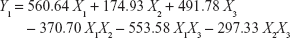Back to Journals » International Journal of Nanomedicine » Volume 13
In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: formulation, characterization, and in vivo performance
Authors Ahmed OAA , Badr-Eldin SM
Received 26 July 2018
Accepted for publication 11 September 2018
Published 11 October 2018 Volume 2018:13 Pages 6325—6335
DOI https://doi.org/10.2147/IJN.S181587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Osama AA Ahmed,1,2 Shaimaa M Badr-Eldin1,3
1Department of Pharmaceutics, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Minia University, Minia, Egypt; 3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Background: Raloxifene hydrochloride (RLX) is approved by the US Food and Drug Administration for the treatment and prevention of osteoporosis, in addition to reducing the risk of breast cancer in postmenopausal women. RLX has the disadvantages of low aqueous solubility, extensive presystemic intestinal glucuronidation, and first-pass metabolism, resulting in a limited bioavailability of only 2%. The aim of this work was to enhance the bioavailability of RLX via the formulation of an in situ nasal matrix (misemgel) comprising micelles made of vitamin E and D-α-tocopheryl polyethylene glycol 1000 succinate and nanosized self-emulsifying systems (NSEMS).
Materials and methods: Optimization of the RLX-loaded NSEMS was performed using a mixture design. The formulations were characterized by particle size and then incorporated into an in situ nasal gel. Transmission electron microscopy, bovine nasal mucosa ex vivo permeation, and visualization using a fluorescence laser microscope were carried out on the RLX in situ misemgel comparing with raw RLX in situ gel. In addition, the in vivo performance was studied in rats.
Results: The results revealed improved permeation parameters for RLX misemgel compared with control gel, with an enhancement factor of 2.4. In vivo studies revealed a 4.79- and 13.42-fold increased bioavailability for RLX in situ misemgel compared with control RLX in situ gel and commercially available tablets, respectively. The obtained results highlighted the efficacy of combining two different formulations to enhance drug delivery and the benefits of utilizing different possible paths for drug absorption.
Conclusion: The developed in situ misemgel matrix could be considered as a promising multifunctional platform for nasal delivery which works based on a dual-absorption mechanism.
Keywords: micelles, nanosized self-emulsifying systems, mixture design, fluorescence laser microscope, permeation, pharmacokinetics
Introduction
Raloxifene hydrochloride (RLX) is a second-generation selective estrogen receptor modulator (SERM) belonging to benzothiophene class and has estrogen agonist effects on bone and cholesterol metabolism. In contrast, it has estrogen antagonist effects on mammary glands and uterine tissue. It has proven efficacy in the prevention of bone loss and breast cancer. RLX was the first SERM to be approved by the US Food and Drug Administration (FDA) for the treatment and prevention of postmenopausal osteoporosis in 1997.1 It was also granted FDA approval for reducing the risk of breast cancer in postmenopausal women in 2007.2 However, RLX has low aqueous solubility. In addition, it undergoes extensive presystemic intestinal glucuronidation and Phase II metabolism in the liver (the first-pass effect). Due to these limitations, RLX has a less oral bioavailability of only 2%.3,4
Arising from the nanotechnology revolution, nanovehicles were introduced in the field of nanomedicine as promising drug delivery systems due to their potential benefits of reducing adverse side effects and enhancing the bioavailability of drugs by improving their solubility and/or permeability.5 Nanosized self-emulsifying systems (NSEMS) and micelles are types of nanoscopic carrier systems that have recently gained attention due to their potential to optimize the bioavailability of a variety of drugs.6–9
NSEMS are thermodynamically stable isotropic mixtures with a high solvent capacity made up of oil, surfactants, and/or co-surfactants that can spontaneously form an oil-in-water emulsion upon exposure to aqueous media under mild agitation.10,11 These systems are suitable vehicles for hydrophobic drugs as they bypass the dissolution step in vivo and therefore enhance the absorption and bioavailability of such drugs.12
Micelles are produced by the aggregation of the ionic or nonionic surfactant monomers that comprise hydrophobic compartments. These compartments are made up of a nonpolar surfactant tail part and amphiphilic shells that deliver drugs to the biological membrane.13 Micelles are considered to be efficient drug carriers owing to their small size and ability to solubilize hydrophobic drugs.14,15 In addition, micelles have shown great potential as carriers of anticancer therapeutics.15–17 Recently, an aqueous-soluble form of natural vitamin E, known as D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), has gained attention as a promising pharmaceutical excipient for developing amphiphilic micelles (alone or mixed with other biomaterials) and are capable of encapsulating hydrophobic drugs.18,19
