Back to Journals » International Journal of Nanomedicine » Volume 14
Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges
Authors Rezaei A , Varshosaz J , Fesharaki M, Farhang A, Jafari SM
Received 21 February 2019
Accepted for publication 14 May 2019
Published 24 June 2019 Volume 2019:14 Pages 4589—4599
DOI https://doi.org/10.2147/IJN.S206350
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Atefe Rezaei,1,2 Jaleh Varshosaz,3 Mehrafarin Fesharaki,4 Armin Farhang,2 Seid Mahdi Jafari5
1Department of Food Science and Technology, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran; 2Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; 3Department of Pharmaceutics, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran; 4Department of Cell Science, Research Center Medical Sciences, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; 5Department of Food Materials and Process Design Engineering, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran
Purpose: Ferulic acid (FA) is a poorly water-soluble natural antioxidant with anticancer activity. This poor solubility limits the application of FA in the food and pharmaceutical industry. Cyclodextrin nanosponges (CD-NSs) are a novel group of cross-linked CD derivatives which can be used to enhance the solubility of low-soluble bioactive compounds.
Methods: In this study, FA was encapsulated into the NSs in the proportion of 1:4 (FA:NS). Diphenyl carbonate was used as a cross-linker in different proportions with β-CD. Characterization of obtained NSs was performed using scanning electron microscopy, X-ray diffraction (XRD), differential scanning calorimetry (DSC), and Fourier transform infrared (FTIR) analysis.
Results: Our results revealed that the solubility of encapsulated FA was increased up to fifteenfold compared with pure FA in the proportion of 1:4 (CD:cross-linker). The results of FTIR, XRD, and DSC confirmed the interaction of FA with NSs. The cytotoxicity of encapsulated FA against MCF7 and 4T1 breast cancer cell lines was investigated using different concentrations of FA in 24, 48, and 72 hrs. The cytotoxicity assay indicated that FA treatment reduced viability and enhanced apoptosis of cancer cells. IC50 value of encapsulated FA (250 ppm) was decreased by threefold when compared with pure FA (750 ppm).
Conclusion: In general, CD-NS was found to be a suitable delivery system for poorly soluble bioactives such as FA.
Keywords: nanoencapsulation, nanosponges, cyclodextrins, ferulic acid, cytotoxicity
Introduction
4-hydroxy,3-methoxy cinnamic acid known as ferulic acid (FA) is a monophenolic compound which is found plentifully in plant cell walls, such as grains, vegetables, and fruits.1 Also, it can be found in the beverages such as beer and coffee.2 FA is present as a free form or linked to hemicellulosic polysaccharides (in grain) or hydroxy acids (in fruits and vegetables).3 It is described to have antioxidant, antimicrobial, anti-angiogenic, anticancer, antidiabetic, wound healing, cholesterol-lowering, and anti-inflammatory activities.2,4–7 In Japan, FA has been approved as a sunscreen due to its photoprotective properties.8 The antioxidant activity of FA is related to its phenolic nucleus and unsaturated side chain which contributes to the formation of resonance compounds and acts as an antiproliferative agent.5
There are many evidences about the antitumor activity of FA against cervical,9 breast,6 colon,10 prostate,11 and osteosarcoma.12 FA performs its anticancer activity by interference in cell cycle and apoptotic pathways11 or inhibition of metastasis in cancer cells.6 Breast cancer is the first reason of cancer mortality in women. The major strategy for the recovery and treatment of cancer is radiotherapy but because of its undesirable efficacy on normal tissues and also other limitations, such as chemoresistance, immune escape, metastasis, and recurrence, researchers are trying to find new drugs from natural plant-based sources.5,6
Natural antioxidants such as FA have indicated cytotoxic traces in various cancers either alone or combined with other bioactive compounds.13 Despite of the many beneficial effects of FA, its application has been limited similar to other hydrophobic phenolic compounds due to low water solubility, low bioavailability, and phytochemical instability.14,15 Therefore, some efforts have been made to improve the solubility and bioavailability of FA using different nanocarriers such as biopolymeric nanoparticles5,7 or electrospun nanofibers.16–18 Panwar et al (2016) encapsulated FA into chitosan nanoparticles and showed that the solubility of FA was increased by 28% and 25% in water and acetic acid (1%, v/v), respectively.5 Other researchers have also encapsulated FA into poly (lactic-co-glycolic acid) (PLGA) nanoparticles,7 chitosan-polycaprolactone nanofibers,18 or poly (d,l-lactide-co-glycolide)/polyethylene oxide nanofibers,16 but the solubility of FA was not investigated.
