Back to Journals » Drug Design, Development and Therapy » Volume 14
Impact of Dexmedetomidine Infusion on Postoperative Acute Kidney Injury in Elderly Patients Undergoing Major Joint Replacement: A Retrospective Cohort Study
Authors Zhu H , Ren A, Zhou K, Chen Q, Zhang M , Liu J
Received 23 August 2020
Accepted for publication 7 October 2020
Published 2 November 2020 Volume 2020:14 Pages 4695—4701
DOI https://doi.org/10.2147/DDDT.S278342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
He Zhu,1 Aolin Ren,1 Kang Zhou,1 Qiuchong Chen,1 Mengjun Zhang,1 Jindong Liu2
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Correspondence: Jindong Liu
Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
, Tel +86 10 8580-2333
Email [email protected]
Purpose: Postoperative acute kidney injury (AKI) is a frequent complication in elderly patients that increases morbidity and mortality. Approximately 1.7 million people die from AKI worldwide every year. Dexmedetomidine (Dex) is often used as an adjunct to multimodal analgesia. Our study investigated whether Dex could safely decrease the incidence of AKI in elderly patients undergoing major joint replacement.
Methods: A single-center retrospective study was conducted in patients aged > 65 years undergoing major joint replacement. Propensity score–matching analysis was used, and a total of 1,006 patients were matched successfully. The primary outcome was the incidence of postoperative AKI. Secondary outcomes included perioperative adverse complications, opioid consumption, time to extubation, and length of hospital stay.
Results: Among the 1,006 patients included, postoperative AKI occurred in 9.3% (n=94). The Dex group (perioperative Dex infusion) had lower incidence of postoperative AKI than the control group (7.2% vs 11.5%, P=0.017). Compared with the control group, the Dex group had less opioid consumption (P< 0.05), reduced time to extubation (P=0.004), and shorter length of hospital stay (P=0.001). The Dex group also showed higher incidence of bradycardia (20.1% vs 15.1%, P=0.038). There were no differences in intraoperative hypotension (19.5% vs 17.5%), postoperative nausea and vomiting (4.2% vs 5.4%), time in PACU (45.0± 6.4 vs 45.5± 6.2 minutes), or rate of ICU admission (9.7% vs 11.1%) between the Dex group and control group (All P> 0.05).
Conclusion: This retrospective study showed Dex infusion in elderly patients undergoing major joint replacement was associated with lower incidence of postoperative AKI, less opioid consumption, and shorter extubation time and hospital stay. However, the Dex group had higher incidence of bradycardia. We found no statistical differences in other perioperative adverse complications between the groups.
Keywords: dexmedetomidine, acute kidney injury, elderly patients, joint replacement
Introduction
Postoperative acute kidney injury (AKI) is a complex group of clinical syndromes. Elderly patients are especially in danger of developing AKI, given the presence of several comorbidities, including hypertension, diabetes, and chronic kidney disease.1,2 AKI has become a globalissue, but has not attracted widespread attention. The occurrence of AKI after surgery leads to an increase in postoperative complications, which brings a heavy financial burden to families.3 A meta-analysis showed that the incidence of AKI and severe AKI requiring dialysis after total hip arthroplasty was 6.3% and 0.5%, respectively.4 Due to the different locations of joint replacement, the incidence of postoperative AKI is 0.5%–21.9%, and the incidence of renal failure caused by hip and knee replacement is 0.8%.5 At present, clinical research on AKI is mostly limited to cardiovascular surgery. There has been little research on AKI after joint replacement.
Dexmedetomidine (Dex) is a highly selective α2-adrenoceptor agonist with sympatholytic, analgesic, dose-dependent, sedative, and anxiolytic properties with minimal respiratory depression.6 Studies have shown that Dex provides neuroprotection and renoprotection in a dose-dependent manner.7,8 Perioperative Dex infusion can provide smooth and hemodynamically stable emergence.9 There has been no study to investigate the effects of Dex in preventing AKI in elderly patients undergoing major joint replacement due to its renal protection.Early identification and intervention of postoperative AKI is important, because there is no good therapy other than renal replacement when the disease becomes severe. We hypothesized that Dex infusion would lead to a reduced incidence of postoperative AKI safely.