Nasal drug administration has attracted considerable attention in the field of drug delivery due to the distinct advantages that it can offer. These advantages include the avoidance of presystemic metabolism and rapid absorption when compared to the oral route, owing to the rich vasculature of the nasal mucosa.20 However, the rapid mucociliary clearance represents a major limitation for the delivery of drugs via the nasal route. To overcome this limitation, formulation approaches, including mucoadhesive and in situ gelling systems, have been utilized to prolong the residence time in the nasal cavity.21,22
In a previously published study, multi-absorption mechanism (MAM) was investigated for the formulation of a topical drug delivery system combining the advantages of both nanoemulsions and micelles. It was hypothesized that MAM would enable the absorption of drugs from the combined system to occur by different possible paths of absorption offered by the individual systems.23 In the present work, the MAM strategy was used for the formulation of an in situ misemgel for the nasal delivery of RLX. The in situ misemgel system is composed of two drug-loaded subsystems, namely, NSEMS and micelles, incorporated in an in situ gelling matrix. This system aims to utilize the benefits of both subsystems and the gelling matrix to the maximum. Both NSEMS and micelles can improve the solubility of the drug. In addition, NSEMS could promote absorption via the transcellular route due to its high lipid content, while micelles could enhance absorption via the paracellular route.23,24 The incorporation of both subsystems in an in situ gelling matrix could enable a prolonged residence time in the nasal cavity, thus allowing contact with the nasal mucosa for a longer period.20
Materials and methods
Materials
RLX, peppermint oil, Tween® 80, n-propranolol, n-butanol, polyethylene glycol (PEG) 200, propylene glycol, gellan gum, TPGS, and HPLC-grade methanol and acetonitrile were purchased from Sigma-Aldrich (St Louis, MO, USA). Sefsol® 218 was kindly supplied by Nikko Chemicals Company, Ltd. (Tokyo, Japan), linoleic acid was purchased from Thermo Fisher Scientific (Waltham, MA, USA), and Kolliphor® RH 40 was purchased from BASF Corp. (Ludwigshafen, Germany).
RLX solubility studies
The solubility of RLX in various oils (Sefsol® 218, peppermint oil, and linoleic acid), surfactants (Kolliphor® RH 40 and Tween® 80), and co-surfactants (n-propranolol, n-butanol, and PEG 200) was studied. Each vehicle (1 g) was added to a vial containing a known excess of RLX (300 mg). The vials were sealed and vortexed for 60 seconds, and then the obtained mixtures were shaken at 30°C±0.5°C until they reached equilibrium in a thermostatically controlled shaking water bath. The mixtures were subjected to centrifugation at 4,000 rpm for 5 minutes and then filtered through a Millipore membrane filter (0.45 μm). The concentration of the drug in the filtrate was then determined using a previously reported HPLC method adopted and validated in our laboratory.25
Emulsification efficiency of surfactants
A specified quantity of each surfactant (Kolliphor® and Tween® 80) was mixed with an equivalent quantity of the selected oil phase (linoleic acid). The mixtures were gently heated at 40°C to ensure homogenization of the components. An accurately weighed quantity of each mixture was diluted with double-distilled water. The feasibility of the emulsion formation was assessed by the number of volumetric flask inversions required to yield a uniform emulsion. The formed emulsions were inspected visually for turbidity, and the spectrophotometric absorbance at 638.2 nm was then determined after standing for 2 hours (UV-1601 PC; Shimadzu Corporation, Kyoto, Japan) using double-distilled water as a blank.26
Formulation and optimization of RLX-loaded NSEMS
RLX-loaded NSEMS were prepared by mixing specified amounts of the three components at 40°C. The mixture was vortexed for 60 seconds to achieve complete homogenization. A specified amount of RLX was then added to each vial and stirred until a clear mixture was obtained.
The optimization of NSEMS was carried out by applying a D-optimal mixture experimental design using Design-Expert® software (version 11; Stat-Ease, Inc., Minneapolis, MN, USA). The independent variables were the percentage concentration of the oil phase X1 (linoleic acid), the surfactant X2 (Kolliphor®:Tween® 80, 1:1, w/w), and the co-surfactant X3 (PEG 200). The concentration of components was adjusted to make up to 100%. The amount of drug was kept constant at 5 mg/g. The range of each of the three components was determined based on a preliminary phase diagram study (data not shown), in which only globule sizes of <200 nm were considered in the nanoemulsion region of the diagram.11 The selected ranges were 20%–60% for both X1 and X2 and 10%–30% for X3. The mean globule size (Y) was used as the response. The design comprised 16 runs, including a high and low level from each variable, centers of edges, constraint plane centroids, axial checkpoint, and an overall center point, as presented in Table 1. The response data were fitted to different mathematical models which are used to fit mixture design. The best fitting model was chosen based on various statistical parameters calculated by the software. The level of statistical significance was set at P≤0.05. Desirability function, followed by numerical optimization, was utilized to detect the composition of the optimum formula with a minimum globule size.