Cyclodextrins (CDs) are oligosaccharides with a cyclic structure that have a hydrophilic outer surface and a hydrophobic central part.19,20 Therefore, they can be used to encapsulate both hydrophobic and hydrophilic bioactive compounds.21–23 Cyclodextrin-based nanosponges (CD-NSs) are nanoporous structures which are obtained through hyper cross-linking of the CDs using different cross-linkers. Recently, CD-NSs are specially used as innovative nanodelivery vehicles to enhance the water solubility of poorly soluble compounds,24,25 to improve their bioavailability,26 and to prolong the release of encapsulated compounds.27 CD-NSs can form colloidal nanosuspensions in aqueous solutions and enhance the release of bioactive compound.28 CD-NSs have a higher capability than the natural CDs to improve the water solubility of hydrophobic bioactive compounds.28,29 Some of the advantages of CD-NSs compared to other solubilization technologies are that CD-NSs are solid particles and can easily be used in formulations, their production is easy and affordable and also have a higher loading capacity than other nanocarriers.
The aim of this study was to encapsulate FA into the CD-NSs for the first time and then, to characterize the obtained NSs in terms of encapsulation efficiency, structure, and physicochemical properties. Also, the toxicity of encapsulated FA against MCF7 and 4T1 cancer cell lines was investigated.
Materials and methods
Β-CD was provided by SD-fine corporation, India. FA and diphenyl carbonate were obtained from Sigma Aldrich (Sigma, USA). DMEM, FBS, PBS, EDTA, and trypsin were purchased from Bioidia corporation, Iran. Penicillin-streptomycin and 3 (4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) were procured from Sigma-Aldrich (Sigma, USA). MCF7 (human breast cancer) and 4T1 (mouse breast cancer) cell lines were purchased from Pasteur Institute of Iran. All other chemicals and reagents were of analytical grade.
Preparation of β-CD-NSs
Three types of β-CD-NSs were produced according to the different molar ratios of β-CD to diphenyl carbonate as cross-linker (1:2, 1:4, and 1:8).28 Briefly, determined amounts of anhydrous β-CD were reacted with calculated amounts of melted diphenyl carbonate at 90ºC for at least 5 hrs. Once the reaction was completed, the obtained compound was cooled at room temperature and washed by extra amount of double distilled water to eliminate unreacted β-CD. After this, the obtained product was Sohxlet extracted with ethanol up to 4 hrs to remove unreacted or impurities of diphenyl carbonate. After purification, the nanosponges were dried under vacuum. This reaction also was done in with sonication for 10 mins.
Preparation of FA-loaded NSs
Obtained NSs from the previous stage were dissolved into distilled water and then calculated amounts of FA were added into the solution in a w/w ratio of 4:1 (NS:FA). Then, this mixture was stirred for 24 hrs in dark conditions at room temperature. The uncomplexed FA was separated by centrifuging the suspension at 2,000 rpm for 10 mins; the supernatant which was containing the complexed (encapsulated) FA was finally freeze dried (Dena Vacuum, Iran) at −40ºC for 24 hrs.
Determination of encapsulation efficiency and loading efficacy
Specified amounts of FA-NSs as well as fixed amounts of NSs were dissolved in 10 mL ethanol and then sonicated for 10 mins at ambient temperature, individually. Next, the solutions were filtered through a 0.45 µm filter. The amount of FA was obtained by reading the solution absorbance using a UV spectrophotometer at 319 nm.30 The dispersion of NS was used as the blank sample. Encapsulation efficiency30 and loading efficacy of FA within NSs were determined according to the following equations:
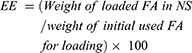
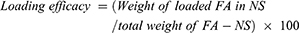 >
>
Analysis of solubility
An excess amount of FA-NS as well as a fixed amount of NS were dispersed in 10 mL distilled water and agitated by a stirrer for 24 hrs at 100 rpm and room temperature, separately. Further, the suspensions were centrifuged at 10,000 rpm for 10 mins. The absorbance of the supernatant was read at 319 nm after filtration through a 0.45 µm filter. The dispersion of NS was used as the blank sample.
