Back to Archived Journals » Reports in Organic Chemistry » Volume 6
Hypervalent iodine reagents for heterocycle synthesis and functionalization
Authors Sun J, Zhang-Negrerie D, Du Y, Zhao K
Received 2 January 2016
Accepted for publication 6 June 2016
Published 9 August 2016 Volume 2016:6 Pages 25—45
DOI https://doi.org/10.2147/ROC.S84894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sean Kerwin
Jiyun Sun,1 Daisy Zhang-Negrerie,2 Yunfei Du,1 Kang Zhao,1
1Tianjin Key Laboratory for Modern Drug Delivery and High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin, 2Concordia International School Shanghai, Shanghai, People’s Republic of China
Abstract: Hypervalent iodine reagents have been vastly applied in many significant oxidative reactions. This surging interest in iodine reagents is mainly due to the very useful oxidizing properties, combined with their benign environmental character and commercial availability. In this review, we focus on the representative transformations that used the common hypervalent iodine reagents as oxidants in heterocycle synthesis and functionalizations, based on the type of the hypervalent iodine reagents.
Keywords: hypervalent iodine reagent, heterocycle synthesis, heterocycle functionalization, oxidative reaction
Introduction
The 1990s witnessed rapid development of hypervalent iodine chemistry. The intense interest is mainly due to the remarkable oxidizing properties of hypervalent iodine reagents and their attractive features such as easy to handle, low toxicity, availability of supply, and environmental benignity.1–20 Two of their most important synthetic applications are in the constructions of heterocyclic skeletons and functionalization of heterocycles, such as three- to seven-membered rings and spiro compounds, under metal-free reaction conditions. Some representative transformations have been shown in Figure 1. In this review, we summarize, with representative examples, the reactions involving various hypervalent iodine (III) and (IV) reagents used as oxidants for the syntheses and functionalization of heterocyclic compounds. The organization of the presentation is based on the type of the hypervalent iodine reagents.
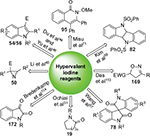  | Figure 1 Representative reactions involving hypervalent iodine reagents. |
Hypervalent iodine (III) reagents
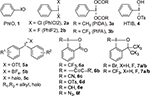
The common classification of hypervalent iodine (III) reagents is according to the type of ligands attached to the iodine atom, as shown in Figure 2.10,16 These broadly applied hypervalent iodine (III) reagents, namely, iodosylarenes 1, (dichloroiodo)arenes 2a and (difluoroiodo)arenes 2b, [bis(acyloxy)iodo]arenes 3, [hydroxy(tosyloxy)iodo]benzene 4 (Koser’s reagent), iodonium salts 5, iodonium ylides and iodonium imides, and the benziodoxole-based hypervalent iodine reagents 6 and 7 (Togni’s reagents), have been found to be powerful and effective oxidants for the synthesis of heterocycles and for facilitating functionalization of heterocyclic compounds via atom transfer reactions.
Iodosylarenes
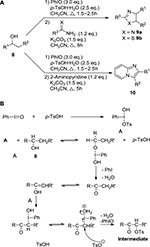
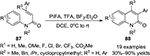
An important synthetic application of iodosobenzene (PhIO) is promoting oxidative annulation during the construction of heterocyclic framework. For example, Ueno et al21 reported a direct preparation of heteroaromatic compounds of imidazoles 9a, thiaozles 9b, and imidazo[1,2-a]pyridines 10 through reactions of alcohol substrates 8 with PhIO catalyzed by p-toluenesulfonic acid monohydrate and followed by further reactions with thioamide, benzamidine, and 2-aminopyridine, respectively, under basic conditions (Figure 3).
In 2010, Fan et al22 described a PhIO-mediated synthesis of the three-membered N-benzoyl aziridines 12 and the five-membered oxazolines 13 through an intramolecular oxidative cyclization of substrates 11 in the presence of catalytic amount of tetra-butylammonium iodide (Figure 4A-a). Similar conditions were applied to the synthesis of the four-membered oxetanes 15 and azetidines 17 from substrates 14 and 16, respectively (Figure 4A-b and -c).23,24 The proposed mechanism has been shown in Figure 4B.
In addition, PhIO can also be used as an efficient oxidant for the functionalization of heterocycles. For example, five- or six-membered lactams 19 could be obtained in moderate yields through the oxidation of cyclic amines 18 with PhIO using H2O as solvent (Figure 5).25
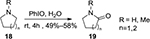  | Figure 5 PhIO-mediated functionalization of cyclic amines. Abbreviations: PhIO, iodosobenzene; rt, room temperature; h, hours. |
Moriarty et al26 reported the oxidation of trimethylsilyl ketene acetals of lactones 20 in methanol, mediated by PhIO, to afford the corresponding α-methoxylated carbonyl compounds 21 in good yields (Figure 6). They also found that reaction of dihydropyran 22 with PhIO in H2O could afford tetrahydro-2-furaldehyde 23 via carbocationic ring contraction (Figure 7). Under the same conditions, cyclohexene and styrene were converted into the corresponding aldehyde products through rearrangement oxidations.27
  | Figure 6 PhIO-mediated oxidation affording α-methoxylated carbonyl compounds. Abbreviation: PhIO, iodosobenzene. |
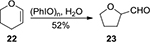  | Figure 7 PhIO-mediated oxidation of dihydropyran. Abbreviation: PhIO, iodosobenzene. |
In the presence of PhIO and I2, N- or O-centered radicals could be generated, respectively, from amides or alcohols.28–30 In 2000, Francisco et al reported the synthesis of homochiral 7-oxa-2-azabicyclo[2.2.1]heptane ring system 28 from specifically protected phosphoramidate derivatives of carbohydrates 24 under the conditions mentioned earlier. Mechanistic studies demonstrated a reaction path involving a hemolytic fragmentation of a hypothetical iodoamide intermediate 26 (Figure 8).30
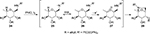  | Figure 8 Synthesis of the homochiral 7-oxa-2-azabicyclo[2.2.1]heptane ring system. Abbreviations: PhIO, iodosobenzene; IHA, intramolecular hydrogen abstraction reaction. |
It is worth noting that the applications of PhIO can be significantly restricted in nonpolar solvents due to low solubility. Therefore, the majority of the known reactions occurs in polar solvents and are catalyzed by a Lewis acid or a transition metal catalyst, with only a few cases reported to be in a nonpolar solvent or without the involvement of a catalyst. One of the rare examples is the formation of lactams 30 in CHCl3 from the cyclic amino acids 29 via initial imine formation followed by oxidative decarboxylation (Figure 9).31
  | Figure 9 PhIO-mediated conversion of proline into 2-pyrrolidone in nonpolar solvent. Abbreviations: PhIO, iodosobenzene, rt, room temperature; d, days. |
(Difluoroiodo)arenes
As fluorinating reagents, (difluoroiodo)arenes (ArIF2) have found many synthetic applications for the syntheses of biologically and pharmaceutically interesting F-containing heterocyclic compounds.32,33 In 1991, Caddick et al32 reported the reaction of 1-(arylthio)glycosides 31 with TolIF2, which afforded various 1-fluoroglycosides 32 in moderate-to-good yields (Figure 10).