Formulation of RLX micelles
RLX-loaded TPGS micelles were prepared using the solvent evaporation technique previously reported by Somagoni et al.23 Accurately weighed amounts of TPGS and RLX were added to 2 mL of ethanol. Distilled water (20 mL) was then added to the clear solution, and the organic solvent was gradually evaporated to form micelles.
Globule size measurement
The mean globule size of the RLX-loaded NSEMS and micelles was determined using a Zetatrac particle size analyzer (Microtrac Inc., Montgomeryville, PA, USA). Each measurement was performed five times after appropriate dilution with double-distilled water, and the mean size was calculated.
Formulation and characterization of RLX in situ nasal misemgel
RLX in situ misemgel was prepared as previously described but with a slight modification.27 In brief, a specified amount of gellan gum (0.2%–0.7%, w/v) was sprinkled over boric/borax buffer (pH 7.4) at 80°C and the mixture was continuously stirred until a clear polymer dispersion was obtained. The formed dispersion was left to cool overnight. The optimized NSEMS and micelles were then incorporated into the obtained dispersion to achieve a final RLX concentration of 5 mg/g. The final misemgel was stored overnight in the refrigerator prior to evaluation. An in situ gel containing the raw drug at the same concentration was prepared for comparison.
For the characterization of the prepared in situ misemgel, the viscosity and gelling factor were determined in simulated nasal fluid (SNF) before and after gelation.27,28 In addition, measurement of the gel strength was carried out by measuring the time, in seconds, taken for a specific weight to penetrate 3 cm into the gel.
Transmission electron microscopy
The optimized RLX-loaded NSEMS, RLX-loaded TPGS micelles, in situ misemgel, and raw RLX in situ gel were studied using transmission electron microscopy (TEM). The sample was diluted with distilled water, and one drop was placed on a carbon-coated grid and the excess was removed with a filter paper. The sample was then stained with uranyl acetate, and the grid was visualized after drying.
Ex vivo permeation studies
Fresh nasal mucosa was carefully excised from bovine nasal cavities obtained from the local slaughterhouse. Mucosal samples were mounted between the two chambers of an automated Franz diffusion cell (MicroettePlus; Hanson Research, Chatsworth, CA, USA) with an area of 1.76 cm2, and allowed to equilibrate for 15 minutes in SNF at pH 6.5 and a temperature of 37°C.28,29 The misemgel or control raw drug-loaded gel (0.1 g) was placed in the donor chamber. SNF (pH 6.8, 7 mL) was used as the diffusion medium in the receptor chamber. The temperature was maintained at 37°C±0.5°C throughout the study, and the agitation rate was set to 400 rpm. At predetermined intervals of 0.5, 1, 2, 4, 6, 8, 12, 18, and 24 hours, aliquots of 1.5 mL were withdrawn and replaced with fresh SNF. The samples were analyzed using a previously reported HPLC method.25 All experiments were performed in triplicate. In addition, steady-state flux, Jss (μg/cm2 h), permeability coefficients, Pc (cm/h), and diffusion coefficients, D (cm2/h), were computed.
Visualization of misemgel nasal mucosal penetration using fluorescence laser microscopy
Rhodamine misemgel was prepared using the method described previously, but using rhodamine (0.15 μmol/mL) instead of RLX. For comparison, a control in situ gel loaded with raw rhodamine was prepared. The prepared gels were applied to freshly excised bovine nasal mucosa, and the penetration of rhodamine across the mucosa was investigated using an automated Franz diffusion cell as described previously. Mucosal tissue was removed after 0.5 and 5 hours and immersed in formalin solution (37%–40%, v/v) for 10 minutes, and then frozen to −20°C. The mucosa was cut into 4 μm-thick longitudinal sections using a microtome (SM2400; Leica Microsystems, Wetzlar, Germany) and then placed on glass cover slips. The prepared samples were finally examined using a Zeiss Axio Observer D1 Inverted DIC fluorescence laser microscope (Carl Zeiss AG, Oberkochen, Germany). The images were acquired with identical acquisition parameters, with minimum excitation and gain.30
In vivo pharmacokinetic performance of RLX in situ misemgel gel
The pharmacokinetic performance of the RLX nasal in situ misemgel was examined in male Wistar rats (n=36), weighing 200–250 g each, and compared to the control raw RLX-loaded in situ gel and commercially available RLX oral tablets. The study protocol was approved by the Research Ethics Committee, Faculty of Pharmacy, King Abdulaziz University, Kingdom of Saudi Arabia. The committee ensures that all animal use complies with the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes and the Guiding Principles in Care and Use of Animals (DHEW publication NIH 80-23). The study included three animal groups (I, II, and III), each containing 12 rats, with all animals in the study receiving an RLX dose of 5 mg/kg.31 Group I (standard control) received orally administered commercially available RLX tablets, group II (positive control) were subjected to intranasal application of raw RLX-loaded in situ gel, and group III (test) were subjected to intranasal application of RLX in situ misemgel. At specified time intervals, blood samples were withdrawn from the tail vein until 24 hours after drug administration. The blood samples were centrifuged at 5,000 rpm for 5 minutes to obtain plasma and stored at −80°C prior to analysis.