Instrumental analysis of FA-loaded nanosponges
The morphology of FA-NSs was investigated by scanning electron microscopy (SEM). The samples were fixed onto the glass slides and prepared by sputtering with a thin film of gold before imaging (HITACHI S-4160, Japan). Particle size was specified using dynamic light scattering by a Zetasizer ZEN 3600 (Malvern, UK). The apparatus worked at a fixed temperature of 25ºC with a scattering angle of 90°. Suitable dilution of the samples was prepared with ethyl acetate before measurement.
Fourier transform infrared (FTIR) spectroscopy analysis of FA, NS, FA-NSs, and diphenyl carbonate were done with a Tensor 27 spectrometer (Bruker, Germany) using the ATR pellet method. The frequency range and the resolution of the instrument were 4000–600 cm−1 and 4 cm−1, respectively. The X-ray diffraction (XRD) patterns of FA, NS, and FA-NSs were determined using a Philips X’Pert diffractometer and Cu Kα radiation (λ=1.54°A). The operating range was 2θ =10–60° and step size was 0.05°. The XRD spectra were analyzed using X’Pert and Origin software. Differential scanning calorimetry (DSC) (STA 503, Germany) was applied for analyzing the thermal properties of FA, NS, and FA-NSs. For the DSC analysis, the heating rate was 20ºC/min and the samples heated from 25ºC to 350ºC under nitrogen gas.
In vitro release of FA
FA release from NSs was studied using a dialysis membrane (molecular weight cutoff between 12,000 and 14,000 Da, Himedia Lab, India) in phosphate buffer (pH=6.8) at 37ºC and 100 rpm in a shaking thermostatic incubator.8 To study the FA release, 15 mg of FA-NS was dispersed in 2 mL phosphate buffer and then poured into the dialysis tube. Then, the dialysis tube was suspended into the 30 mL phosphate buffer. At specified time intervals, 5 mL of the solution was removed for sampling and substituted with the same volume of the release medium to keep a fixed volume. The absorbance of the samples was recorded by UV spectrophotometer at 319 nm after filtration through a 0.45 µm filter.
The cumulative amount (%) of released FA from NSs was obtained using the following equation:
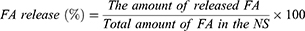 >
>
Cell cytotoxicity of FA-loaded nanosponges
The cell cytotoxicity of FA-NSs, blank NS, and free FA were specified using MTT assay in MCF7 (human breast cancer) and 4T1 (mouse breast cancer) cell lines. Briefly, cells were grown in 90% DMEM supplemented with 10% FBS, sodium bicarbonate, L-glutamine, non-essential amino acid, sodium pyruvate, 100 mg/mL streptomycin, and 100 U/mL penicillin incubated at the temperature of 37°C and under 5% CO2 atmosphere. Further, the cells were gathered using 0.25 w/v% trypsin-EDTA solution and seeded in a 96-well culture plate at a density of 5×103 cell/well and incubated for 24 hrs to confirm the attachment of the cells. Then, 100 µL of different concentration (5, 10, 20, 40, 80, 125, 250, 500, 750, and 1,000 µM) from FA-NS, free FA, and blank NS in the medium culture were added into the wells and incubated for 24, 48, and 72 hrs for evaluating a time and dose-depending manner. Next, cell viability was estimated by MTT assay at 570 nm.31 Untreated cells were taken as control. The cell viability was calculated as:
Finally, half maximal inhibitory concentration (IC50) of FA-NSs and free FA was obtained.
Statistical analysis
All the experiments were performed in triplicates and data were expressed as mean value± standard deviation. Statistical analysis was done using the SPSS program (version 20). The significance of difference (P<0.05) was analyzed using one-way ANOVA.