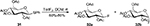  | Figure 10 Synthesis of various 1-fluoroglycosides with TolIF2. Abbreviations: rt, room temperature; DCM, dichloromethane. |
Upon treating the iodoaldyl substituted four-, five-, and six-membered cyclic ethers 33–35 with TolIF2, the five-, six-, and seven-membered cyclic ethers 36–38 were stereoselectively synthesized in moderate-to-good yields (Figure 11).34
  | Figure 11 Ring-expansion reactions induced by TolIF2. Abbreviations: eq., equivalent; rt, room temperature; h, hour; DCM, dichloromethane. |
Dichloroiodoarene
(Dichloroiodo)arenes (ArICl2) have been used as chlorinating reagents to carry out modification of various heterocyclic compounds. For example, reaction of N-protected pyrrolidine 39 with 4-nitrobenzeneiododichloride afforded α-hydroxy-β,β-dichloropyrrolidine 40 as the main product via a complicated ionic mechanism involving a C(sp3)–H bond activation process (Figure 12). This oxidation gave an α,β,β-oxidation pattern relative to the nitrogen of the heterocycle.35
  | Figure 12 Synthesis of α-hydroxy-β,β-dichloropyrrolidine with 4-NO2PhICl2. Abbreviation: eq., equivalent. |
An effective system consisting of a combination of PhICl2 and Pb(SCN)2 was developed by Prakash et al36 for convenient thiocyanation of various enol silyl ethers 41 (Figure 13).
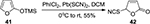  | Figure 13 PhICl2/Pb(SCN)2-mediated thiocyanation of enol silyl ethers leading to lactone 42. Abbreviations: rt, room temperature; DCM, dichloromethane. |
Recently, Hepples et al37 reported a Lewis base-catalyzed chlorination method for the diazocarbonyl compound 43a and isatin-3-hydrazone 43b by using PhICl2, both of which led to the same product 44 (Figure 14).
  | Figure 14 Lewis base-catalyzed chlorination facilitated by PhICl2. Abbreviations: eq., equivalent; rt, room temperature; min, minutes; DCM, dichloromethane. |
The common feature of these reactions is the transfer of the two chlorine ligands from PhICl2 in a germinal fashion rather than vicinal.37,38
In 2014, He et al39 reported a method for the direct synthesis of oxazolidin-2-ones 46 and imidazolidin-2-ones 48 from 1,3-diols 45 and 3-amino alcohols 47 using combined PhICl2 and NaN3 (Figure 15).
  | Figure 15 (A) Direct synthesis of oxazolidin-2-ones and imidazolidin-2-ones using PhICl2 and NaN3. (B) Proposed mechanism. Abbreviations: eq., equivalent; h, hours. |
[Bis(acyloxy)iodo]arenes
[Bis(acyloxy)iodo]arenes (ArI(OCOR)2), notably the easily prepared and commercially available phenyliodine diacetate (PIDA) and phenyliodine bis(trifluoroacetate) (PIFA), have been widely used as oxidizing reagents in various syntheses of heterocycles. In this review, the applications of PIDA and PIFA are presented based on the type of heterocycles obtained.
Three-membered heterocyclic products
In 2009, our group reported the synthesis of the smallest unsaturated N-containing heterocycle, namely, 2H-azirine 50, via PIDA-mediated intramolecular oxidative azirination of the substituted enamine derivatives 49 under mild conditions (Figure 16).40 A similar strategy was later applied to the one-pot synthesis of isoxazoles from enaminones.41
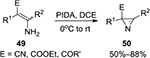  | Figure 16 PIDA-mediated synthesis of 2H-azirine derivatives from enamines. Abbreviations: PIDA, phenyliodine diacetate; rt, room temperature; DCE, 1,2-dichloroethane. |
Five-membered heterocyclic compounds
Pyrrole
Mediated by PIFA, the synthesis of polysubstituted pyrroles 52 was achieved via a tandem dimerization/cyclocondensation of enaminones 51 (Figure 17).42 Asymmetrical polysubstituted pyrroles were obtained from enamine esters or ketones mediated by PIDA in the presence of BF3·Et2O.43
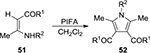  | Figure 17 PIFA-mediated synthesis of polysubstituted pyrroles 52. Abbreviation: PIFA, phenyliodine bis(trifluoroacetate). |
Indole
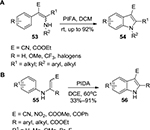
In 2006, the syntheses of N-arylated and N-alkylated indoles 54 from enamine derivatives 53 were realized through a PIFA-mediated intramolecular oxidative C(sp2)–N bond formation (Figure 18A).44 The same strategy was also applied to the synthesis of carbazolones via PIFA-mediated intramolecular cyclization of 2-aryl enaminones.45 In 2009, a variety of functionalized indoles 56 were synthesized from N-aryl enamines 55 via PIDA-mediated oxidative C(sp2)–C(sp2) involving no transition metals (Figure 18B).46
Azole
In 2007, Das et al47 reported the condensation of α-hydroxy ketones 57 with aldehydes and ammonium acetate by using PIDA as the sole oxidant. The reaction furnished the cyclized imidazole product 58 through an oxidative C(sp2)–N bond formation (Figure 19). Various 2-arylbenzimidazoles and benzimidazoles were later synthesized adopting the same methodology.48
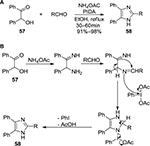  | Figure 19 (A) PIDA-mediated synthesis of imidazoles via condensation of α-hydroxy ketones with aldehydes and NH4OAc. (B) Proposed mechanism. Abbreviations: PIDA, phenyliodine diacetate; min, minutes. |
In 1996, Kotali49 realized the synthesis of aminoindazole derivatives 60 from the o-aminoaryl ketone acylhydrazones 59 via PIDA-mediated N–N bond formation (Figure 20).
  | Figure 20 PIDA-mediated synthesis of aminoindazole derivatives. Abbreviation: PIDA, phenyliodine diacetate. |
In 2012, intramolecular oxidative C–O coupling of N-(4-alkoxy-phenyl) and N-(4-acetamido-phenyl) benzamides was found to afford the benzoxazole products in high yields under metal-free conditions by using PIFA as an oxidant and TMSOTf as a catalyst (Figure 21).50
  | Figure 21 PIFA/TMSOTf-mediated synthesis of benzoxazole derivatives. Abbreviations: PIFA, phenyliodine bis(trifluoroacetate); rt, room temperature; TMSOTf, trimethylsilyl trifluoromethanesulfonate. |
Upon treating β-monosubstituted enamines 63 with PIFA, an intermolecular cross-coupling occurred and was succeeded by condensation to provide the 4,5-disubstituted 2-(trifluoromethyl)oxazoles 64 (Figure 22).51 In this approach, the trifluoromethyl moiety in one of the PIFA ligands was incorporated into the final products at the C2 position.
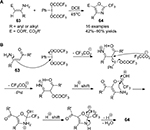  | Figure 22 (A) PIFA-mediated synthesis of 2-trifluoromethyl oxazole derivatives. (B) Proposed mechanism. Abbreviation: PIFA, phenyliodine bis(trifluoroacetate); DCE, 1,2-dichloroethane. |
In 2010, Saito et al52 reported the oxidative cycloisomerization of propargylamide derivatives 65, mediated by PIDA in AcOH or AcOH-HFIP and affording the corresponding 2,5-disubstituted oxazoles 66 (Figure 23).
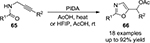  | Figure 23 PIDA-mediated synthesis of 2,5-disubstituted oxazoles in AcOH or AcOH-HFIP. Abbreviations: PIDA, phenyliodine diacetate; rt, room temperature. |
Treating anthranilamides 67a or salicylamides 67b with PIDA in the presence of potassium hydroxide, the 2-benzimidazolones 68a and 2-benzoxazolones 68b were, respectively, obtained in good yields (Figure 24). The postulated mechanistic pathway suggested an initial Hofmann-type rearrangement followed by a sequential intramolecular cyclization of the intermediate isocyanate.53
  | Figure 24 PIDA/KOH-mediated synthesis of 2-benzimidazolones and 2-benzoxazolones. Abbreviation: PIDA, phenyliodine diacetate. |
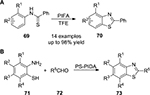
In 2008, PIFA-mediated intramolecular cyclization of the thiobenzamides 69 resulting in the benzothiazoles 70 via reactive intermediates of aryl radical cations was described (Figure 25A).54 Later on, Kumar et al55 applied the polymer-supported PIDA to construct the benzothiazoles 73 from the corresponding o-amino benzenethiol components 71 and aldehydes 72 (Figure 25B).