Extraction of plasma samples was carried out by vortex mixing 200 μL of plasma sample with 100 μL of methanol and 200 μL of acetonitrile for 10 seconds. The obtained mixture was subjected to centrifugation for 20 minutes at 5,300 rpm, and 20 μL of the supernatant was injected for analysis using a HPLC method modified from the one previously described by Yang et al and validated in our laboratory.32 An HPLC Agilent 1200 system was used with a diode array detector. The separation was performed on an Agilent Zorbax Eclipse Plus C18 column, 5 μm, 4.6×150 mm (Agilent Technologies, Santa Clara, CA, USA). The mobile phase was composed of an ammonium acetate buffer at pH 4 and acetonitrile (67:33, v/v), and the flow rate was 1 mL/min. An in vivo calibration curve was constructed for the range of 0.5–100 μg/mL.
Kinetica™ software (Version 4; Thermo Fisher Scientific, Waltham, MA, USA) was used to compute various pharmacokinetic parameters including the maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), and area under the plasma concentration-time curve (AUC). The computed parameters were statistically analyzed using SPSS® v16 software (SPSS Inc., Chicago, IL, USA). One-way ANOVA, followed by Tukey’s honestly significant difference multiple comparisons test, was performed on Cmax and AUC. The level of significance was considered to be P<0.05.
Results and discussion
Solubility studies
To present the drug in solution, NSEMS should spontaneously yield a clear monophasic liquid upon mixing with water and should possess good solvent characteristics.33 Amongst the oils screened, linoleic acid exhibited the highest solubility of RLX (9.07±0.81 mg/mL), and therefore, it was selected for further investigations. Regarding the surfactants screened, Kolliphor® RH 40 exhibited a higher solubility of RLX (2.17±0.18) compared to Tween® 80 (1.74±0.05). It is worth noting that with regard to surfactants, the main selection criteria are emulsification efficiency and bioactivity, with drug solubility coming next in terms of importance. Therefore, the final selection of surfactants is based on emulsification efficiency rather than drug solubility.4 PEG 200 was found to have the highest ability to solubilize RLX amongst the screened co-surfactants (2.44±0.21), and therefore, it was considered for further studies aimed at improving drug loading and reducing the time required for self-emulsification.
Emulsification efficiency of surfactants
Efficient emulsification was achieved by a small number of flask inversions (one flask inversion takes 1 second) and a high percentage of transmittance, that is, >90%.4 The results revealed that both the screened surfactants, Kolliphor® RH 40 (hydrophilic–lipophilic balance [HLB] 14–16) and Tween® 80 (HLB 15), showed a high emulsification ability for the selected oil (linoleic acid) with a percentage transmittance of 98.6% and 94.9%, respectively. The number of flask inversions required to achieve a homogenous emulsion was less than ten for both surfactants. The comparable emulsification ability of both surfactants could be due to their similar HLB values. It is worth noting that both surfactants have previously been reported to have both safety and bio-enhancing effects.34,35 Thus, it was decided to include both surfactants in further investigations in a ratio of 1:1 (w/w).
Optimization of RLX-loaded NSEMS
To obtain optimized RLX-loaded NSEMS with minimized globule sizes, D-optimal mixture experimental design was used. The experimental runs, independent variable levels, and mean globule size are compiled in Table 1. The RLX-loading capacity for all experiments was kept constant at 5 mg/g. The loading capacity was selected to be less than the minimum determined loading capacity for all prepared systems to avoid drug precipitation during the study.7 The variables and mean response were correlated using different polynomial equations that were computed using the experimental design software. Regression analysis revealed that the mean globule size best fit the quadratic model. This was evidenced by the higher R-squared statistic of 0.8956 when compared to the linear and special cubic models (R-squared values of 0.8202 and 0.8565, respectively). ANOVA of the results revealed the significance of the applied model, as evidenced by the P-value being <0.05. A lack-of-fit F-value of 3.00 implies that the lack of fit is not significant relative to the pure error. A nonsignificant lack of fit confirms fitting of data to the model. In addition, an adequate precision ratio of 9.234 (>4; the desirable value) ensures that the proposed model could be used to navigate the design space.36 A two-dimensional contour plot illustrating the effect of mixture components on the globule size of the prepared NSEMS is illustrated in Figure 1. The areas not included in the regression analysis due to the constraints applied in design are represented as white areas in the figure. The quadratic equation relating the mean globule size (Y1) to the variables was generated as follows:
|
|
The positive sign on the coefficients of individual components indicates their positive effect on the particle size. The highest coefficient of X1 indicates that the oil percentage has the most potent influence on particle size. This result is in accordance with previous studies which confirmed that the globule size of self-emulsifying systems increased with increasing oil content.37 The negative coefficients for the interaction terms indicate the negative effect of the combination of components on globule size. The optimum formulation composition was generated by utilizing the desirability approach followed by numerical optimization as follows: 24.55% linoleic acid, 60% Kolliphor® RH 40:Tween® 80 (1:1, w/w), and 15.45% PEG 200. The predicted globule size (186.16 nm) was in good agreement with the measured size (183.45 nm), indicating that there was no significant difference at P<0.05.