Results and discussion
Solubility and encapsulation efficiency of FA loaded into nanosponges
Three different proportions of CD:cross-linker (1:2, 1:4, 1:8) were used for the preparation of nanosponges. The solubility, loading capacity, and encapsulation efficiency of FA were evaluated to find the suitable proportion of CD:cross-linker. As can be seen in Figure 1, the solubility of FA increased after encapsulation into the NSs. Also, FA solubility was enhanced by increasing the proportion of CD:cross-linker from 1:2 to 1:4. This higher solubility may be due to entrapment of FA as an inclusion complex within CDs and in the porous structure of NSs. In the proportion of 1:4, the higher cross-linker resulted in a more porous structure compared to lower proportions (1:2). By increasing the proportion up to 1:8, the solubility was decreased which could be due to the hyper cross-linking of CDs preventing the interaction of FA with inclusion cavity.8,24,32 Also, it can be due to saturation solubility of FA in NSs33,34 The proportion of 1:4 (CD:cross-linker) produced NSs with the highest solubility of FA; the solubility increased up to 15 folds compared with free FA.
 | Figure 1 Solubility of ferulic acid (FA) at the different proportions of cyclodextrin:cross-linker. Abbreviation: NS, nanosponge. |
The loading capacity and encapsulation efficiency of FA are presented in Table 1. By increasing the proportion of CD:cross-linker from 1:2 to 1:4, loading capacity and encapsulation efficiency were remarkably enhanced which may be due to the more porous structures resulted from the higher cross-linking; while by increasing the proportion to 1:8, encapsulation efficiency and loading capacity did not change significantly (P>0.05) possibly because of the hyper cross-linking of CDs.
 | Table 1 Encapsulation efficiency and loading capacity of ferulic acid (FA) at different proportions of cyclodextrin (CD): cross-linker |
According to the higher encapsulation efficiency, loading capacity and also solubility results, the proportion of 1:4 (CD:cross-linker) was selected to produce the NSs for encapsulation of FA and the characterization stages at the following steps.
Characterization results of FA loaded into nanosponges
SEM and particle size
SEM images of FA-NSs are presented in Figure 2. As can be seen, the obtained NSs had a porous structure. The particle size analysis indicated that 78.4% of the samples with sonication treatment and 88.2% the samples without sonication had a particle size around 369±27 and 443±44 nm, respectively. Therefore, the sonicated samples which had a lower particle size were selected for further analysis.
Results of FTIR analysis
The FTIR spectra of the samples are presented in Figure 3. Characteristic peaks of FA were detected at 3,437 cm−1 (-OH stretching), 1692 cm−1 (C=O stretching vibration), 1,619, 1,514 cm−1 (C=C aromatic ring), and 1,272, 1,205 cm−1 (C-H bending modes). These results were similar to the FTIR peaks presented earlier for FA.35 In the spectra of CD and diphenyl carbonate, the characteristic peaks of each component are differentiable. The NS spectrum is highly similar to CD spectrum and their difference is only in the intensity of peaks. In the spectrum of FA-NS, the characteristic peaks of FA with a little shifting (1,683, 1,626, 1,595, 1,518, 1,269, and 1,206 cm−1) are visible which indicate FA has been properly incorporated into the NSs.
 | Figure 3 FTIR spectra of β-cyclodextrin nanosponges (A), ferulic acid (B), ferulic acid-loaded nanosponges (C), β-cyclodextrin (D), and diphenyl carbonate (E). |
XRD results
Figure 4 indicates the XRD pattern of FA, NS, and FA-NSs. The sharp characteristic peaks of FA revealed its crystalline structure (Figure 4B). The XRD pattern of FA-NS (Figure 4C) has different crystalline structures compared with its original constituents, namely, FA and NS (Figure 4A) which confirms the complex formation. Furthermore, the reduction in the intensity and difference in diffraction peaks and interplanar spacing indicates successful encapsulation of FA into the nanoporous structures of NS.