Methods
Study Design and Participants
This retrospective study (Jiangsu, China; XYFY2020-KL050-01) was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. We used propensity score–matching analysis, and 1,006 elderly patients who had undergone major joint replacement at the Affiliated Hospital of Xuzhou Medical University from October 2013 to October 2019 were included finally. The study was registered in the American Clinical Trial Registry (NCT04132921) and the requirement for informed consent waived, because this retrospective trial was limited to preexisting data without intervention.Inclusion criteria were age ≥65 years and unilateral joint replacement. Exclusion criteria were lack of clinically relevant data, patients who had undergone emergency surgery, patients with severe liver/kidney dysfunction, heart failure, or severe arrhythmia.
Data Collection
All information was obtained from the electronic medical record and anesthesia systems. Researchers were trained before data collection, and two independent investigators verified all data. We mainly collected demographic data (sex, age, BMI, American Society of Anesthesiologists [ASA] classification, and preoperative comorbidities), general conditions during the operation, laboratory-examination results, incidence of AKI, postoperative outcomes, including nausea and vomiting, length of hospitalization, time to extubation (time from end of operation to removal of tracheal tube), time in the postanesthesia care unit (PACU), and whether the patient had been admitted to the ICU after surgery.
Dex administration was defined as a loading dose of 0.5–1.0μg/kg within 10–15 minutes or intraoperative continuous infusion at a rate of 0.2–0.7μg/kg/h. The primary outcome variable was prevalence of postoperative AKI defined according to the criteria from Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. AKI can be diagnosed if one of the following conditions is met: increase in serum creatinine ≥0.3 mg/dL within 48 hours or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days. Baseline serum creatinine was defined as the most recent value prior to surgery, and the peak value was defined as the highest serum creatinine within 7 days following surgery. A simplified MDRD formula was used to calculate the estimated glomerular filtration rate (eGFR): 186 × serum creatinine value−1.154 × age−0.203 × 0.742 (female). Low eGFR was defined as eGFR <60mL/min/1.73m2, hypotension as mean arterial pressure <65mmHg for >5 minutes, bradycardia as heart rate <50 beats per minute, hypoproteinemia as albumin <35g/L and thrombocytopenia as platelet count <100×109 L.
Elderly patients >65 years old often have accompanying basic diseases, and the impact of comorbidities on disease outcomes can be measured by a well-established and validated 20-item risk-scoring tool: the Charlson Comorbidity Index (CCI). The second edition of the CCI is a combined age–comorbidity score that adds the factor of age (Table 1). Based on their scores, patients were divided into two groups in our study: those with scores ≤3 and those with scores of >3. This work was completed by two members of the team reviewing the surgical medical record system. All enrolled patients were anesthetized by general anesthesia at the discretion of the attending anesthesiologist, and intraoperative monitoring was performed with electrocardiography, pulse oximetry, invasive surveillance of arterial blood pressure, and bispectral index. In order to reduce the use of anesthetic drugs and postoperative complications, most patients underwent general anesthesia combined with femoral nerve block, sciatic nerve block, or lumbar plexus block. All invasive operations were performed by senior anesthesiologists.
 |
Table 1 Weighted scores for each item of the 20-item Charlson Comorbidity Index |
Statistical Analyses
For continuous variables, the Kolmogorov–Smirnov test was used to assess normality, normally distributed continuous variables as means ± SD, and abnormally distributed continuous variables as medians (IQR). Categorical variables are summarized as frequencies and proportions. Unpaired t-tests were used normally distributed continuous variables, Mann–Whitney U-test for variables without normal distribution, and x2 or Fisher’s exact tests for categorical data as appropriate. Propensity-score methods can effectively control for baseline confounding by balancing measured baseline confounders and risk factors. We identified 900 patients who had been treated with Dex and 576 who had not. Finally, we successfully matched 503 pairs who had propensity score within 0.02, based on age, sex, BMI, ASA classification, and CCI score. Propensity-score matching was successful in achieving balance across all measured covariates.
Sample size was based on a 23.2% incidence of AKI in hospitalized patients according to KDIGO criteria.10 We assumed a 35% reduction in postoperative AKI incidence in the Dex group. Sample size was calculated using PASS11 software, and 736 patients were required to provide 80% power to detect a two-sided difference, with a type I error probability of 0.05. Ultimately, we included 1,006 patients for analysis. Statistical analysis was performed using SPSS version 23.0 (IBM, Armonk, NY, USA). All statistical tests were two-tailed, and P<0.05 was considered to indicate statistical significance.
