Back to Journals » Drug Design, Development and Therapy » Volume 9
Hyaluronic acid abrogates ethanol-dependent inhibition of collagen biosynthesis in cultured human fibroblasts
Authors Donejko M, Przylipiak A , Rysiak E, Miltyk W, Galicka E, Przylipiak J, Zaręba I, Surazynski A
Received 8 July 2015
Accepted for publication 15 September 2015
Published 24 November 2015 Volume 2015:9 Pages 6225—6233
DOI https://doi.org/10.2147/DDDT.S91968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Magdalena Donejko,1 Andrzej Przylipiak,1 Edyta Rysiak,2 Wojciech Miltyk,3 Elżbieta Galicka,1 Jerzy Przylipiak,4 Ilona Zaręba,2 Arkadiusz Surazynski2
1Department of Esthetic Medicine, 2Department of Medicinal Chemistry, 3Department of Pharmaceutical Analysis, Faculty of Pharmacy, Medical University of Białystok, 4Medical Office, Białystok, Poland
Introduction: The aim of the study was to evaluate the effect of ethanol on collagen biosynthesis in cultured human skin fibroblasts, and the role of hyaluronic acid (HA) in this process. Regarding the mechanism of ethanol action on human skin fibroblasts we investigated: expression of β1 integrin and insulin-like growth factor 1 receptor (IGF-IR), signaling pathway protein expression: mitogen-activated protein kinases (MAPKs), protein kinase B (Akt), nuclear factor kappa B (NF-κB) transcription factor, cytotoxicity assay and apoptosis, metalloproteinase activity, as well as the influence of HA on these processes.
Materials and methods: Collagen biosynthesis, activity of prolidase, DNA biosynthesis, and cytotoxicity were measured in confluent human skin fibroblast cultures that have been treated with 25, 50, and 100 mM ethanol and with ethanol and 500 µg/mL HA. Western blot analysis and zymography were performed to evaluate expression of collagen type I, β1 integrin receptor, IGF-IR, NF-κB protein, phospho-Akt protein, kinase MAPK, caspase 9 activity, and matrix metalloproteinases (MMP-9 and MMP-2).
Results: Ethanol in a dose-dependent manner lead to the impairment of collagen biosynthesis in fibroblast cultures through decreasing prolidase activity and expression of β1 integrin and IGF-IR. This was accompanied by an increased cytotoxicity, apoptosis and lowered expression of the signaling pathway proteins induced by β1 integrin and IGF-IR, that is, MAPK (ERK1/2) kinases. The lowered amount of synthesized collagen and prolidase activity disturbance may also be due to the activation of NF-κB transcription factor, which inhibits collagen gene expression. It suggests that the decrease in fibroblast collagen production may be caused by the disturbance in its biosynthesis but not degradation. The application of HA has a protective effect on disturbances caused by the examined substances. It seems that regulatory mechanism of ethanol-induced collagen aberration take place at the level of collagen biosynthesis, since no effect of ethanol and HA was found on process of collagen degradation by MMP-2 and MMP-9.
Conclusion: This study provides evidence that ethanol impairs collagen metabolism in human skin fibroblasts, leading to a significant decrease in the amount of produced protein. This mechanism probably is due to downregulation of prolidase activity, expression of β1 integrin and IGF-IR receptors, and the signaling pathway proteins induced by these receptors.
Keywords: collagen, ethanol, hyaluronic acid, fibroblast
Introduction
Ethanol (ethyl alcohol) exposure can cause a variety of local and systematic abnormalities. Organs that are particularly affected by ethanol intoxication are lungs, kidney, and liver.1 It has been shown that ethanol intoxication induces oxidative stress resulting in modulation of expression of metalloproteinases and tissue inhibitors of matrix metalloproteinases (MMPs) in cardiac and hepatic cells contributing to changes in cell phenotype to fibroblast-like with further consequence in tissue collagen accumulation and fibrosis.2,3 Several studies documented the ethanol-dependent oxidative damage in a liver. It was found that the mechanism of this process may involve mitochondrial function and cell survival.1 Therefore, the cytotoxicity of ethanol may contribute to cell apoptosis or may induce proinflammatory cytokines.4 Alcohol was found to inhibit MCF-7 human mammary carcinoma cell growth in vitro.5
It seems that ethanol especially affects biosynthesis of collagen type I. This process occurs mainly in hepatic cells but it is also observed in skin cells. Exposure of skin to ethanol led to raise many dermatological abnormalities like palmar erythema, plethoric facies, infections, and flushing.6 Biopsies obtained from skin of alcohol abuse donors supported the tissue structure abnormality and isolated fibroblasts evoked prolonged time of proliferation.6 Some data suggest that ethanol may affect wound healing by interference with collagen I and III biosynthesis.7 Ethanol is commonly used in cosmetics, pharmaceutics, and disinfectants.8,9 A large variety of alcohols are used as diluents, preservatives, ingredients of moisturizing substances or fragrance in cosmetic applied on the skin.9,10 The concentration of ethanol in product for typical application may vary from less than 1% to 96.9%.