Lactone
In 2007, Dohi et al56 developed a direct construction of the biologically important aryl lactone 76 from carboxylic acid 74 using combined PIDA and KBr (Figure 26). The aryl group in the substrate was understood to be indispensable due to the benzyl radical intermediate 75 as suggested by the mechanism. The aryl lactone product 76 was achieved via hydrogen abstraction and then cyclization.
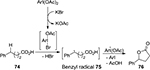  | Figure 26 PIDA/KBr-mediated synthesis of aryl lactones. Abbreviation: PIDA, phenyliodine diacetate. |
Spiro heterocycles and bisindolines
In 2012, Wang et al57 reported a PIFA-mediated synthesis of spirooxindoles 78 from anilide derivatives 77 bearing an appropriate α-arylaminocarbonyl group (Figure 27). These processes feature a metal-free oxidative C(sp2)–C(sp3) bond formation, followed by oxidative spirocyclization.
Recently, Zhang et al58 reported a PIFA-mediated cascade annulation of internal alkyne 79, affording the spiro heterocycle 80 (Figure 28). This process encompasses not only two sequential C–N/C–O bond formations but also the insertion of a carbonyl oxygen, all in one pot.
In 2014, Kim et al59 realized a cascade intramolecular oxidative diamination of olefins 81 by using PIDA as an oxidant and a halide as an additive, leading to the synthesis of a variety of bisindolines 82 (Figure 29).
Six- and seven-membered heterocycles
A PIFA-mediated oxidative C(sp2)–C(sp2) bond formation between two aryl rings was reported by Kita et al.60 Later, this oxidative coupling strategy was widely applied to the conversion of various biaryl substrates tethered by a relatively labile linker attached to the heterocycles, such as a silaketale, sulfide, sulfoxide, sulfone, or dibenzylether.61–63 For example, Moreno et al64 described an efficient synthesis of benzo[c]phenanthridine 84 and phenanthridinone 86 from properly substituted benzylnaphthylamine 83 and naphthylbenzamide 85, respectively, through a PIFA-mediated intramolecular oxidative C–C bond formation between the two electron-rich phenyl rings (Figure 30).
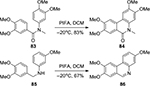  | Figure 30 PIFA-mediated synthesis of benzo[c]phenanthridine and phenanthridinone. Abbreviation: PIFA, phenyliodine bis(trifluoroacetate); DCM, dichloromethane. |
Liu et al65 reported the syntheses of a variety of 3-arylquinolin-2-one compounds 88 from the N-methyl-N-phenylcinnamamides 87. The reactions involved an exclusive 1,2-aryl migration along with a metal-free oxidative C–C bond formation, mediated by PIFA in the presence of a Lewis acid (Figure 31).65
In 2001, Arisawa et al66 reported a PIFA-mediated direct intramolecular cyclization of α-(aryl)alkyl-β-dicarbonyl compounds 89 leading to the spirobenzannulated products 90. Both meta- and para-substituted phenol ether derivatives containing cyclic or acyclic 1,3-dicarbonyl moieties on the side chain underwent the annulation in a facile manner (Figure 32).
  | Figure 32 PIFA-mediated direct intramolecular cyclization of α-(aryl)alkyl-β-dicarbonyl compounds. Abbreviations: PIFA, phenyliodine bis(trifluoroacetate); TFE, 2,2,2-Trifluoroethanol. |
In 1990, Kikugawa and Kawase67 reported an intramolecular oxidative C(sp2)–N bond formation in substrates 91, which contained a methoxyamide side chain on the aromatic ring, to give the N-aryl-N-methoxyamides 92 (Figure 33) via a nitrenium ion intermediate. This oxidative amidation protocol was later applied in many explorations of novel means to construct heterocyclic framework.68–70
  | Figure 33 PIFA-mediated synthesis of N-aryl-N-methoxyamides via an intramolecular oxidative C–N bond formation. Abbreviation: PIFA, phenyliodine bis(trifluoroacetate). |
Starting from N-methoxybenzamide 93 and alkyne 94, Misu and Togo71 developed a straightforward synthesis of isoquinolones 95 using PIDA generated in situ through an intermolecular organocatalytic annulation (Figure 34).
  | Figure 34 Synthesis of isoquinolones from N-methoxybenzamide and diphenyl acetylene mediated by PIDA generated in situ. Abbreviations: PIDA, phenyliodine diacetate; rt, room temperature. |
The indenocarboxamides 96 could be converted to the fused indeno-1,4-diazepinones 97 through intramolecular oxidative C–N bond formations mediated by PIFA (Figure 35).72 Moreover, various PIFA-promoted intramolecular amidation reactions have been developed for the formation of five-, six-, and seven-membered heterocycles.72–75
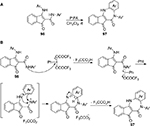  | Figure 35 (A) PIFA-mediated synthesis of the fused indeno-1,4-diazepinones. (B) Proposed mechanism. Abbreviations: PIFA, phenyliodine bis(trifluoroacetate); rt, room temperature. |
In 2014, Zhao and Du described a PIDA-mediated oxidative coupling of the two aryl groups in either 2-acylamino-N-phenylbenzamides 98 or 2-hydroxy-N-phenylbenzamides 100 to afford the dibenzodihydro-1,3-diazepin-2-ones 99 and dibenzo[d,f][1,3]oxazepin-6(7H)-ones 101, respectively (Figure 36). The reaction sequence involves an oxidative C(sp2)–C(sp2) aryl–aryl bond formation, C(sp2)–C/O bond cleavage, and an intramolecular lactamization/lactonization. The unique feature of this conversion is the concomitant insertion of the ortho-substituted N or O atom into the tether, realized for the first time.76
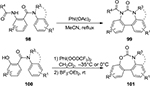  | Figure 36 I (III)-mediated formation of dibenzodihydro-1,3-diazepin-2-ones and dibenzo[d,f][1,3]oxazepin-6(7H)-ones. Abbreviation: rt, room temperature. |
A variety of systems involving PIDA/PIFA have been developed to realize functionalization of heterocyclic compounds. Some representative examples are discussed later.
Iodination
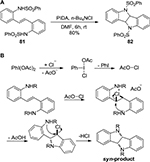
By using a combination of PIFA and I2, Benhida et al77 developed an iodination method suitable for electron-deficient heterocyclic compounds including substituted indoles 102 (Figure 37) and coumarins. Moreover, the methodologies offered reaction conditions mild enough to ensure the survival of sensitive protecting group such as acetyl and tert-butyldimethylsilyl. The methods were also applied to the iodination of substituted pyrazoles in providing the corresponding 4-iodopyrazole derivatives.78
  | Figure 37 PIFA/I2-mediated iodination of indole derivatives to 3-iodoindoles 103. Abbreviations: PIFA, phenyliodine bis(trifluoroacetate); rt, room temperature; h, hours; DCM, dichloromethane. |
Likewise, PIFA-mediated direct cyanations of various heterocyclic compounds including pyrroles, thiophenes, and indoles were realized using trimethylsilyl cyanide as a source of CN.79 For example, cyanation of N-tosylpyrroles 104 at the C2 position was achieved by using trimethylsilyl cyanide along with PIFA with moderate-to-excellent selectivity (Figure 38).
