 100%.
Drug Toxicity
The remaining embryos in each well of 12-well plates were continuously treated with compounds for another 24 h, the toxic effects of compounds on the zebrafish embryo development were observed under the Nikon SMZ 1500 Fluorescence microscope.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from 30 to 50 embryos that were treated with 5 μM Deox B 7,4, 5 μM PTK787, or DMSO for 24 h using TRIzol® (Roche, Basel, Switzerland) according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara, Otsu, Japan). The quantification of gene expression was performed in triplicates using the iQ™ SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) and the Mastercycler® Realplex system (Eppendorf, Hamburg, Germany). Primers used in this study have been listed in Table 1. β-actin was used as an internal reference. The relative gene expression was quantified based on the comparative threshold cycle method (2−ΔΔCt). All experiments were performed in triplicate.
 |
Table 1 Primer Sequences Used in This Study |
Statistical Analysis
All results were presented as means ± standard error of the mean. Differences between the two groups were evaluated using a t-test. For multiple comparisons, analysis of variance was employed. GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis and graphical representation of the data. P <0.05 was regarded as statistically significant (*), P <0.01 was considered as statistically very significant (**), and P <0.001 was considered as statistically highly significant (***).
Results
Angiogenesis Drug Screening
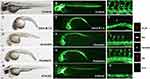
The transgenic zebrafish line Tg (fli1a:EGFP) was used for angiogenesis drug screening. Among 240 compounds from the Natural Products Collection Library, 5 compounds, dihydromunduletone, Deox B 7,4, ononetin, mundoserone, and pomiferin, exhibited strong anti-angiogenic activity on the zebrafish embryogenesis (Table 2). Dihydromunduletone inhibits angiogenic vessel growth in the zebrafish, which has been reported in the previous study.17 The zebrafish embryos in the vehicle group developed complete ISVs, DA, and DLAV, while the formation of ISVs, DA, and DLAV in the zebrafish embryos treated with PTK787 was completely inhibited. In comparison of the vehicle group to the positive controls, the embryos treated with Deox B 7,4, ononetin, or pomiferin presented a few incomplete ISVs, and the primary sprouts of DA can be occasionally observed (Figure 1). The chemical structures of Deox B 7,4, ononetin, and pomiferin have been presented in Figure 2. Twelve compounds showed toxic activity on the zebrafish embryos, mundulone, celastrol, purpurogallin-4-carboxylic acid, purpurogallin, and agaric acid caused 100% mortality; 4-nonylphenol caused 80% mortality; madecassic acid caused 40% mortality. 7-desacetoxy-6,7-dehydrogedunin, obliquin, and rhamnetin exhibited organ-specific (skin, muscle, heart, and fin) toxicity. Moreover, 3-methylcatechol inhibited pigmentation, while xanthone inhibited hatching (Table 2).
 |
Table 2 Summary of the Initial Screening of 240 Compounds from the Natural Products Collection Library |
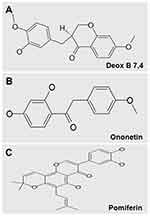 |
Figure 2 Chemical structures of Deox B 7,4 (A), ononetin (B) and pomiferin (C). Deoxysappanone B 7,4ʹ-Dimethyl Ether, Deox B 7,4. |
Deox B 7,4 Inhibited the Formation of ISVs in the Zebrafish Embryos in a Dose-Dependent Manner
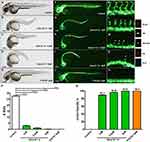
The dose-response effect of Deox B 7,4 on ISVs formation of the zebrafish was further investigated. After the Tg (fli1a:EGFP)y1 embryos were treated with 1 μM, 2.5 μM, and 5 μM of Deox B 7,4, respectively, for 24 h, the number of complete ISVs formed in the zebrafish embryos at 48 hpf declined accordingly (Figure 3). The number of complete ISVs in the zebrafish embryos treated with Deox B 7,4 was significantly reduced as compared to the number of ISVs in the vehicle group, and the inhibition rates of 1, 2.5, or 5 μM Deox B 7,4 on the formation of ISVs were up to 89.13%, 96.02%, or 99.64%, respectively (Figure 3P and Q).