 | Figure 4 X-ray diffraction (XRD) of β-cyclodextrin nanosponges (A), ferulic acid (B), and ferulic acid-loaded nanosponges (C). |
DSC results
Thermal properties of FA, NS, and FA-NSs were determined using DSC and are depicted in Figure 5. FA showed an endothermic point at 174.2ºC related to the melting point of FA followed by an exothermic effect corresponding to the thermal decomposition at higher temperatures (Figure 5B). Thermal analysis of NS and FA-NSs indicated that their thermograms are very similar and showed an endothermic point at 297.9ºC due to the CD dehydration and decomposition. The components of NS did not show separate endothermic points which reveals the formation of NSs (Figure 5A). FA-NSs did not show the endothermic point of FA that again confirms FA has been completely included into the NSs and also there is a tight interaction between CD and FA (Figure 5C).26,8
 | Figure 5 DSC thermograms of β-cyclodextrin nanosponges (A), ferulic acid (B), and ferulic acid-loaded nanosponges (C). |
Results of in vitro release of FA
The in vitro release profile of FA from NSs is presented in Figure 6. As can be seen, the NSs have been resulted in a controlled and slow release of FA. The initial burst release in the first hour (around 15%) indicated surface loading of FA and then followed by a slow release up to 70% after 24 hrs. This slow release may be due to the inclusion complexation of FA into the NSs and also cross-linking structure of NSs; this confirms the optimum complexation between FA and NSs. Other researchers have also reported a slow release of encapsulated compounds from NSs.8,32
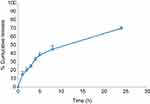 | Figure 6 In vitro release profile of ferulic acid from cyclodextrin nanosponges. |
In vitro cytotoxicity results of FA loaded into nanosponges
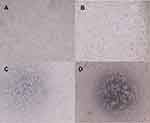
The in vitro cytotoxicity of FA, FA-NSs, and blank NS was investigated against MCF7 (human breast cancer) and 4T1 (mouse breast cancer) cell lines and cell viability was specified using MTT assay (Figure 7). For this purpose, the mentioned cancer cell lines were treated with different concentrations of FA-NSs at different times (24, 48, 72 hrs). The concentrations of 5, 10, 20, 40, and 80 µM of FA-NS did not have a significant effect (P>0.05) on the viability of cells; therefore, we used higher concentrations. Our results revealed that the toxicity of FA-NSs was concentration-dependent and by increasing the concentration of FA-NS up to 500 µM, the cell viability decreased; the higher concentrations did not have a significant influence (P>0.05) on cell viability. Other researchers have also indicated that the higher concentration of phenolic compounds such as FA may be unprofitable and even result in oxidative damage of ordinary cells because these compounds can act as pro-oxidants in high concentrations.29,35 Also, the toxicity of FA-NSs was time dependence meaning that by increasing the time from 24 to 72 hrs, the cell viability was decreased. As can be seen in Figure 7, 250 µM of FA-NS in both MCF7 and 4T1 cell lines caused around 50% inhibition of cell proliferation (IC50). For free FA, the concentration of 750 µM was determined as IC50. Panwar et al (2016) indicated that ME-180 cervical cancer cells after treatment with 40 µM of FA-loaded chitosan nanoparticles showed a 50% inhibition in their growth. This low concentration was due to the synergistic effect of FA and chitosan nanoparticles. Our results indicated that encapsulation of FA into the NSs enhanced its toxicity and decreased the inhibitory concentration. Encapsulation of FA into the NSs leads to a higher solubility and stability of FA and may have contributed to higher anticancer properties of FA. The higher solubility of FA-NSs may be resulted in higher cell permeability and also higher toxicity.34 In vitro cytotoxicity of blank NS revealed that it was nontoxic.