Results
A total of 1,640 elderly patients who had been scheduled to undertake elective major joint replacement surgery were screened from October 2013 to October 2019 at the Affiliated Hospital of Xuzhou Medical University. There were 164 patients excluded according to our exclusion criteria and 1,476 eligible patients included. In order to balance measured baseline confounders and risk factors, propensity score–matching analysis was used and 1,006 matched successfully. The specific flow diagram for patient selection is presented in Figure 1.
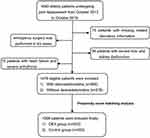 |
Figure 1 Study flowchart. |
Baseline Characteristics
There were no significant differences in sex, age, BMI, ASA classification, CCI scores, comorbidities (hypertension, diabetes, low eGFR, anemia, cardiac disease), chronic smoking, or alcoholism between the two groups (All P>0.05). We analyzed preoperative laboratory information and found significant differences in albumin and platelet counts. For ease of analysis and interpretation, we represented these related risk factors as binary predictor variable. There was no difference for hypoproteinemia or thrombocytopenia (Tables 2 and 3).
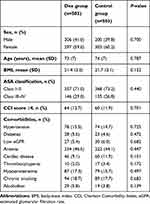 |
Table 2 Baseline variables of patients with or without dexmedetomidine infusion |
 |
Table 3 Preoperative laboratory information |
Outcomes
Our study showed no differences in surgery or anesthesia procedure (Table 4). However, there were significant significant differences in sufentanil and remifentanil consumption between the groups (P<0.05). No differences were seen in propofol, rocuronium, cisatracurium, sevoflurane, or NSAID use. We compared the choice of nerve block, and no differences were found for femoral nerve block (37.0% vs 40.0%, P=0.331), sciatic nerve block (20.1% vs 22.9%, P=0.282), or lumbar plexus block (11.7% vs 9.3%, P=0.218) (Table 4).Postoperative AKI occurred in 9.3% (n=94) of all patients, and the Dex group was associated with lower incidence of postoperative AKI than the control group (7.2% vs 11.5%, P=0.017; Table 5).
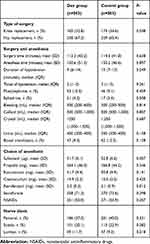 |
Table 4 Surgery- and anesthesia-related information |
 |
Table 5 Perioperative outcomes |
Compared with the control group, the Dex group had higher incidence of bradycardia (20.1% vs 15.1%, P=0.038). We found that the Dex group had shorter time (minutes) to extubation (18.6±2.2 vs 19.0±2.3, P=0.004) and shorter length (days) of hospital stay (19.5±2.0 vs 19.9±1.9, P=0.001). Nausea and vomiting are the most common complications after general anesthesia, and 21 patients inthe Dex group and 27in the control group experienced mild nausea and vomiting upon checking the anesthesia record. No significant difference was observed between the two groups (4.2% vs 5.4%, P=0.375). There were no differences in intraoperative hypotension (19.5% vs 17.5%), time (minutes) in PACU (45.0±6.4 vs 45.5±6.2), or rate of ICU admission (9.7% vs 11.1%) between the Dex group and control group (all P>0.05, Table 5).
Discussion
AKI has become a global issue, however, attention paid to it is still not enough. Elderly patients are growing in numbers and often have such diseases as hypertension and diabetes, which increase the burden on the kidneys.11,12 Therefore, for elderly patients, a comprehensive anesthesia and surgical evaluation should be performed before surgery. A study has shown that Dex infusion in pediatric patients after congenital heart surgery is associated with decreased incidence of AKI.13 However, the impact of Dex on AKI after major joint replacement is still unknown, and our study showed that Dex infusion decreased the incidence of postoperative AKI safely in elderly patients after total knee or hip arthroplasty.
AKI is a common complication caused by multiple factors, and its risk factors include older age, high BMI, hypoproteinemia, anemia, decrease in GFR, use of colloids, and decreased mean arterial pressure.14–17 Many factors are still controversial, and one possible mechanism is hemodynamic changes leading to an imbalance of oxygen supply and demand in organs, with ischemia–reperfusion injury eventually leading to AKI.18,19 Si et al20 recently demonstrated that Dex appears to act, at least in part, by upregulating SIRT3 to inhibit mitochondrial damage and cell apoptosis and thereby protect against renal ischemia–reperfusion injury, which is consistent with our study. However, Oh et al found no significant association between Dex use and AKI after joint replacement, and a possible explanation that should be considered is this retrospective observational study included only patients who received spinal anesthesia.