Bifunctionalization of glycals 106, including homogeneous azidization and selenylation, has been realized by Mironov et al80 through the reaction of glycals with PIDA in the presence of TMSN3 and Ph2Se2 (Figure 39).
  | Figure 39 PIDA-mediated homogeneous azidization and selenylation of glycals. Abbreviations: PIDA, phenyliodine diacetate; h, hours; DCM, dichloromethane. |
[Hydroxy-(organosulfonyloxy)iodo]arenes
Recently, Kawai et al81 described a new method for the synthesis of biologically significant trifluoromethyl-2-isoxazoline N-oxides 111. This conversion is realized through the intramolecular oxidative N–O coupling in β-trifluoromethyl-β-hydroxy ketoximes 109, generated from trifluoromethyl-β-keto alcohols 108, and mediated by [hydroxy(tosyloxy)iodo]benzene (Figure 40).81
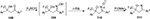  | Figure 40 HTIB-mediated synthesis of trifluoromethyl-2-isoxazoline-N-oxides. Abbreviation: HTIB, [hydroxy(tosyloxy)iodo]benzene. |
Treatment of 2H-chromene 112 with [hydroxy(tosyloxy)iodo]benzene in methanol could introduce a methoxyl group at the C4 position to afford 4-methoxy-2H-chromene 113 (Figure 41).82
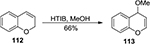  | Figure 41 HTIB-mediated synthesis of 4-methoxy-2H-chromene. Abbreviation: HTIB, [hydroxy(tosyloxy)iodo]benzene. |
Benziodoxole-based hypervalent iodine reagents
During the last decade, studies on the development of the λ3 iodine benziodoxolone reagents and their applications in facilitating organic transformations have attracted the attention of many synthetic chemists. Some representative examples are presented in this section.
In 2006, Eisenberger et al83 reported the first use of benziodoxole-derived reagents 5a and 6b for CF3 transfer. Later on, many practical applications of this class of hypervalent iodine (III) were developed.84,85
In 2014, Wang et al86 described an intramolecular carbotrifluoromethylation of alkynes 114 by using Togni’s reagent in the presence of Cu(I). A variety of trifluoromethylated heterocycles, such as 2H-chromene derivatives 115 and 117, 1,2-dihydroquinoline derivative 116, and the 2H-chromene five-membered cyclic product 118, were synthesized with great substituent tolerance and high selectivity (Figure 42).
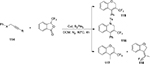  | Figure 42 Intramolecular carbotrifluoromethylation of alkynes with Togni’s reagent and Cu(I). Abbreviations: h, hours; DCM, dichloromethane. |
Due to the multiple reactive sites in indoles, trifluoromethylation of indole derivatives presents a challenge in synthetic chemistry. Shimizu et al87 developed a direct C2-selective trifluoromethylation of indole derivatives 119 with 2-trifluoromethyl indole 120 as the product by using Togni’s reagent (Figure 43). Later on, a method for the trifluoromethylation of indole compounds to afford the fused tricyclic indoles was established.88
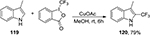  | Figure 43 Trifluoromethylation of indole derivatives with Togni’s reagent. Abbreviations: rt, room temperature; h, hours. |
In 2014, Zhang and Studer89 reported a method for the synthesis of the biologically important 1-trifluoromethylated isoquinolines 122. This transformation starts from the β-aryl-α-isocyano-acrylates 121 and uses Togni’s reagent as the CF3 radical precursor to afford the products in moderate-to-excellent yield, in the absence of any transition metal (Figure 44).
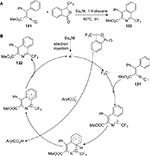  | Figure 44 (A) Synthesis of biologically important 1-trifluoromethylated isoquinolines with Togni’s reagent. (B) Proposed mechanism. Abbreviation: h, hours. |
Recently, by using Togni’s reagent and a simple catalyst CuI, Wang et al90 reported an elegant method for the aryltrifluoromethylation of N-phenylcinnamamides 123, where a series of CF3-containing 3,4-dihydroquinolin-2(1H)-ones 124 were obtained regioselectively and diastereoselectively (Figure 45). The same conversion from N-arylcinnamamides to CF3-containing dihydroquinolin-2(1H)-ones was also realized under visible light conditions.91
  | Figure 45 Aryltrifluoromethylation of N-phenylcinnamamides by using Togni’s reagent and copper catalyst. Abbreviation: h, hours. |
Another widely applied benziodoxole reagent is the [(triisopropylsilyl)ethynyl]benziodoxolone (TIPS-EBX) for its role in introducing alkynyl groups. Although TIPS-EBX had been prepared in 1996,92 the first significant application was not reported until 2009 by Brand et al.93 Direct alkynylation of indole and pyrrole heterocycles 125 was achieved with good functional group tolerance by using TIPS-EBX in the presence of gold as catalyst (Figure 46).94
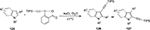  | Figure 46 Direct alkynylation of indole and pyrrole heterocycles by using TIPS-EBX. Abbreviation: TIPS-EBX, [(triisopropylsilyl)ethynyl]benziodoxolone. |
Recently, cobalt(III)-catalyzed C2-alkynylation of indoles 128 using hypervalent iodine–alkyne reagents was reported (Figure 47).95 This efficient protocol provided a variety of indole derivatives 129 bearing a C2 alkynyl linker, which can be connected to a series of synthetically useful functional groups such as −F, −Cl, −Br, −CO2Me, or −CN.
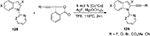  | Figure 47 Selective cobalt(III)-catalyzed alkynylation of indoles using hypervalent iodine-alkyne reagents. Abbreviations: TFE, 2,2,2-Trifluoroethanol; h, hours; Cp*, cyclopentadienyl. |
Applying TMS-EBX in the presence of tertiary amines, a metal-free alkynylation of various heterocyclic compounds 130–133 can be realized under mild conditions and affords the corresponding alkynylated heterocyclic compounds 134–137 containing a quaternary carbon in high yields (Figure 48).96
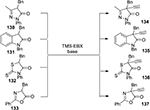  | Figure 48 Metal-free alkynylation of various heterocyclic compounds with TMS-EBX. |
In the presence of CsF, cycloaddition between the iodonium ylides 139 and the ortho-silyl aryltriflates 138 afforded a series of benzofurans 140 at room temperature in moderate-to-good yields (Figure 49).97
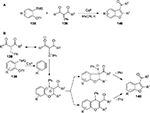  | Figure 49 (A) Cycloaddition of ortho-silyl aryltriflates and iodonium ylides. (B) Proposed mechanism. Abbreviation: rt, room temperature. |
Aryliodonium imides in the presence of metal complexes were reported to efficiently introduce another nitrogen atom into the nitrogen-containing heterocycle compounds. Figure 50 depicts the selective addition of the imido moiety to the N atom of pyridine rings 141 through a Ru-catalyzed N–N bond formation.98
  | Figure 50 Ru-catalyzed nitrogen atom transfer. Abbreviations: h, hours; DCM, dichloromethane. |
Arylation of heterocycles with diaryliodonium salts, whether at a carbon or a heteroatom, has drawn much attention from synthetic chemists. One of the most representative examples is the arylation of indole derivatives. In 2006, Deprez et al99 developed a method to carry out arylation of indoles 144 at C2 through a palladium-catalyzed reaction using diaryliodonium salts (Figure 51). This reaction was proven to be compatible with free N–H indoles 144, such that no by-product from N-arylation was observed.