Deox B 7,4 Regulated the Expression of Angiogenesis-Related Genes During Zebrafish Embryogenesis
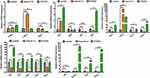
In order to unveil the molecular mechanisms underlying the Deox B 7,4-mediated inhibition of growth of angiogenic vessels in the zebrafish, qRT-PCR was performed to evaluate the expression of the genes that participated in the angiogenesis-associated signaling pathways in embryos, ie, delta-like ligand 4 (dll4), neurogenic locus notch homolog protein 1 (notch1a), hes-related family basic helix-loop-helix transcription factor with YRPW motif 2 (hey2), ephrin B2 (efnb2), slit guidance ligand (slit) 2, slit3, roundabout guidance receptor (robo) 1, robo2, robo4, vascular endothelial growth factor Aa (vegfaa), vegfr-1, vegfr-2, fibroblast growth factor receptor (fgfr) 1, fgfr2, fgfr3, fgfr4, cyclooxygenase-2 (COX2), matrix metallopeptidase 9 (mmp9), protein tyrosine phosphatase, receptor type B (ptp-rb), phosphoinositide-3-kinase regulatory subunit 2 (pik3r2). As shown in Figure 4, Deox B 7,4 treatment significantly suppressed the expression of dll4, hey2, efnb2, slit2, slit3, robo1, robo2, robo4, fgfr3, COX2, ptp-rb, and pik3r2; Deox B 7,4 treatment markedly promoted the expression of fgfr1, vegfr-2, and mmp9 as compared to the expression of genes in the vehicle group. No significant difference in the expression of notch1a, fgfr2, fgfr4, vegfaa, and vegfr-1 was observed between the vehicle group and the Deox B 7,4-treated group.
Discussion
Pathological angiogenesis has been reported in a range of angiogenesis-dependent diseases, including cancer,18 psoriasis,19 diabetic retinopathy,20 rheumatoid arthritis,21 and endometriosis.22 Tumor angiogenesis provides the tumor with a continuous supply of blood and nutrients in the tumor microenvironment, hence promotes tumor progression. Anti-angiogenesis strategies are considered as a promising new form of cancer treatment.3 Several anti-angiogenesis NP drugs have been approved by the Food and Drug Administration and continue to be used for cancer treatment;23–25 however, the wide range of side effects caused by these anti-angiogenesis agents limits their broad application and acceptability.26,27 Using the transgenic zebrafish strain Tg (fli1a:EGFP)y1, we screened 240 compounds from the Natural Products Collection Library and identified five lead compounds (dihydromunduletone, Deox B 7,4, ononetin, mundoserone, and pomiferin) with strong anti-angiogenic activity on angiogenic vessel-growth of the zebrafish. Of these five leads, dihydromunduletone has been demonstrated to inhibit the intersegmental vessel growth in the zebrafish and possesses antitumor activity against prostate cancer.13 Besides, the anti‑angiogenic activity of Mundoserone has been reported in our previous study.14
In this study, we used zebrafish strain Tg (fli1a:EGFP)y1 as the in vivo model for screening of natural products because of its high genetic, pharmacologic and physiologic similarity with humans. Besides, the zebrafish is usually small, optical transparency and produces a large number of embryos. These characters define zebrafish as an ideal in vivo model for drug discovery compared with other animals, such as rats and mice.28,29 The zebrafish strain Tg (fli1a:EGFP)y1 is a transgenic zebrafish strain which exhibits vasculature-specific expression of EGFP in tail and trunk during embryonic and larval development. This strain has been frequently used as the in vivo model for studying the angiogenic activity of nature products.10,30
The anti-angiogenic activity of mundoserone on zebrafish embryogenesis has been previously reported by a study conducted by our group.14 Deox B 7,4 seemed to exhibit the most robust anti-angiogenic activity on angiogenic vessel-growth of the zebrafish as compared to the anti-angiogenic activity exhibited by the other four compounds (Figure 1). In this study, we further evaluated Deox B 7,4 for its anti-angiogenic efficacy and mechanism of action.