Analysis of perioperative adverse outcomes showed no significant differences in intraoperative hypotension, nausea and vomiting, time in PACU, or rate of ICU admission. The Dex group had higher incidence of bradycardia than the control group, but the occurrence of bradycardia was transient and the percentage of patients requiring intervention very low. Compared with the control group, the Dex group had shorter time to extubation and shorter length of hospital stay, meaning an optimal dose of Dex infusion was not associated with obvious residual sedation, which is similar to a recent study.21
Total hip or knee arthroscopy is a common orthopedic procedure, and postoperative pain delays discharge and increases medical cost.22,23 From the perspective of enhanced recovery after surgery, we have to achieve significant clinical and economic benefits after surgery.24 According to Sun et al,25 elderly patients in a Dex group reported significantly lower numeric rating–scale pain scores at 3, 12, 24, and 48 hours after surgery and significantly improved Richards–Campbell Sleep Questionnaire results during the first 3 days after major elective noncardiac surgery. Studies have shown satisfactory analgesic effects of Dex in total hip or knee arthroscopy, but some experimental conclusions are controversial.26–28 The dose of Dex we chose was similar to previous research: the Dex administration was a loading dose of 0.5–1.0μg/kg within 10–15 minutes or intraoperative continuous infusion at 0.2–0.7μg/kg/h according to anesthesia system records. Consistently with previous research results,29 intraoperative Dex was associated with a small but clinically important reduction in opioid consumption. Due to the limitations of retrospective studies, we could not collect additional postoperative opioid-use data, which may limit the accuracy of our conclusions.
Additionally, this was a single-center retrospective study, which has some limitations. Demographic, anesthesia-, and surgery-related data were collected from electronic medical records and doses and infusion rate of Dex cannot be accurately assessed; therefore, large-sample randomized controlled trials are still needed. We also noticed the impact of tourniquets on postoperative AKI, but as this was a retrospective experiment, we could not accurately assess the use of tourniquets in knee arthroplasty.
Conclusion
Our study showed that Dex infusion was associated with lower incidence of postoperative AKI, less opioid consumption, and shorter extubation time and length of hospital stay. Except for transient bradycardia during the operation, Dex infusion did not produce any severe adverse complications.
Data-Sharing Statement
Individual participant data underlying published results reported in this study can be accessed with approval from the corresponding author after 6 months of publication of the main results. The study protocol, statistical analysis plan, and clinical study report will also be available.
Acknowledgment
This work was supported by the Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University. No commercial funding was received.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gameiro J, Fonseca JA, Neves M, et al. Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8(1):22. doi:10.1186/s13613-018-0369-7
2. Regenbogen SE, Cain-Nielsen AH, Norton EC, et al. Costs and consequences of early hospital discharge after major inpatient surgery in older adults. JAMA Surg. 2017;152(5):e170123. doi:10.1001/jamasurg.2017.0123
3. Angeli P, Bezinover D, Biancofiore G, et al. Acute kidney injury in liver transplant candidates: a position paper on behalf of the liver intensive care group of Europe. Minerva Anestesiol. 2017;83(1):88–101.