 | Figure 7 MTT viability assay for (A) MCF7 and (B) 4T1 cell lines treated with different formulations of ferulic acid (FA)-loaded cyclodextrin nanosponges (NS) and various interval times at 570 nm. |
Figures 8 and 9 show the impact of different formulations on cell morphology after 24 hrs. The morphology of cancer cells treated with NS was very similar to control and displayed a normal morphology (Figure 8A and C and Figure 9A and C). Our results revealed that the morphology of cancer cell lines which were treated with 250 µM of free FA (Figures 8B and 9B) did not show a significant change compared with the control cells; while for the cancer cell lines treated with 250 µM of FA-NS (Figures 8D and 9D), they had significant structural changes compared with other formulations and also induced 50% cell death. Panwar et al (2015) reported that the cytotoxicity of encapsulated FA into the chitosan nanoparticles was higher than the free FA against cervical cancer cell lines and resulted in cell apoptosis along with cytoplasmic remnants and damaged wrinkled cells.5 Other researchers have also indicated that FA caused cell apoptosis, enhanced lipid peroxidation, affected on the mitochondrial activity, disrupted the cell organelle such as mitochondria and resulted in the cell death.36,31
Conclusion
In this study, we successfully encapsulated FA into the NSs which resulted in a significant improvement of the FA solubility up to 15 folds compared with the free form of FA. The physicochemical and structural characterization using FTIR, XRD, and DSC revealed that FA was properly encapsulated into the NS structures. MTT assay showed an enhanced antiproliferation activity of FA-NS as compared to free FA against MCF7 and 4T1 breast cancer cell lines. In vitro release data indicated a slow controlled release of FA from NSs. Our results confirmed that CD-NS is a suitable nano delivery system for FA which could increase its cytotoxicity, solubility, and anticancer potential.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Assadpour E, Jafari SM, Esfanjani AF. Protection of phenolic compounds within nanocarriers. CAB Rev. 2017;12(057):1–8. doi:10.1079/PAVSNNR201712057
2. Nankar R, Prabhakar PK, Doble M. Hybrid drug combination: combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine. 2017;37:10–13. doi:10.1016/j.phymed.2017.10.015
3. Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109(4):691–702. doi:10.1016/j.foodchem.2008.02.039
4. Yang GW, Jiang JS, Lu WQ. Ferulic acid exerts anti-angiogenic and anti-tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int J Mol Sci. 2015;16(10):24011–24031. doi:10.3390/ijms161024011
5. Panwar R, Sharma AK, Kaloti M, Dutt D, Pruthi V. Characterization and anticancer potential of ferulic acid-loaded chitosan nanoparticles against ME-180 human cervical cancer cell lines. Appl Nanosci. 2016;6(6):803–813. doi:10.1007/s13204-015-0502-y
6. Zhang X, Lin D, Jiang R, Li H, Wan J, Li H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol Rep. 2016;36(1):271–278. doi:10.3892/or.2016.4804
7. Bairagi U, Mittal P, Singh J, Mishra B. Preparation, characterization and in-vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev Ind Pharm. 2018;44:1783–1796. doi:10.1080/03639045.2018.1496448.
8. Shende PK, Gaud RS, Bakal R, Patil D. Effect of inclusion complexation of meloxicam with beta-cyclodextrin- and beta-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf B Biointerfaces. 2015;136:105–110. doi:10.1016/j.colsurfb.2015.09.002
9. Gao J, Yu H, Guo W, et al. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018;18:102. doi:10.1186/s12935-018-0595-y
10. Janicke B, Hegardt C, Krogh M, et al. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr Cancer. 2011;63(4):611–622. doi:10.1080/01635581.2011.538486
11. Eroglu C, Secme M, Bagci G, Dodurga Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol. 2015;36(12):9437–9446. doi:10.1007/s13277-015-3689-3
12. Wang T, Gong X, Jiang R, Li H, Du W, Kuang G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am J Transl Res. 2016;8(2):968–980.
13. Celinska-Janowicz K, Zareba I, Lazarek U, et al. Constituents of propolis: chrysin, caffeic acid, p-coumaric acid, and ferulic acid induce PRODH/POX-dependent apoptosis in human tongue squamous cell carcinoma cell (CAL-27). Front Pharmacol. 2018;9:336. doi:10.3389/fphar.2018.00336
14. Faridi Esfanjani A, Assadpour E, Jafari SM. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci Technol. 2018;76:56–66. doi:10.1016/j.tifs.2018.04.002
15. Faridi Esfanjani A, Jafari SM. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf B Biointerfaces. 2016;146:532–543. doi:10.1016/j.colsurfb.2016.06.053
16. Vashisth P, Kumar N, Sharma M, Pruthi V. Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biophys Rep. 2015;8:36–44. doi:10.1016/j.btre.2015.08.008
17. Aceituno-Medina M, Mendoza S, Rodríguez BA, Lagaron JM, López-Rubio A. Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. J Funct Foods. 2015;12:332–341. doi:10.1016/j.jff.2014.11.028
18. Poornima B, Korrapati PS. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr Polym. 2017;157:1741–1749. doi:10.1016/j.carbpol.2016.11.056
19. Assadpour E, Jafari SM. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit Rev Food Sci Nutr. 2018;1–47. doi:10.1080/10408398.2018.1484687
20. Gharibzahedi SMT, Jafari SM. 7 - Nanocapsule Formation by Cyclodextrins. Nanoencapsulation Technologies for the Food and Nutraceutical Industries. San Diego: Academic Press; 2017:187–261.