Formulation and characterization of RLX micelles
Vitamin E TPGS, an aqueous-miscible form of vitamin E, has been reported to successfully form a micellar drug delivery system due to its special amphiphilic structure which comprises a hydrophobic vitamin E part and a hydrophilic PEG chain.38 Therefore, the advantages of both parts are combined, meaning that it is able to extend the half-life of the drug in plasma and enhance the cellular uptake of drugs.39 TPGS micellar systems have proven to increase the solubility of various drugs because TPGS has a high HLB of 13.2. Moreover, vitamin E TPGS has a proven antioxidant activity.40 It is worth noting that RLX has previously been reported to possibly undergo oxidative degradation during storage.41,42 Thus, selecting TPGS to use in the formation of RLX micelles could not only offer the advantage of enhancing drug solubility but also limit the possible formation of its oxidative degradation products. TPGS was used at a higher concentration than the reported critical micelle concentration of 0.02% (w/w) to ensure the formation of thermodynamically stable micelles. Ethanol was selected as the solvent due to its good water miscibility and low vapor pressure, facilitating solvent removal, and because it has been labeled by the FDA as a “generally recognized as safe” substance. The gradual change of solvent from an organic to an aqueous one helped TPGS and RLX aggregate into micelles rather than precipitating out from the solution into the bulk. The prepared micelles exhibited a micellar size in the nano range of 23.32±1.59 nm and were able to dramatically enhance drug solubility.
Formulation and characterization of the RLX in situ nasal misemgel
The RLX in situ misemgel was prepared as a viscous liquid and showed an increase in viscosity and gelling factor after the addition of SNF with variations in the gellan gum concentrations of 0.2%–0.7%. At gellan gum concentrations of 0.4%–0.7% (w/v), the formulations were transformed from a viscous liquid into a pourable gel after the addition of SNF. The optimum concentration of gellan gum was found to be 0.6% (w/v). The characterization of the in situ misemgel was carried out to determine the most desirable viscosity to facilitate instillation into the nose as a liquid, and then undergo gelation to improve the drug residence time.27,43 The gelation of gellan gum after the addition of SNF could be attributed to the cross-linking of gellan gum helices with the SNF solution cations.44
Transmission electron microscopy
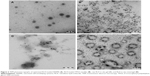
Optimized RLX-loaded NSEMS, RLX-loaded TPGS micelles, in situ misemgel, and raw RLX in situ gel were investigated using TEM. The results revealed that the optimized RLX-loaded NSEMS (Figure 2A) showed separate single RLX-loaded NSEMS droplets with a spherical outline. The results for the RLX-loaded TPGS micelles (Figure 2B) revealed a wide range of cluster sizes for the RLX-loaded TPGS micelles with larger clumps that could be interpreted as micelle aggregates. The raw RLX in situ gel showed discrete crystals of RLX distributed in the in situ gel matrix (Figure 2C), and the RLX in situ misemgel (Figure 2D) showed both clusters of RLX-loaded TPGS micelles and NSEMS droplets with some micelle clusters distributed on the outer surface of the NSEMS droplets.