4. Thongprayoon C, Kaewput W, Thamcharoen N, et al. Acute kidney injury in patients undergoing total hip arthroplasty: a systematic review and meta-analysis. J Clin Med. 2019;8(1):66. doi:10.3390/jcm8010066
5. Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Engl. 2017;99(4):307–312. doi:10.1308/rcsann.2016.0324
6. Giovannitti JA, Thoms SM, Crawford,JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62(2):31–38. doi:10.2344/0003-3006-62.1.31
7. Chen Y, Zhang X, Zhang B, et al. Dexmedetomidine reduces the neuronal apoptosis related to cardiopulmonary bypass by inhibiting activation of the JAK2-STAT3 pathway. Drug Des Devel Ther. 2017;11:2787–2799. doi:10.2147/DDDT.S140644
8. Gu J, Zhao H, Watts HR, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15(3):R153. doi:10.1186/cc10283
9. Aouad MT, Zeeni C, Al Nawwar R, et al. Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth Analg. 2019;129(6):1504–1511. doi:10.1213/ANE.0000000000002763
10. Liu BC, Tang RN, Liu ZH. Current clinical research of acute kidney injury in China. Chin Med J (Engl). 2015;128(9):1268–1271. doi:10.4103/0366-6999.156148
11. Baek SH, Lee SW, Kim SW, et al. Frailty as a predictor of acute kidney injury in hospitalized elderly patients: a single center, retrospective cohort study. PLoS One. 2016;11(6):e0156444. doi:10.1371/journal.pone.0156444
12. Patschan D, Schwarze K, Henze E, et al. Acute kidney injury-associated systemic inflammation is aggravated in insulin-dependent diabetes mellitus. J Clin Med Res. 2019;11(10):720–723. doi:10.14740/jocmr3852
13. Kwiatkowski DM, Axelrod DM, Sutherland SM, Tesoro TM, Krawczeski CD. Dexmedetomidine is associated with lower incidence of acute kidney injury after congenital heart surgery. Pediatr Crit Care Med. 2016;17(2):128–134. doi:10.1097/PCC.0000000000000611
14. Gharaibeh KA, Hamadah AM, Sierra RJ, et al. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am. 2017;99(21):1819–1826. doi:10.2106/JBJS.16.01027
15. Aksoy R, Adademir T, Yilmaz E, et al. Is hypoalbuminemia a predictor for acute kidney injury after coronary bypass grafting in diabetes mellitus patients? Braz J Cardiovasc Surg. 2019;34(5):565–571. doi:10.21470/1678-9741-2018-0291
16. Choi YJ, Kim SO, Sim JH, Hahm KD. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg. 2016;122(6):1923–1928. doi:10.1213/ANE.0000000000001003
17. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–134. doi:10.1056/NEJMoa1204242
18. Salmasi V, Maheshwari K, Yang D, et al. Relationship between Intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65.
19. Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. doi:10.1097/ALN.0000000000000765
20. Si Y, Bao H, Han L, et al. Dexmedetomidine attenuation of renal ischaemia-reperfusion injury requires sirtuin 3 activation. Br J Anaesth. 2018;121(6):1260–1271. doi:10.1016/j.bja.2018.07.007
21. Lee HS, Yoon HY, Jin HJ, Hwang SH. Can dexmedetomidine influence recovery profiles from general anesthesia in nasal surgery? Otolaryngol Head Neck Surg. 2017;158(1):43–53. doi:10.1177/0194599817733735
22. Panigrahi R, Roy R, Mahapatra AK, Prasad A, Priyadarshi A, Palo N. Intra-articular adjuvant analgesics following knee arthroscopy: comparison between single and double dose dexmedetomidine and ropivacaine a multicenter prospective double-blind trial. Orthop Surg. 2015;7(3):250–255. doi:10.1111/os.12182
23. El Baz MM, Farahat TEM. Efficacy of adding dexmedetomidine to intra-articular levobupivacaine on postoperative pain after knee arthroscopy. Anesth Essays Res. 2019;13(2):254–258. doi:10.4103/aer.AER_23_19
24. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl3):iii62–iii72. doi:10.1093/bja/aew362
25. Sun Y, Jiang M, Ji Y, Sun Y, Liu Y, Shen W. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Devel Ther. 2019;13:2911–2922. doi:10.2147/DDDT.S208703
26. Kundu R, Dehran M, Chandralekha C, Trikha A, Nag,HL. Safety and analgesic efficacy of intravenous dexmedetomidine in arthroscopic knee surgery. Anesth Essays Res. 2015;9(3):391–396. doi:10.4103/0259-1162.161820
27. Oh TK, Park JW, Shin HJ, et al. Perioperative sedative use is not associated with acute kidney injury after total hip or knee arthroplasty. Ann Transl Med. 2019;7(11):237. doi:10.21037/atm.2019.04.66
28. Sharma B, Rupal S, Swami AC, Lata S. Effect of addition of dexmedetomidine to ropivacaine 0.2% for femoral nerve block in patients undergoing unilateral total knee replacement: A randomised double-blind study. Indian J Anaesth. 2016;60(6):403–408. doi:10.4103/0019-5049.183392
29. Shin HJ, Do SH, Lee JS, Kim TK, Na HS. Comparison of intraoperative sedation with dexmedetomidine versus propofol on acute postoperative pain in total knee arthroplasty under spinal anesthesia: a randomized trial. Anesth Analg. 2018;129(6):1512–1518. doi:10.1213/ANE.0000000000003315
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