Deox B 7,4 is a compound with anti-leukemic activity.31 In the present study, we found that Deox B 7,4 can also function as an anti-angiogenic agent to inhibit the vessel growth of the zebrafish. The Tg (fli1a:EGFP)y1 embryos at 24 hpf were exposed to 1, 2.5, and 5 μM of Deox B 7,4, respectively, for 24 h, the number of complete ISVs formed in the zebrafish embryos at 48 hpf was observed under a fluorescence microscope. Deox B 7,4 treatment in a dose-dependent manner inhibited the ISVs formation in the zebrafish embryos, and almost completely inhibited the ISVs formation at 5 μM (99.64% inhibition, Figure 3). These findings suggest that Deox B 7,4 possesses anti-angiogenic activities and has a therapeutic potential for the treatment of angiogenesis-dependent disorders, especially cancer.
Pro-angiogenic factors, VEGF and FGF, can stimulate angiogenesis through specific binding to cell surface-expressed receptors with tyrosine kinase activity. The activation of receptor kinases leads to downstream signaling cascades that regulate various aspects of endothelial cells like differentiation, proliferation, and migration.32 In this study, we detected the gene expression of vegfaa, VEGF and FGF receptors vegfr-1, vegfr-2, fgfr1, fgfr2, fgfr3, and fgfr4 in the zebrafish embryos treated with Deox B 7,4. We observed that fgfr3 was significantly down-regulated in the zebrafish embryos as compared to the levels observed in the vehicle group (Figure 4), suggesting that Deox B 7,4 may exert anti-angiogenesis activity via suppressing fgfr3 in the zebrafish. The up-regulation of vegfr-2 and fgfr1 may be attributed to the stress-response of Deox B 7,4 treatment. The FGF signaling pathway is responsible for the maintenance of vascular integrity through enhancement of the stability of VE-cadherin at adherens junctions. Protein tyrosine phosphatases (PTPs) are involved in the maintenance of the VE-cadherin-catenin complex at adherens junctions.33 Blockade of the FGF signaling pathway has been demonstrated to accelerate the degradation of PTP non-receptor type 11 (PTPN11) and disrupts the PTPN11/VE-cadherin interaction, leading to the loss of endothelial junction integrity and loss of endothelial cell elongation ability.33,34 In addition, PTP has been reported to regulate the phosphoinositide 3-kinase (PI3K) signaling pathway through dephosphorylation of pik3r2.35 In this study, the expression of ptp-rb and pik3r2 in the zebrafish was inhibited by Deox B 7,4 treatment, which is consistent with the results of the previous studies.
COX-2 and matrix metallopeptidases are the vital regulators of inflammatory angiogenesis; the inhibition of expression of COX-2 and expression of mmp9 has been shown to contribute to reduced angiogenesis in endothelial cells.36 Consistently, our data showed that COX-2 expression in the zebrafish was significantly inhibited by Deox B 7,4 treatment (Figure 4). The expression of mmp9 was substantially up-regulated by Deox B 7,4 treatment, suggesting that the anti-angiogenesis potential of Deox B 7,4 in the zebrafish embryos might not dependent on inhibition of mmp9 expression. However, the molecular mechanism of the upregulation of mmp9 needs further research.
The crucial roles of secreted slit proteins and their respective robo receptors during angiogenesis have been highlighted in a number of studies. Interaction of ROBO1/2 and SLITs, especially SLIT2, favors angiogenesis by promoting endothelial cell motility and cell polarity.37,38 Vertebrate ROBO4 is reported to control angiogenesis and blood vessel permeability.39,40 In human endothelial cells, ROBO1/ROBO4 heterodimer promotes cell migration.41 As a common cause of preeclampsia, hypoxia can significantly enhance the levels of SLIT3, ROBO1, and ROBO4 in placental endothelial cells.42 In this study, the potential anti-angiogenic agent Deox B 7,4 significantly inhibited the expression of slit2, slit3, robo1, robo2, and robo4 in the zebrafish embryos (Figure 4).