Ex vivo permeation studies
Figure 3 illustrates the mean cumulative percentage of RLX that permeated through the freshly excised bovine nasal mucosa from the RLX in situ misemgel compared to the control RLX in situ gel. In addition, the permeation parameters were calculated (Figure 3, inset). It was evident that the misemgel formulation significantly improved the cumulative amount of permeated RLX when compared with the raw RLX in situ gel. The gradual controlled release exhibited by both formulations could be attributed to the inclusion of either the drug or the drug-loaded subsystems (NSEMS and micelles) in the in situ gelling matrix that was formed upon contact with the SNF. The computed permeation parameters showed a significant increase in diffusion and permeability coefficients of the misemgel compared with the control gel at a 95% level of significance (Figure 3, inset). In addition, the misemgel showed an enhancement factor of 2.4 compared to the raw RLX gel. The higher permeation of RLX from the misemgel could be attributed to the enhanced solubility of the drug, in addition to the utilization of maximum pathways of drug absorption. With regard to the micelles, TPGS could enhance the absorption via the paracellular route by temporarily disturbing the cell arrangement; in addition, the NSEMS could promote the transcellular absorption by passive diffusion due to lipid content.23,24,45
Visualization of misemgel nasal mucosal penetration using fluorescence laser microscopy
To visualize the extent of permeation of the prepared misemgel through the nasal mucosa, a fluorescence laser microscope was used and rhodamine was used as a model fluorescent agent to imitate the behavior of RLX in the formulation.46 The images showed limited fluorescence after 0.5 hours for both the misemgel and control gel formulations, whereas after 5 hours, the misemgel formulation clearly showed widespread intense green fluorescence in the mucosal tissue compared to the control gel, as depicted in Figure 4. The laser microscopy results indicated the enhancement of penetration through the nasal mucosal layers. Thus, the fluorescence laser microscopy images could support the hypothesis of the MAM that could be achieved by combining the two drug delivery subsystems, micelles and NSEMS. These results correlate well with the previously discussed ex vivo permeation studies.
  | Figure 4 Fluorescence laser microscopy images of permeation from rhodamine-labeled in situ misemgel and raw rhodamine gel through the bovine nasal mucosa after 0.5 and 5 hours. Magnification 400×. |
In vivo pharmacokinetic performance of RLX in situ misemgel
The in vivo pharmacokinetic performance of the RLX in situ nasal misemgel was assessed in rats and compared to the control RLX in situ nasal gel and oral RLX commercially available tablets. The RLX concentrations spiked in plasma exhibited a linear correlation with the peak areas, with a coefficient (r) of 0.9996. Validation of the assay demonstrated an acceptable precision with the relative SD being in the range of 9.1%–11.4% and 9.6%–10.9% for the intra-day assay and the inter-day assay, respectively. The RLX-fortified plasma samples showed that the extraction recovery ranged from 90.8%±6.4% to 101%±8.6%. The mean plasma concentrations of RLX following the oral administration of commercially available RLX tablets, the intranasal administration of control RLX gel, and the intranasal administration of RLX in situ misemgel are illustrated in Figure 5.
The RLX in situ misemgel reached its peak plasma concentration after 0.25 hour, while both the tablets and control gel reached their peak concentrations after 0.5 hour, respectively, indicating that the misemgel enabled enhanced drug absorption (Figure 5, inset). This result is in good agreement with the ex vivo permeation studies.
The Cmax and AUC of both the gel formulations were significantly higher than that of the tablet formulations, at P<0.05. In addition, the misemgel demonstrated a significantly higher Cmax and AUC (P<0.05) when compared to the raw RLX gel (Figure 5, inset). The RLX in situ misemgel relative bioavailability showed a 4.79- and 13.42-fold increase when compared to the raw RLX gel and the commercially available RLX tablets, respectively. The significantly higher absorption demonstrated by the RLX nasal gel formulations when compared to the oral tablets could be ascribed to utilizing the nasal route of administration, therefore overcoming the presystemic intestinal and hepatic first-pass of the drug. In addition, the higher absorption of RLX from the misemgel compared with the raw RLX gel is accredited to the improved solubility and permeability offered by the two delivery subsystems, micelles and NSEMS, incorporated into the in situ gel formulation. The in vitro studies in the present work proved the capability of both systems to improve RLX solubility either via micellization or via emulsification (NSEMS). Moreover, the enhanced permeation of RLX from the misemgel could highlight the potential benefits of the nanosize and the different paths of drug absorption offered by both micelles (paracellular route) and NSEMS (transcellular route) upon combining both in an in situ gel formulation. The multifunctional dual-absorption strategy applied in this study shows potential for the design and formulation of more effective drug delivery systems.
Conclusion
The multifunctional dual-absorption strategy applied in this study was successful in improving the delivery and bioavailability of RLX. The strategy utilized micelles- and NSEMS-loaded in situ gel for RLX nasal delivery. The RLX-loaded misemgel showed improved penetration of RLX through bovine nasal mucosa. Assessment of the pharmacokinetic parameters of the misemgel in rats revealed that the Cmax and AUC of the misemgel were significantly higher (P<0.05) when compared with commercially available RLX tablets and the control raw RLX gel. The enhanced permeation and pharmacokinetic parameters demonstrated by the RLX misemgel highlight the potential benefit of using the dual-absorption strategy offered by both micelles (paracellular route) and NSEMS (transcellular route) in the design and formulation of drug delivery systems.