Hyaluronic acid (HA) was found as a beneficial agent in wound healing and inflammatory processes. It was supported by in vivo and in vitro studies.11,12 Approximately half of produced hyaluronan is synthesized by fibroblasts and occurs in skin extracellular matrix. Binding of HA to cluster of differentiation 44 (CD44) and receptor for hyaluronic acid-mediated motility (RHAMM) on fibroblast may affect the signaling thereby regulating cell growth, differentiation, adherence, and collagen synthesis.13 Synthesis of collagen in fibroblast can be triggered in a variety of ways, one of which is a prolidase-dependent mechanism.12
The mechanism of HA protective action on collagen metabolism disturbances in ethanol exposure is not known. Therefore, we focused on the mechanism of ethanol-induced downregulation of collagen biosynthesis and the role of HA in this process. To explore and characterize potential negative consequence(s) of ethanol exposure to skin fibroblasts we examined its effect on expression on collagen type I, β1 integrin and insulin-like growth factor 1 receptor (IGF-IR), phosphorylated mitogen-activated protein kinases (MAPKs: ERK1/ERK2), protein kinase B (Akt), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), caspase 9, and activity of prolidase in cultured human skin fibroblasts.
Materials and methods
Cell culture
Primary Dermal Fibroblast: Normal, Human, Adult ATCC® PCS-201-012™ were purchased from ATCC (Manassas, VA, USA) and HA sodium salt H5542 was purchased from Sigma-Aldrich (St Louis, MO, USA). The cells were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA), 2 mM glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin at 37°C in a 5% CO2 incubator. Cells were used between passages 10 and 14 for all experiments. Ethical permission from Medical University Bialystok- ethical commission was obtained for the use human cell lines.
Collagen synthesis
Incorporation of radioactive precursor into proteins was measured after labeling confluent fibroblasts cultured in growth medium with varying concentrations of ethanol and HA 500 μg/mL for 24 hours in serum-free medium with the 5[3H] proline (5 μCi/mL, 28 Ci/mmol). Quantity of incorporated label into collagen was determined by digesting proteins with purified Clostridium histolyticum collagenase according to the method of Peterkofsky and Diegelmann.14
Determination of prolidase activity
Prolidase activity was measured using the method described by Myara et al.15 Protein concentration was measured by the method of Lowry et al.16 Cells were harvested before being centrifugated at 200× g for 15 minutes. The supernatant was refused. Then the pellet was resuspended in (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (50 mM, pH 7.8) and disrupted by sonication (3×10 seconds at temperature of 0°C). After that followed centrifugation at 12,000× g for 30 minutes at 4°C and protein was determined in supernatant, and activity of prolidase was assayed. Then 100 μL samples of supernatant of the cell extract were mixed with HEPES (100 μL, 50 mM, pH 7.8) which contained MnCl2 (to achieve the final concentration 1 mM of MnCl2). Samples were incubated at 37°C for 24 hours before being initiated with 100 μL of 94 mM glycyl-proline (Gly-Pro) to achieve 47 mM of final concentration Gly-Pro. Then samples were incubated at 7°C for 60 minutes and the reaction was stopped by adding 0.45 M trichloroacetic acid (TCA) (1 mL portion). TCA was added to parallel unused tubes at time “zero”. Then followed centrifugation of samples for 15 minutes at 10,000× g. Determination of proline occurred through addition of TCA/supernatant (0.5 mL) to the mixture (1:1) of glacial acetic acid/Chinard’s reagent. Then entireness was incubated at 90°C for 10 minutes. Colorimetry (absorbance at 515 nm) was used for determination of proline toward the proline standards. Results were expressed as nanomoles of proline released per minute per milligram of protein.