The Dll4/Notch signaling pathway controls arterial-venous differentiation and angiogenic tip cell selection during embryonic vascular development.43–46 In endothelial cells, activation of dll4/Notch transcriptionally enhances efnb2 expression, while decreased dll4/Notch function leads to ectopic arterial expression of efnb2 and arteriovenous malformations (AVMs).47 Furthermore, hey1 and hey2 are also canonical targets of Notch in endothelial cells.47–49 In this study, dll4 and Notch-targeted hey2 and efnb2 were markedly down-regulated in the zebrafish embryos by Deox B 7,4 treatment (Figure 4), suggesting Deox B 7,4 treatment may result in AVMs during vascular development.
There were several limitations to this study. First, Deox B 7,4 exhibited multiple severe effects on the zebrafish embryogenesis in this study. Deox B 7,4 treatment not only resulted in the inhibition of ISVs formation but also led to ocular dysplasia and skeletal dysplasia in the zebrafish embryos, suggesting the roles of Deox B 7,4 treatment in the zebrafish embryogenesis are complex and may be involved in multiple mechanisms. Further studies are needed that focus on the side effects caused by Deox B 7,4 treatment and the underlying mechanisms. Second, the safety and the toxicity of Deox B 7,4 treatment for the juvenile zebrafish and the adult zebrafish with full development of major organ systems remain unknown. As a potential anti-angiogenic drug, these effects of Deox B 7,4 treatment should be investigated in future studies. Third, the in vitro anti-angiogenic functions of human endothelial cells were not conducted in this study. Therefore, this is a preliminary study about the anti-angiogenic functions of Deox B 7,4 and much investigation is still warranted before its clinical use.
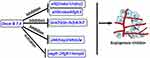
In conclusion, five leads out of 240 compounds from the Natural Products Collection Library were identified with anti-angiogenic activities. Among them, treatment with Deox B 7,4 inhibited the formation of ISVs in the zebrafish embryos in a dose-dependent manner. The expression profile of angiogenesis-related genes suggested that Deox B 7,4 treatment may exert anti-angiogenic functions by inhibiting the slit2/robo1/2, the slit3/robo4, the cox2/ptp-rb/pik3r2, and the dll4/hey2/efnb2a signaling pathways as well as activation of vegfr-2/fgfr1/mmp9 (Figure 5). Further investigations need to be conducted in the future to unveil how Deox B 7,4 mediates the above-mentioned signaling pathways during vascular development.
Data Sharing Statement
All data generated or analyzed during this study have been included in this published article.
Ethics Approval and Consent to Participate
All protocols comply with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The protocol was approved by Institutional Animal Care and Use Committee of the Shanghai Research Center for Model Organisms (IACUC No. 2015-0022).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer – a brief overview. Adv Biol Regul. 2015;57:1–9. doi:10.1016/j.jbior.2014.09.013
2. Birbrair A, Zhang T, Wang ZM, et al. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307(1):C25–38. doi:10.1152/ajpcell.00084.2014
3. Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. 2017;5(2):34. doi:10.3390/biomedicines5020034
4. Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–219. doi:10.2147/vhrm.2006.2.3.213