Acknowledgment
The authors thank the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, for technical support and for funding this project (grant no G-658-166-37).
Disclosure
The authors report no conflicts of interest in this work.
References
Saini D, Fazil M, Ali MM, Baboota S, Ali J. Formulation, development and optimization of raloxifene-loaded chitosan nanoparticles for treatment of osteoporosis. Drug Deliv. 2015;22(6):823–836. | ||
Waters EA, Mcneel TS, Stevens WM, Freedman AN. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134(2):875–880. | ||
Kosaka K, Sakai N, Endo Y, et al. Impact of intestinal glucuronidation on the pharmacokinetics of raloxifene. Drug Metab Dispos. 2011;39(9):1495–1502. | ||
Elsheikh MA, Elnaggar YS, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: optimization and in vivo appraisal. Int J Nanomedicine. 2012;7:3787–3802. | ||
Nazari-Vanani R, Azarpira N, Heli H, Karimian K, Sattarahmady N. A novel self-nanoemulsifying formulation for sunitinib: Evaluation of anticancer efficacy. Colloids Surf B Biointerfaces. 2017;160:65–72. | ||
Qian J, Meng H, Xin L, et al. Self-nanoemulsifying drug delivery systems of myricetin: Formulation development, characterization, and in vitro and in vivo evaluation. Colloids Surf B Biointerfaces. 2017;160:101–109. | ||
Ahmed OA, Badr-Eldin SM, Tawfik MK, Ahmed TA, El-Say KM, Badr JM. Design and optimization of self-nanoemulsifying delivery system to enhance quercetin hepatoprotective activity in paracetamol-induced hepatotoxicity. J Pharm Sci. 2014;103(2):602–612. | ||
Ahmed OA, Afouna MI, El-Say KM, Abdel-Naim AB, Khedr A, Banjar ZM. Optimization of self-nanoemulsifying systems for the enhancement of in vivo hypoglycemic efficacy of glimepiride transdermal patches. Expert Opin Drug Deliv. 2014;11(7):1005–1013. | ||
Abourehab MA, Ahmed OA, Balata GF, Almalki WH. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood-brain barrier. Int J Nanomedicine. 2018;13:3679–3687. | ||
Devraj R, Williams HD, Warren DB, Porter CJ, Pouton CW. Choice of nonionic surfactant used to formulate type IIIA self-emulsifying drug delivery systems and the physicochemical properties of the drug have a pronounced influence on the degree of drug supersaturation that develops during in vitro digestion. J Pharm Sci. 2014;103(4):1050–1063. | ||
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391(1–2):203–211. | ||
Leichner C, Menzel C, Laffleur F, Bernkop-Schnürch A. Development and in vitro characterization of a papain loaded mucolytic self-emulsifying drug delivery system (SEDDS). Int J Pharm. 2017;530(1–2):346–353. | ||
Hekmat A, Attar H, Seyf Kordi AA, Iman M, Jaafari MR. New oral formulation and in vitro evaluation of docetaxel-loaded nanomicelles. Molecules. 2016;21(9):1265. | ||
Tripodo G, Chlapanidas T, Perteghella S, et al. Mesenchymal stromal cells loading curcumin-INVITE-micelles: a drug delivery system for neurodegenerative diseases. Colloids Surf B Biointerfaces. 2015;125:300–308. | ||
Wu J, Zhang H, Hu X, et al. Reduction-sensitive mixed micelles assembled from amphiphilic prodrugs for self-codelivery of DOX and DTX with synergistic cancer therapy. Colloids Surf B Biointerfaces. 2018;161:449–456. | ||
Zhang P, Zhang H, He W, Zhao D, Song A, Luan Y. Disulfide-linked amphiphilic polymer-docetaxel conjugates assembled redox-sensitive micelles for efficient antitumor drug delivery. Biomacromolecules. 2016;17(5):1621–1632. | ||
Hu X, Liu R, Zhang D, Zhang J, Li Z, Luan Y. Rational design of an amphiphilic chlorambucil prodrug realizing self-assembled micelles for efficient anticancer therapy. ACS Biomater Sci Eng. 2018;4(3):973–980. | ||
Wegmann M, Parola L, Bertera FM, et al. Novel carvedilol paediatric nanomicelle formulation: in-vitro characterization and in-vivo evaluation. J Pharm Pharmacol. 2017;69(5):544–553. | ||
Wang S, Chen R, Morott J, Repka MA, Wang Y, Chen M. mPEG-b-PCL/TPGS mixed micelles for delivery of resveratrol in overcoming resistant breast cancer. Expert Opin Drug Deliv. 2015;12(3):361–373. | ||
Saindane NS, Pagar KP, Vavia PR. Nanosuspension based in situ gelling nasal spray of carvedilol: development, in vitro and in vivo characterization. AAPS PharmSciTech. 2013;14(1):189–199. | ||