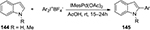  | Figure 51 Diaryliodonium salts-mediated arylation of indoles at C2. Abbreviations: rt, room temperature; h, hours. |
As arylation using diaryliodonium salts would inevitably generate one equivalent of an iodoarene as a side product, it makes this approach unattractive with regard to atom economy. Recently, a Cu-catalyzed tandem C−H/N−H arylation of indoles 146 was discovered, which incorporated both aryl groups from the reagent diaryliodonium salts while providing novel indoles 147 (Figure 52).100
  | Figure 52 Cu-catalyzed tandem C–H/N–H arylation of indoles with diaryliodonium salts. Abbreviations: eq., equivalent; DMEDA, N,N’-Dimethyl-1,2-ethanediamine. |
A significant amount of efforts have been devoted to the arylation of N-containing heterocycles by using diaryliodonium salts and metal catalysts. For example, a Pd-mediated arylation of benzotriazol 148 and a Cu-mediated N-arylation of indole 150, cyclohexylamine 152, and the four-membered lactam 154 were realized. Selected examples are presented in Figure 53.101–105
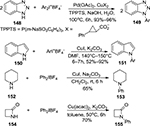  | Figure 53 Arylation of N-containing heterocycles with diaryliodonium salts. Abbreviations: rt, room temperature; h, hours. |
In 2013, Wang et al106 realized a Cu(OTf)2-catalyzed regioselective synthesis of polysubstituted quinolines from three components including the diaryliodonium salt 156, the nitrile 157, and the alkyne 158 (Figure 54). It is worth noting that the aryl group of the diaryliodonium serves as a C2 building block in this reaction.
  | Figure 54 A Cu(OTf)2-catalyzed, three-component regioselective synthesis of polysubstituted quinolones. Abbreviations: eq., equivalent; DCE, 1,2-dochloroethane. |
Hypervalent iodine (V) reagents
Among the iodine (V) compounds, Dess–Martin periodinane (DMP) and 2-iodoxybenzoic acid (IBX) are the two most practical and therefore most widely applied oxidants for their mild characteristics. A large range of syntheses and functionalization of heterocyclic compounds have been achieved in recent years through the applications of iodine (V) reagents.
Dess–Martin periodinane
DMP was first introduced in 1984.107 The most special property of it is its ability to realize selective oxidation of primary and secondary alcohols to their respective aldehydes and ketones.108,109 Some applications have been formulated based on this property. For example, when treated with DMP in a hydrocarbon solvent, cleavage of the glycol C–C bond in 1,2-diols 160 takes place, leading to the formation of a more complex molecule 162 (Figure 55).110
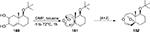  | Figure 55 Oxidative cleavage of the glycol C–C bond with DMP. Abbreviations: DMP, Dess–Martin periodinane; rt, room temperature; h, hours. |
Another example is the synthesis of the 2-substituted benzothiazoles 164 in high yields, which is facilitated by DMP through an intramolecular oxidative cyclization of the thioformanilides 163 in CH2Cl2. The mild reaction environment plays a key role as the reaction proceeds via a thiol radical intermediate (Figure 56).111
  | Figure 56 Synthesis of 2-substituted benzothiazoles with DMP. Abbreviations: DMP, Dess–Martin periodinane; rt, room temperature; min, minutes. |
Iodoxybenzoic acid
Certain heterocyclic compounds such as isoxazolidines, [1,2]oxazinanes, and 3,5-disubstituted isoxazolines could be synthesized through radical cyclization by using IBX as a single-electron transfer (SET) oxidant. The cyclizations brought about with this protocol could occur in an intramolecular as well as intermolecular manner.
In 2005, Janza and Studer112 described the generation of alkoxyamidyl radicals initiated by IBX as an SET oxidant from the acylated alkoxyamines 165. The stereoselective 5-exo and 6-exo reactions with these N-heteroatom-centered radicals led to the isoxazolidines 166a and the [1,2]oxazinanes 166b in good-to-excellent yields (Figure 57).
  | Figure 57 IBX-mediated stereoselective 5-exo and 6-exo formations of isoxazolidines and [1,2]oxazinanes. Abbreviations: IBX, 2-iodoxybenzoic acid; DMSO, dimethyl sulfoxide; min, minutes. |
In 2004, Das et al113 reported the preparation of the 3,5-disubstituted isoxazolines 169, achieved via an SET reaction consisting of multiple components of 167 and 168 using IBX as an oxidant (Figure 58). The reaction proceeded through a substituted aldoxime intermediate followed by a 1,3-dipolar addition of an alkene.113
  | Figure 58 IBX-mediated SET synthesis of isoxazolines involving multiple components. Abbreviations: IBX, 2-iodoxybenzoic acid; SET, single-electron transfer; DCM, dichloromethane. |
Recently, Bredenkamp et al114 reported a new example of IBX-promoted direct functionalization of the indoles 170 to the isatins 172. The reagent mixture 171 (NaI/IBX-SO3K containing a substituted sulfonyl of IBX) was employed to trigger this oxidative process (Figure 59).114
  | Figure 59 Direct functionalization of indoles to isatins by NaI/IBX-SO3K. Abbreviation: DMSO, dimethyl sulfoxide. |
Conclusion
During the past several decades, hypervalent iodine reagents have been widely used in the syntheses and functionalization of heterocyles. The low production cost has made many of them commercially available, and the low toxicity, being transition metal-free, renders them environmentally friendly. But most importantly, it is their powerful oxidizing properties under mild reaction conditions along with high chemoselectivity that have driven hypervalent iodine chemistry to expand its territory in the field of synthetic chemistry.
Acknowledgments
We acknowledge the National Natural Science Foundation of China (#21472136), Tianjin Research Program of Application Foundation and Advanced Technology (#15JCZDJC32900), and the National Basic Research Project (2014CB932201) for financial support.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Stang PJ, Zhdankin VV. Organic polyvalent iodine compounds. Chem Rev. 1996;96(3):1123–1178. | ||
Moriarty RM, Prakash OM. Synthesis of heterocyclic compounds using organohypervalent iodine reagents. Adv Heterocycl Chem. 1997;69:1–87. | ||
Togo H, Katohgi M. Synthetic uses of organohypervalent iodine compounds through radical pathways. Synlett. 2001;5:0565–0581. | ||
Zhdankin VV, Stang PJ. Recent developments in the chemistry of polyvalent iodine compounds. Chem Rev. 2002;102(7):2523–2584. | ||
Moreno I, Tellitu I, Herrero MT, SanMartín R, Domínguez E. New perspectives for iodine(III) reagents in (hetero)biaryl coupling reactions. Curr Org Chem. 2002;6:1433–1452. | ||
Wirth T. Hypervalent iodine chemistry in synthesis: scope and new directions. Angew Chem Int Ed Engl. 2005;44(24):3656–3665. | ||