5. Mousa SA, Davis PJ. Angiogenesis and Anti-Angiogenesis Strategies in Cancer Therapeutics. Academic Press; 2017.
6. Shen B. A new golden age of natural products drug discovery. Cell. 2015;163(6):1297–1300. doi:10.1016/j.cell.2015.11.031
7. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. doi:10.1021/np200906s
8. Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol. 2002;1(1 Pt 1):41–48. doi:10.1089/154065802761001293
9. Mandrekar N, Thakur NL. Significance of the zebrafish model in the discovery of bioactive molecules from nature. Biotechnol Lett. 2009;31(2):171–179. doi:10.1007/s10529-008-9868-1
10. Crawford AD, Liekens S, Kamuhabwa AR, et al. Zebrafish bioassay-guided natural product discovery: isolation of angiogenesis inhibitors from East African medicinal plants. PLoS One. 2011;6(2):e14694. doi:10.1371/journal.pone.0014694
11. Pantel J, Williams SY, Mi D, et al. Development of a high throughput screen for allosteric modulators of melanocortin-4 receptor signaling using a real time camp assay. Eur J Pharmacol. 2011;660(1):139–147. doi:10.1016/j.ejphar.2011.01.031
12. Cross LM, Cook MA, Lin S, Chen J-N, Rubinstein AL. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscle Thromb Vasc Biol. 2003;23(5):911–912. doi:10.1161/01.ATV.0000068685.72914.7E
13. Wang C, Tao W, Wang Y, et al. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol. 2010;58(3):418–426. doi:10.1016/j.eururo.2010.05.024
14. Chen K, Wang C, Fan Y, et al. Identification of mundoserone by zebrafish in vivo screening as a natural product with anti-angiogenic activity. Exp Ther Med. 2018;16(6):4562–4568. doi:10.3892/etm.2018.6748
15. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi:10.1002/aja.1002030302
16. Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248(2):307–318. doi:10.1006/dbio.2002.0711
17. Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1(3):257–267. doi:10.1016/S1535-6108(02)00042-9
18. Xu H, Zhang Y, Pena MM, Pirisi L, Creek KE. Six1 promotes colorectal cancer growth and metastasis by stimulating angiogenesis and recruiting tumor-associated macrophages. Carcinogenesis. 2017;38(3):281–292. doi:10.1093/carcin/bgw121
19. Malecic N, Young HS. Excessive angiogenesis associated with psoriasis as a cause for cardiovascular ischaemia. Exp Dermatol. 2017;26(4):299–304. doi:10.1111/exd.13310
20. Abu El-Asrar AM, Struyf S, Mohammad G, et al. Osteoprotegerin is a new regulator of inflammation and angiogenesis in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(7):3189–3201. doi:10.1167/iovs.16-20993
21. Lu Y, Yu SS, Zong M, et al. Glucose-6-phosphate isomerase (g6pi) mediates hypoxia-induced angiogenesis in rheumatoid arthritis. Sci Rep. 2017;7:40274. doi:10.1038/srep40274
22. Rakhila H, Al-Akoum M, Bergeron ME, et al. Promotion of angiogenesis and proliferation cytokines patterns in peritoneal fluid from women with endometriosis. J Reprod Immunol. 2016;116:1–6. doi:10.1016/j.jri.2016.01.005
23. Liang F, Han Y, Gao H, et al. Kaempferol identified by zebrafish assay and fine fractionations strategy from dysosma versipellis inhibits angiogenesis through VEGF and FGF pathways. Sci Rep. 2015;5:14468. doi:10.1038/srep14468
24. Yang GW, Jiang JS, Lu WQ. Ferulic acid exerts anti-angiogenic and anti-tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int J Mol Sci. 2015;16(10):24011–24031. doi:10.3390/ijms161024011
25. Zhao D, Qin C, Fan X, Li Y, Gu B. Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur J Pharmacol. 2014;723:360–367. doi:10.1016/j.ejphar.2013.10.069
26. Oh WK, McDermott D, Porta C, et al. Angiogenesis inhibitor therapies for advanced renal cell carcinoma: toxicity and treatment patterns in clinical practice from a global medical chart review. Int J Oncol. 2014;44(1):5–16. doi:10.3892/ijo.2013.2181
27. Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised Phase 3 trial. Lancet Oncol. 2014;15(3):286–296. doi:10.1016/S1470-2045(14)70030-0
28. Pitchai A, Rajaretinam RK, Freeman JL. Zebrafish as an emerging model for bioassay-guided natural product drug discovery for neurological disorders. Medicines (Basel). 2019;6(2):61.