21. Rezaei A, Nasirpour A. Encapsulation of curcumin using electrospun almond gum nanofibers: fabrication and characterization. Int J Food Prop. 2018;21(1):1608–1618. doi:10.1080/10942912.2018.1503300
22. Tejashri G, Amrita B, Darshana J. Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharm. 2013;63(3):335–358. doi:10.2478/acph-2013-0021
23. Sherje AP, Dravyakar BR, Kadam D, Jadhav M. Cyclodextrin-based nanosponges: a critical review. Carbohydr Polym. 2017;173:37–49. doi:10.1016/j.carbpol.2017.05.086
24. Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery - Physicochemical characterization, drug release, stability and cytotoxicity. J Drug Deliv Sci Technol. 2018;45:45–53. doi:10.1016/j.jddst.2018.03.004
25. Rezaei A, Fathi M, Jafari SM. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019;88:146–162. doi:10.1016/j.foodhyd.2018.10.003
26. Mendes C, Meirelles GC, Barp CG, Assreuy J, Silva MAS, Ponchel G. Cyclodextrin based nanosponge of norfloxacin: intestinal permeation enhancement and improved antibacterial activity. Carbohydr Polym. 2018;195:586–592. doi:10.1016/j.carbpol.2018.05.011
27. Shende P, Deshmukh K, Trotta F, Caldera F. Novel cyclodextrin nanosponges for delivery of calcium in hyperphosphatemia. Int J Pharm. 2013;456(1):95–100. doi:10.1016/j.ijpharm.2013.08.012
28. Swaminathan S, Pastero L, Serpe L, et al. Cyclodextrin-based nanosponges encapsulating camptothecin: physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm. 2010;74(2):193–201. doi:10.1016/j.ejpb.2009.11.003
29. Maurya DK, Devasagayam TP. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol. 2010;48(12):3369–3373. doi:10.1016/j.fct.2010.09.006
30. Panwar R, Raghuwanshi N, Srivastava AK, Sharma AK, Pruthi V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater Sci Eng C. 2018;92:381–392. doi:10.1016/j.msec.2018.06.055
31. Karthikeyan S, Kanimozhi G, Prasad NR, Mahalakshmi R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol In Vitro. 2011;25(7):1366–1375. doi:10.1016/j.tiv.2011.05.007
32. Coviello V, Sartini S, Quattrini L, Baraldi C, Gamberini MC, La Motta C. Cyclodextrin-based nanosponges for the targeted delivery of the anti-restenotic agent DB103: a novel opportunity for the local therapy of vessels wall subjected to percutaneous intervention. Eur J Pharm Biopharm. 2017;117:276–285. doi:10.1016/j.ejpb.2017.04.028
33. Ansari KA, Vavia PR, Trotta F, Cavalli R. Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech. 2011;12(1):279–286. doi:10.1208/s12249-011-9584-3
34. Dora CP, Trotta F, Kushwah V, et al. Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr Polym. 2016;137:339–349. doi:10.1016/j.carbpol.2015.10.080
35. Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi:10.1016/j.freeradbiomed.2004.04.034
36. Ezhuthupurakkal PB, Ariraman S, Arumugam S, et al. Anticancer potential of ZnO nanoparticle-ferulic acid conjugate on Huh-7 and HepG2 cells and diethyl nitrosamine induced hepatocellular cancer on Wistar albino rat. Nanomedicine. 2018;14(2):415–428. doi:10.1016/j.nano.2017.11.003
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.