Zhdankin VV. Benziodoxole-based hypervalent iodine reagents in organic synthesis. Curr Org Synth. 2005;2(1):121–145. | ||
Moriarty RM. Organohypervalent iodine: development, applications, and future directions. J Org Chem. 2005;70(8):2893–2903. | ||
Richardson RD, Wirth T. Hypervalent iodine goes catalytic. Angew Chem Int Ed. 2006;45(27):4402–4404. | ||
Zhdankin VV, Stang PJ. Chemistry of polyvalent iodine. Chem Rev. 2008;108(12):5299–5358. | ||
Merritt EA, Olofsson B. Diaryliodonium salts: a journey from obscurity to fame. Angew Chem Int Ed Engl. 2009;48(48):9052–9070. | ||
Zhdankin VV. Hypervalent iodine(III) reagents in organic synthesis. ARKIVOC. 2009;i:1–62. | ||
Duschek A, Kirsch SF. 2-iodoxybenzoic acid–a simple oxidant with a dazzling array of potential applications. Angew Chem Int Ed. 2011;50(7):1524–1552. | ||
Brand JP, Gonzalez DF, Nicolai S, Waser J. Benziodoxole-based hypervalent iodine reagents for atom-transfer reactions. Chem Commun (Camb). 2011;47(1):102–115. | ||
Brown M, Farid U, Wirth T. Hypervalent iodine reagents as powerful electrophiles. Synlett. 2013;24:0424–0431. | ||
Parra A, Reboredo S. Chiral hypervalent iodine reagents: synthesis and reactivity. Chemistry. 2013;19(51):17244–17260. | ||
Zheng Z, Zhang-Negrerie D, Du Y, Zhao K. The applications of hypervalent iodine(III) reagents in the constructions of heterocyclic compounds through oxidative coupling reactions. Sci China Chem. 2014;57(2):189–214. | ||
Narayan R, Manna S, Antonchick AP. Hypervalent iodine(III) in direct carbon–hydrogen bond functionalization. Synlett. 2015;26:1785–1803. | ||
Narayan R, Matcha K, Antonchick AP. Metal-free oxidative C–C bond formation through C–H bond functionalization. Chemistry. 2015;21(42):14678–14693. | ||
Charpentier J, Fruh N, Togni A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem Rev. 2015;115(2):650–682. | ||
Ueno M, Nabana T, Togo H. Novel oxidative α-tosyloxylation of alcohols with iodosylbenzene and p-toluenesulfonic acid and its synthetic use for direct preparation of heteroaromatics. J Org Chem. 2003;68(16):6424–6426. | ||
Fan R, Wang H, Ye Y, Gan J. PhIO/Bu4NI mediated oxidative cyclization of amidoalkylation adducts for the synthesis of N-benzoyl aziridines and oxazolines. Tetrahedron Lett. 2010;51:453–456. | ||
Ye Y, Zheng C, Fan R. Solvent-controlled oxidative cyclization for divergent synthesis of highly functionalized oxetanes and cyclopropanes. Org Lett. 2009;11(14):3156–3159. | ||
Ye Y, Wang H, Fan R. Stereoselective construction of highly functionalized azetidines via a [2 + 2]-cycloaddition. Org Lett. 2010;12(12):2802–2805. | ||
Moriarty RM, Vaid RK, Duncan MP. Hypervalent iodine oxidation of amines using iodosobenzene: synthesis of nitriles, ketones and lactams. Tetrahedron Lett. 1988;29(52):6913–6916. | ||
Moriarty RM, Rani N, Condeiu C, Duncan MP, Prakash O. Hypervalent iodine oxidation of trimethylsilyl ketene acetals: a convenient route to α-methoxylation of esters and lactones. Synth Commun. 1997;27(18):3273–3277. | ||
Moriarty RM, Prakash O, Duncan MP, Vaid RK, Rani N. Oxidation of some olefinic compounds using iodosobenzene. J Chem Res Synop. 1996;9:432–433. | ||
Francisco CG, Herrera AJ, Suarez E. Intramolecular hydrogen abstraction reaction in carbohydrate chemistry. Synthesis of chiral 2,7-dioxabicyclo[2.2.1]heptane and 6,8-dioxabicyclo[3.2.1]octane ring systems. Tetrahedron Lett. 2000;41:7869–7873. | ||
Francisco CG, Freire R, González CC, León EI, Riesco-Fagundo C, Suárez E. Fragmentation of carbohydrate anomeric alkoxy radicals. synthesis of polyhydroxy piperidines and pyrrolidines related to carbohydrates. J Org Chem. 2001;66(5):1861–1866. | ||
Francisco CG, Herrera AJ, Suarez E. Intramolecular hydrogen abstraction promoted by N-radicals: synthesis of chiral 7-oxa-2-azabicyclo[2.2.1]heptane and 8-oxa-6-azabicyclo[3.2.1] octane ring systems. Tetrahedron. 2000;11:3879–3882. | ||
Ochiai M, Inenaga M, Nagao Y. Oxidative decarboxylation of cyclic amino acids and dehydrogenation of cyclic secondary amines with iodosobenzene. Tetrahedron Lett. 1988;29(52):6917–6920. | ||
Caddick S, Motherwell WB, Wilkinson JA. A concise method for the preparation of glycosyl fluorides via displacement reactions of 1-arylthioglycosides with 4-methyl(difluoroiodo)benzene. J Chem Soc Chem Commun. 1991:674–675. | ||
Caddick S, Gazzard L, Motherwell WB, Wilkinson JA. Preparation of 1-fluoroglycosides from l-arylthio and 1-arylselenoglycosides using 4-methyl(difluoroiodo)benzene. Tetrahedron. 1996;52:149–156. | ||
Inagaki T, Nakamura Y, Sawaguchi M, Yoneda N, Ayuba S, Hara S. Fluorinative ring-expansion of cyclic ethers using p-iodotoluene difluoride. Stereoselective synthesis of fluoro cyclic ethers. Tetrahedron Lett. 2003;44:4117–4119. | ||
Salamant W, Hulme C. Unique one step, multicomponent α,β,β-oxidations of carbamates with Willgerodt-like hypervalent iodine reagents – an example of triple C–H bond activation. Tetrahedron Lett. 2006;47:605–609. | ||
Prakash O, Kaur H, Batra H, Rani N, Singh SP, Moriarty RM. α-thiocyanation of carbonyl and β-dicarbonyl compounds using (dichloroiodo)benzene-lead(II) thiocyanate. J Org Chem. 2001;66(6):2019–2023. | ||
Hepples C, Murphy GK. Synthesis of 3,3-dichloro-2-oxindoles from isatin-3-p-tosylhydrazones and (dichloroiodo)benzene. Tetrahedron Lett. 2015;56:4971–4974. | ||
Coffey KE, Moreira R, Abbas FZ, Murphy GK. Synthesis of 3,3-dichloroindolin-2-ones from isatin-3-hydrazones and (dichloroiodo)benzene. Org Biomol Chem. 2015;13(3):682–685. | ||
He T, Gao WC, Wang WK, Zhang C. Synthesis of oxazolidin-2-ones and imidazolidin-2-ones directly from 1,3-diols or 3-amino alcohols using iodobenzene dichloride and sodium azide. Adv Synth Catal. 2014;356:1113–1118. | ||
Li X, Du Y, Liang Z, Li X, Pan Y, Zhao K. Simple conversion of enamines to 2H-azirines and their rearrangements under thermal conditions. Org Lett. 2009;11(12):2643–2646. | ||
Zheng Y, Yang C, Zhang-Negrerie D, Du Y, Zhao K. One-pot synthesis of isoxazoles from enaminones: an application of Fe(II) as the catalyst for ring expansion of 2H-azirine intermediates. Tetrahedron Lett. 2013;54:6157–6160. | ||
Zhang PF, Chen ZC. Hypervalent iodine in synthesis 72: a tandem dimerisation-cyclocondensation of enaminones with [bis(trifluoroacetoxy)iodo]benzene: an effective method for the synthesis of highly substituted pyrroles. J Chem Res Synop. 2001:150–152. | ||
Wang JY, Liu SP, Yu W. Synthesis of polysubstituted pyrroles via PhI(OAc)2-mediated oxidative coupling of enamine esters and ketones. Synlett. 2009;15:2529–2533. | ||