Hosny KM, Hassan AH. Intranasal in situ gel loaded with saquinavir mesylate nanosized microemulsion: preparation, characterization, and in vivo evaluation. Int J Pharm. 2014;475(1–2):191–197. | ||
Pawar D, Jaganathan KS. Mucoadhesive glycol chitosan nanoparticles for intranasal delivery of hepatitis B vaccine: enhancement of mucosal and systemic immune response. Drug Deliv. 2016;23(1):185–194. | ||
Somagoni J, Boakye CH, Godugu C, et al. Nanomiemgel – a novel drug delivery system for topical application – in vitro and in vivo evaluation. PLoS One. 2014;9(12):e115952. | ||
Ghori MU, Mahdi MH, Smith AM, Conway BR. Nasal drug delivery systems: An overview. Am J Pharmacol Sci. 2015;3(5):110–119. | ||
Trontelj J, Vovk T, Bogataj M, Mrhar A. HPLC analysis of raloxifene hydrochloride and its application to drug quality control studies. Pharmacol Res. 2005;52(4):334–339. | ||
Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329(1–2):166–172. | ||
Sayed EG, Hussein AK, Khaled KA, Ahmed OA. Improved corneal bioavailability of ofloxacin: biodegradable microsphere-loaded ion-activated in situ gel delivery system. Drug Des Devel Ther. 2015;9:1427–1435. | ||
Farid RM, Etman MA, Nada AH, Ebian AA. Formulation and in vitro evaluation of salbutamol sulphate in situ gelling nasal inserts. AAPS PharmSciTech. 2013;14(2):712–718. | ||
Martinac A, Filipović-Grčić J, Voinovich D, Perissutti B, Franceschinis E. Development and bioadhesive properties of chitosan-ethylcellulose microspheres for nasal delivery. Int J Pharm. 2005;291:69–77. | ||
Ahmed TA, El-Say KM, Aljaeid BM, Fahmy UA, Abd-Allah FI. Transdermal glimepiride delivery system based on optimized ethosomal nano-vesicles: Preparation, characterization, in vitro, ex vivo and clinical evaluation. Int J Pharm. 2016;500(1–2):245–254. | ||
Evista. Scientific discussion. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000184/WC500031007.pdf. Accessed May 4, 2018. | ||
Yang ZY, Zhang ZF, He XB, Zhao GY, Zhang YQ. Validation of a novel HPLC method for the determination of raloxifene and its pharmacokinetics in rat plasma. Chromatographia. 2007;65(3–4):197–201. | ||
Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212(2):233–246. | ||
Buggins TR, Dickinson PA, Taylor G. The effects of pharmaceutical excipients on drug disposition. Adv Drug Deliv Rev. 2007;59(15):1482–1503. | ||
Chen ML. Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev. 2008;60(6):768–777. | ||
Al-Mahallawi AM, Abdelbary AA, Aburahma MH. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int J Pharm. 2015;485(1–2):329–340. | ||
Liu Y, Zhang P, Feng N, Zhang X, Wu S, Zhao J. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2009;365(1–2):136–142. | ||
Yang C, Wu T, Qi Y, Zhang Z. Recent advances in the application of Vitamin E TPGS for drug delivery. Theranostics. 2018;8(2):464–485. | ||
Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. | ||
Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of Vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49(2):175–186. | ||
Hartauer KJ, Arbuthnot GN, Baertschi SW, et al. Influence of peroxide impurities in povidone and crospovidone on the stability of raloxifene hydrochloride in tablets: identification and control of an oxidative degradation product. Pharm Dev Technol. 2000;5(3):303–310. | ||
Shirkhedkar AA, Rajput JK, Rajput DK, Surana SJ. Stability-Indicating RP-TLC/densitometry determination of raloxifene hydrochloride in bulk material and in tablets. Chromatography Research International. 2012;2012(5):1–7. | ||
Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv. 2003;10(3):185–191. | ||
Paulsson M, Hägerström H, Edsman K. Rheological studies of the gelation of deacetylated gellan gum (Gelrite) in physiological conditions. Eur J Pharm Sci. 1999;9(1):99–105. | ||
Tomita M, Shiga M, Hayashi M, Awazu S, Horie T, Ishizawa T. Enhancement of colonic drug absorption by the paracellular permeation route. Pharm Res. 1988;5(6):341–346. | ||
Jain A, Mehra NK, Nahar M, Jain NK. Topical delivery of enoxaparin using nanostructured lipid carrier. J Microencapsul. 2013;30(7):709–715. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.