29. Crawford AD, Esguerra CV, de Witte PA. Fishing for drugs from nature: zebrafish as a technology platform for natural product discovery. Planta Med. 2008;74(6):624–632. doi:10.1055/s-2008-1034374
30. Lam HW, Lin HC, Lao SC, et al. The angiogenic effects of angelica sinensis extract on huvec in vitro and zebrafish in vivo. J Cell Biochem. 2008;103(1):195–211. doi:10.1002/jcb.21403
31. Bernard D, Gebbia M, Prabha S, et al. Select microtubule inhibitors increase lysosome acidity and promote lysosomal disruption in acute myeloid leukemia (aml) cells. Apoptosis. 2015;20(7):948–959. doi:10.1007/s10495-015-1123-3
32. Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22(4):201–207. doi:10.1016/S0165-6147(00)01676-X
33. Hatanaka K, Lanahan AA, Murakami M, Simons M, Ushio-Fukai M. Fibroblast growth factor signaling potentiates ve-cadherin stability at adherens junctions by regulating shp2. PLoS One. 2012;7(5):e37600. doi:10.1371/journal.pone.0037600
34. Sauteur L, Krudewig A, Herwig L, et al. Cdh5/ve-cadherin promotes endothelial cell interface elongation via cortical actin polymerization during angiogenic sprouting. Cell Rep. 2014;9(2):504–513. doi:10.1016/j.celrep.2014.09.024
35. Pestell RG. New roles of cyclin d1. Am J Pathol. 2013;183(1):3–9. doi:10.1016/j.ajpath.2013.03.001
36. Scoditti E, Calabriso N, Massaro M, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through mmp-9 and cox-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527(2):81–89. doi:10.1016/j.abb.2012.05.003
37. Dubrac A, Genet G, Ola R, et al. Targeting nck-mediated endothelial cell front-rear polarity inhibits neovascularization. Circulation. 2016;133(4):409–421. doi:10.1161/CIRCULATIONAHA.115.017537
38. Rama N, Dubrac A, Mathivet T, et al. Slit2 signaling through robo1 and robo2 is required for retinal neovascularization. Nat Med. 2015;21(5):483–491. doi:10.1038/nm.3849
39. Bedell VM, Yeo SY, Park KW, et al. Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102(18):6373–6378. doi:10.1073/pnas.0408318102
40. Jones CA, Nishiya N, London NR, et al. Slit2-robo4 signalling promotes vascular stability by blocking arf6 activity. Nat Cell Biol. 2009;11(11):1325–1331. doi:10.1038/ncb1976
41. Sheldon H, Andre M, Legg JA, et al. Active involvement of robo1 and robo4 in filopodia formation and endothelial cell motility mediated via wasp and other actin nucleation-promoting factors. FASEB J. 2009;23(2):513–522. doi:10.1096/fj.07-098269
42. Liao WX, Laurent LC, Agent S, Hodges J, Chen DB. Human placental expression of slit/robo signaling cues: effects of preeclampsia and hypoxia. Biol Reprod. 2012;86(4):111. doi:10.1095/biolreprod.110.088138
43. Lawson ND, Scheer N, Pham VN, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128(19):3675–3683.
44. Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi:10.1038/nature05571
45. Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445(7129):781–784. doi:10.1038/nature05577
46. Shirota H, Klinman DM. Recent progress concerning cpg DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014;13(2):299–312. doi:10.1586/14760584.2014.863715
47. Iso T, Maeno T, Oike Y, et al. Dll4-selective notch signaling induces ephrinb2 gene expression in endothelial cells. Biochem Biophys Res Commun. 2006;341(3):708–714. doi:10.1016/j.bbrc.2006.01.020
48. Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic hlh transcription factor hesr-1 and downregulates vegfr-2/kdr expression. Microvasc Res. 2002;64(3):372–383. doi:10.1006/mvre.2002.2443
49. Rochon ER, Wright DS, Schubert MM, Roman BL. Context-specific interactions between notch and alk1 cannot explain alk1-associated arteriovenous malformations. Cardiovasc Res. 2015;107(1):143–152. doi:10.1093/cvr/cvv148
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.