Du YF, Liu RH, Linn G, Zhao K. Synthesis of N-substituted indole derivatives via PIFA-mediated intramolecular cyclization. Org Lett. 2006;8(26):5919–5922. | ||
Ban X, Pan Y, Lin Y, Wang S, Du Y, Zhao K. Synthesis of carbazolones and 3-acetylindoles via oxidative C–N bond formation through PIFA-mediated annulation of 2-aryl enaminones. Org Biomol Chem. 2012;10(18):3606–3609. | ||
Yu WQ, Du YF, Zhao K. PIDA-mediated oxidative C–C bond formation: novel synthesis of indoles from N-aryl enamines. Org Lett. 2009;11(11):2417–2420. | ||
Das B, Srinivas Y, Holla H, Krishnaiah M, Narender R. Hypervalent iodine-mediated efficient synthesis of imidazoles. Chem Lett. 2007;36:1270–1271. | ||
Du LH, Wang YG. A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis. 2007;5:675–678. | ||
Kotali A. Synthesis and electron impact mass spectra of 3-substituted 2-acylaminoindazoles. J Heterocycl Chem. 1996;33:605–606. | ||
Yu Z, Ma L, Yu W. Phenyliodine bis(trifluoroacetate) mediated intramolecular oxidative coupling of electron-rich N-phenyl benzamides. Synlett. 2012;23:1534–1540. | ||
Zhao F, Liu X, Qi R, et al. Synthesis of 2-(Trifluoromethyl)oxazoles from β-monosubstituted enamines via PhI(OCOCF3)2-mediated trifluoroacetoxylation and cyclization. J Org Chem. 2011;76(24):10338–10344. | ||
Saito A, Matsumoto A, Hanzawa Y. PIDA-mediated synthesis of oxazoles through oxidative cycloisomerization of propargylamides. Tetrahedron Lett. 2010;51:2247–2250. | ||
Prakash O, Batra H, Kaur H, et al. Hypervalent iodine oxidative rearrangement of anthranilamides, salicylamides and some β-substituted amides: a new and convenient synthesis of 2-benzimidazolones, 2-benzoxazolones and related compounds. Synthesis. 2001;4:541–543. | ||
Downer-Riley NK, Jackson YA. Conversion of thiobenzamides to benzothiazoles via intramolecular cyclization of the aryl radical cation. Tetrahedron. 2008;64:7741–7744. | ||
Kumar A, Maurya RA, Ahmad P. Diversity oriented synthesis of benzimidazole and benzoxa/(thia)zole libraries through polymer-supported hypervalent iodine reagent. J Comb Chem. 2009;11(2):198–201. | ||
Dohi T, Takenaga N, Goto A, Maruyama A, Kita Y. Direct lactone formation by using hypervalent iodine(III) reagents with KBr via selective C–H abstraction protocol. Org Lett. 2007;9(16):3129–3132. | ||
Wang J, Yuan Y, Xiong R, Zhang-Negrerie D, Du Y, Zhao K. Phenyliodine bis(trifluoroacetate)-mediated oxidative C−C bond formation: synthesis of 3-hydroxy-2-oxindoles and spirooxindoles from anilides. Org Lett. 2012;14(9):2210–2213. | ||
Zhang X, Yang C, Zhang-Negrerie D, Du Y. Hypervalent-iodine-mediated cascade annulation of diarylalkynes forming spiro heterocycles under metal-free conditions. Chemistry. 2015;21(13):5193–5198. | ||
Kim H, Cho SH, Chang S. Intramolecular oxidative diamination and aminohydroxylation of olefins under metal-free conditions. Org Lett. 2012;14(6):1424–1427. | ||
Kita Y, Gyoten M, Ohtsubo M, Tohma H, Takada T. Non-phenolic oxidative coupling of phenol ether derivatives using phenyliodine(III) bis(trifluoroacetate). Chem Commun. 1996:1481–1482. | ||
Taylor SR, Ung AT, Pyne SG, Skelton BW, White AH. Intramolecular versus intermolecular oxidative couplings of ester tethered di-aryl ethers. Tetrahedron. 2007;63:11377–11385. | ||
Takada T, Arisawa M, Gyoten M, Hamada R, Tohma H, Kita Y. Oxidative biaryl coupling reaction of phenol ether derivatives using a hypervalent iodine(III) reagent. J Org Chem. 1998;63:7698–7706. | ||
Tohma H, Morioka H, Takizawa S, Arisawa M, Kita Y. Efficient oxidative biaryl coupling reaction of phenol ether derivatives using hypervalent iodine(III) reagents. Tetrahedron. 2001;57:345–352. | ||
Moreno I, Tellitu I, Etayo J, SanMartin R, Dominguez E. Novel applications of hypervalent iodine: PIFA mediated synthesis of benzo[c]phenanthiridines and benzo[c]phenanthridinones. Tetrahedron. 2001;57:5403–5411. | ||
Liu L, Lu H, Wang H, et al. PhI(OCOCF3)2-mediated C–C bond formation concomitant with a 1,2-aryl shift in a metal-free synthesis of 3-arylquinolin-2-ones. Org Lett. 2013;15(12):2906–2909. | ||
Arisawa M, Ramesh NG, Nakajima M, Tohma H, Kita Y. Hypervalent iodine(III)-induced intramolecular cyclization of α-(Aryl)alkyl-β-dicarbonyl compounds: a convenient synthesis of benzannulated and spirobenzannulated compounds. J Org Chem. 2001;66(1):59–65. | ||
Kikugawa Y, Kawase M. An electrophilic aromatic substitution by N-methoxyamides via hypervalent iodine intermediates. Chem Lett. 1990;4:581–582. | ||
Romero AG, Darlington WH, Jacobsen EJ, Mickelson JW. Oxidative cyclization of acyclic ureas with bis(trifluoroacetoxy)iodobenzene to generate N-substituted 2-benzimidazolinones. Tetrahedron Lett. 1996;37(14):2361–2364. | ||
Herrero MT, Tellitu I, Dominguez E, Moreno I, SanMartín R. A novel and efficient iodine(III)-mediated access to 1,4-benzodiazepin-2-ones. Tetrahedron Lett. 2002;43:8273–8275. | ||
Correa A, Tellitu I, Dominguez E, Moreno I, SanMartin R. An efficient, PIFA mediated approach to benzo-, naphtho-, and heterocycle-fused pyrrolo[2,1-c][1,4]diazepines. An advantageous access to the antitumor antibiotic DC-81. J Org Chem. 2005;70(6):2256–2264. | ||
Misu Y, Togo H. Novel preparation of 2,1-benzothiazine derivatives from sulfonamides with [hydroxy(tosyloxy)iodo]arenes. Org Biomol Chem. 2003;1(8):1342–1346. | ||
Malamidou-Xenikaki E, Spyroudis S, Tsanakopoulou M, Hadjipavlou-Litina D. A convenient approach to fused indeno-1,4-diazepinones through hypervalent iodine chemistry. J Org Chem. 2009;74(19):7315–7321. | ||
Serna S, Tellitu I, Dominguez E, Moreno I, SanMartin R. Iodine(III)-mediated aromatic amidation vs olefin amidohydroxylation. The amide N-substituent makes the difference. Tetrahedron. 2004;60:6533–6539. | ||
Correa A, Tellitu I, Dominguez E, SanMartin R. A metal-free approach to the synthesis of indoline derivatives by a phenyliodine(III) bis(trifluoroacetate)-mediated amidohydroxylation reaction. J Org Chem. 2006;71(21):8316–8319. | ||
Zhu J, Xie H, Chen Z, Li S, Wu Y. Synthesis of N-substituted 2-fluoromethylbenzimidazoles via bis(trifluoroacetoxy)iodobenzene-mediated intramolecular cyclization of N,N’-disubstituted fluoroethanimidamides. Synlett. 2009;20:3299–3302. | ||
Shang S, Zhang-Negrerie D, Du Y, Zhao K. Intramolecular metal-free oxidative Aryl–Aryl coupling: an unusual hypervalent-iodine-mediated rearrangement of 2-substituted N-phenylbenzamides. Angew Chem Int Ed. 2014;53(24):6216–6219. | ||
Benhida R, Blanchard P, Fourrey J-L. A mild and effective iodination method using iodine in the presence of bis-(trifluoroacetoxy)iodobenzene. Tetrahedron Lett. 1998;39:6849–6852. | ||
Vasilevsky SF, Klyatskaya SV, Tretyakov EV, Elguero J. Ethyl vinyl ether – an agent for protection of the pyrazole NH-fragment. A convenient method for the preparation of N-unsubstituted 4-Alkynylpyrazoles. Heterocycles. 2003;60(4):879–886. | ||
Dohi T, Morimoto K, Takenaga N, et al. Direct cyanation of heteroaromatic compounds mediated by hypervalent iodine(III) reagents: in situ generation of PhI(III)-CN species and their cyano transfer. J Org Chem. 2007;72(1):109–116. | ||
Mironov YV, Sherman AA, Nifantiev NE. Homogeneous azidophenylselenylation of glycals using TMSN3–Ph2Se2–PhI(OAc)2. Tetrahedron Lett. 2004;45:9107–9110. | ||
Kawai H, Okusu S, Tokunaga E, Shibata N. Enantioselective synthesis of 5-trifluoromethyl-2-isoxazolines and their N-oxides by [Hydroxy(tosyloxy)iodo]benzene-mediated oxidative N–O coupling. Eur J Org Chem. 2013:6506–6509. | ||
Ahmad A, Silva LF Jr. Synthesis of chromanes and 4H-chromenes: exploring the oxidation of 2H-chromenes and dihydro-1-benzoxepines by hypervalent iodine(III). Synthesis. 2012;44(23):3671–3677. | ||
Eisenberger P, Gischig S, Togni A. Novel 10-I-3 hypervalent iodine-based compounds for electrophilic trifluoromethylation. Chemistry. 2006;12(9):2579–2586. | ||
Stanek K, Koller R, Togni A. Reactivity of a 10-I-3 hypervalent iodine trifluoromethylation reagent with phenols. J Org Chem. 2008;73(19):7678–7685. | ||
Kieltsch I, Eisenberger P, Togni A. Mild electrophilic trifluoromethylation of carbon- and sulfur-centered nucleophiles by a hypervalent iodine(III)–CF3 reagent. Angew Chem Int Ed. 2007;46(5):754. | ||
Wang Y, Jiang M, Liu J-T. Copper-catalyzed intramolecular carbotrifluoromethylation of alkynes for the construction of trifluoromethylated heterocycles. Chemistry. 2014;20(47):15315–15319. | ||
Shimizu R, Egami H, Nagi T, Chae J, Hamashima Y, Sodeoka M. Direct C2-trifluoromethylation of indole derivatives catalyzed by copper acetate. Tetrahedron Lett. 2010;51:5947–5949. | ||
Zhu H, Liu H, Feng X, et al. Copper(I)-promoted trifluoromethylation of indole derivatives with concomitant C–C bond formation to access fused tricyclic indoles. Tetrahedron Lett. 2015;56:1703–1705. | ||
Zhang B, Studer A. 1-Trifluoromethylated isoquinolines via radical trifluoromethylation of isonitriles. Org Biomol Chem. 2014;12(48):9895–9898. | ||
Wang Q, Han G, Liu Y, Wang Q. Copper-catalyzed aryltrifluoromethylation of N-phenylcinnamamides: access to trifluoromethylated 3,4-dihydroquinolin-2(1H)-ones. Adv Synth Catal. 2015;357:2464–2468. | ||
Gao F, Yang C, Gao G-L, Zheng L, Xia W. Visible-light induced trifluoromethylation of N-arylcinnamamides for the synthesis of CF3-containing 3,4-disubstituted dihydroquinolinones and 1-azaspiro[4.5]decanes. Org Lett. 2015;17(14):3478–3481. | ||
Zhdankin VV, Kuehl CJ, Krasutsky AP, Bolz JT, Simonsen AJ. 1-(Organosulfonyloxy)-3(1H)-1,2-benziodoxoles: preparation and reactions with alkynyltrimethylsilanes. J Org Chem. 1996;61(19):6547–6551. | ||
Brand JP, Charpentier J, Waser J. Direct alkynylation of indole and pyrrole heterocycles. Angew Chem Int Ed Engl. 2009;48(49):9346–9349. | ||
Kaschel J, Werz D. Ethynyl benziodoxolone (EBX): installing alkynes the reversed way. Angew Chem Int Ed Engl. 2015;54(31):8876–8878. | ||
Zhang Z-Z, Liu B, Wang C-Y, Shi B-F. Cobalt(III)-catalyzed C2-selective C−H alkynylation of indoles. Org Lett. 2015;17(16):4094–4097. | ||
Kamlar M, Císařová I, Veselý J. Alkynylation of heterocyclic compounds using hypervalent iodine reagent. Org Biomol Chem. 2015;13(10):2884–2889. | ||
Huang X-C, Liu Y-L, Liang Y, Pi S-F, Wang F, Li J-H. Cycloaddition of arynes with iodonium ylides: a mild and general route for the synthesis of benzofuran derivatives. Org Lett. 2008;10(8):1525–1528. | ||
Jiang Y, Zhou G-C, He G-L, He L, Li J-L, Zheng S-L. Ruthenium(II)-porphyrin catalyzed selective N-imidation of aromatic nitrogen heterocycles. Synthesis. 2007;10:1459–1464. | ||
Deprez NR, Kalyani D, Krause A, Sanford MS. Room temperature palladium-catalyzed 2-arylation of indoles. J Am Chem Soc. 2006;128(15):4972–4973. | ||
Modha SG, Greaney MF. Atom-economical transformation of diaryliodonium salts: tandem C−H and N−H arylation of indoles. J Am Chem Soc. 2015;137(4):1416–1419. | ||
Beletskaya IP, Davydov DV, Moreno-Manas M. Pd- and Cu-catalyzed selective arylation of benzotriazole by diaryliodonium salts in water. Tetrahedron Lett. 1998;39:5621–5622. | ||
Beletskaya IP, Davydov DV, Gorovoy MS. Palladium- and copper-catalyzed selective arylation of 5-aryltetrazoles by diaryliodonium salts. Tetrahedron Lett. 2002;43:6221–6223. | ||
Davydov DV, Beletskaya IP, Semenov BB, Smushkevich YI. Regioselective arylation of N-tributylstannylated 5-substituted tetrazoles by diaryliodonium salts in the presence of Cu(OAc)2. Tetrahedron Lett. 2002;43:6217–6219. | ||
Kang S-K, Lee S-H, Lee D. Copper-catalyzed N-arylation of amines with hypervalent iodonium salts. Synlett. 2000;7:1022–1024. | ||
Zhou T, Chen Z-C. Hypervalent iodine in synthesis 85: an efficient method for the synthesis of N-arylbenzimidazoles by the copper- catalyzed N-arylation of benzimidazole with diaryliodonium salts. Heteroat Chem. 2002;13(7):617–619. | ||
Wang Y, Chen C, Peng J, Li M. Copper(II)-catalyzed three-component cascade annulation of diaryliodoniums, nitriles, and alkynes: a regioselective synthesis of multiply substituted quinolines. Angew Chem Int Ed Engl. 2013;52(20):5323–5327. | ||
Dess DB, Martin JC. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J Org Chem. 1983;48:4156–4158. | ||
Ladziata U, Zhdankin VV. Hypervalent iodine(V) reagents in organic synthesis. ARKIVOC. 2006;ix:26–58. | ||
Wirth T. IBX-new reactions with an old reagent. Angew Chem Int Ed Engl. 2001;40(15):2812–2814. | ||
Candela Lena JI, Martín Hernando JI, Rosario Rico Ferreira del M, Altinel E, Arseniyadis S. Tandem glycol cleavage-intramolecular [4 + 2] cycloadditions mediated by Dess-Martin periodinane. Synlett. 2001;5:597–600. | ||
Bose DS, Idrees M. Hypervalent iodine mediated intramolecular cyclization of thioformanilides: expeditious approach to 2-substituted benzothiazoles. J Org Chem. 2006;71(21):8261–8263. | ||
Janza B, Studer A. Stereoselective cyclization reactions of IBX-generated alkoxyamidyl radicals. J Org Chem. 2005;70(17):6991–6994. | ||
Das B, Holla H, Mahender G, Banerjee J, Reddy MR. Hypervalent iodine-mediated interaction of aldoximes with activated alkenes including Baylis–Hillman adducts: a new and efficient method for the preparation of nitrile oxides from aldoximes. Tetrahedron Lett. 2004;45:7347–7350. | ||
Bredenkamp A, Mohr F, Kirsch SF. Synthesis of isatins through direct oxidation of indoles with IBX-SO3K/NaI. Synthesis. 2015;47:1937–1943. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.