DNA biosynthesis
The effect of the studied substances on fibroblast [3H]-thymidine incorporation was examined. In 24-well tissue culture dishes, cells were plated at 1×105 cells/well, using 1 mL growth medium in each well. Forty-eight hours later (1.6±0.1×105 cells/well), various concentrations of ethanol with or without 500 μg/mL hyaluronan were added for 24 hours at 37°C to the culture wells. Thereafter 0.5 μCi [3H]-thymidine (6.7 Ci/mmol) was added to the wells and cultures were incubated at 37°C for 4 hours. Subsequently, the cells were rinsed three times with 1 mL 0.05 M Tris–HCl and twice with 5% TCA. This was followed by cell lysis in 1 mL 0.1 M NaOH containing 1% sodium dodecyl sulfate (SDS). Next, 4 mL scintillation liquid was added and radioactivity was measured in scintillation counter.
Cytotoxicity assay
Cell vitality was assayed by using the methyl thiazolyl tetrazolium (MTT) assay, which is based on the conversion of the yellow tetrazolium bromide MTT to the purple formazan derivative, by the mitochondrial enzyme succinate dehydrogenase in viable cells. At the end of the incubation period, the medium was transferred to 96-well trays for cytokine determination. The cells were washed with 0.2 mL of phosphate-buffered saline and assayed for the cytotoxicity in the same tray. For the treated cells, toxicity was expressed as a percentage.
SDS-PAGE
The Laemmli method was used to perform slab 10% sodium dodecyl sulfate-polyacryylamide gel electrophoresis (SDS-PAGE) electrophoresis.17 Samples of cell supernatants (20 μg of protein) were analyzed. As standards we used: myosin (200 kDa), galactosidase (116.2 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), and ovalbumin (45 kDa) (Bio-Rad, Berkeley, CA, USA).
Western blot analysis
The Laemmli method was used to perform slab SDS-PAGE electrophoresis. Equal portions of cell supernatants (20 μg of protein) were analyzed. We condensed ten times culture medium using centricons (3 kDa cut off), mixed with Laemmli sample buffer (containing 2.5% SDS, without reducing agent). Electrophoresis was performed with equal portions (100 μg) of protein. After electrophoresis the cells were equilibrated in 25 mM Tris with 0.2 M glycine in 20% methanol (v/v) for 5 minutes. Samples were transmitted to nitrocellulose 0.2 μm pore sized at 100 mA for 1 hour. For this procedure LKB 2117 Multiphor II Electrophoresis System Multiphor (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) was used. We incubated the nitrocellulose for 1 hour with: monoclonal anti-collagen I antibody (concentration of 1:1,000), monoclonal anti-phospho-Akt antibody (concentration of 1:1,000), polyclonal anti-NF-κB antibody (concentration of 1:1,000), monoclonal anti-phospho-MAPK antibody (ERK1/2) (concentration of 1:1,000), monoclonal anti-caspase 9 antibody (concentration of 1:1,000). The antibodies were dissolved in solution containing 5% dried milk, in Tris-buffered saline with Tween 20 (TBS-T) (20 mmol/L Tris–HCl buffer, pH 7.4, containing 150 mmol/L NaCl and 0.05% Tween 20). To analyze phospho-MAPK (ERK1/ERK/2) and phospho-AKT, anti-mouse IgG (whole molecule) alkaline phosphatase conjugate was added at concentration of 1:5,000 in TBS-T. To analyze NF-κB and caspase-9 second antibody-alkaline phosphatase conjugated, anti-rabbit IgG (whole molecule) was added at concentration of 1:5,000 in TBS-T. To analyze collagen type I and β-actin second antibody-alkaline phosphatase conjugated, anti-goat IgG (whole molecule) was added at concentration of 1:5,000 in TBS-T. The entire mixture was incubated for 30 minutes under slow shaking. After that the nitrocellulose was washed with TBS-T (5×5 minutes) and submitted to 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium liquid substrate reagent. The intensity of the bands was quantified by densitometric analysis.
Zymography
Gelatinolytic activity was measured using the method of Unemori and Werb.18 We condensed ten times culture medium using centricons (3 kDa cut off), mixed with sample buffer (containing 2.5% SDS, without reducing agent). Electrophoresis was performed with equal amounts of protein (20 μg) on polyacrylamide gels (10%) under nonreducing circumstances. Gels were impregnated with gelatin (1 mg/mL). Incubation of gel following the electrophoresis to remove SDS was performed for 30 minutes in 2% Triton X-100. After that followed incubation in buffer containing Tris–HCl 50 mM, pH 8 with CaCl2 5 mM (substrate buffer) for 18–24 hours at 37°C. After that Coomassie Brilliant Blue R250 (Thermo Fisher Scientific, Waltham, MA, USA) was used to perform staining.
Statistical methods
The mean values for at least three assays ± standard deviation (±SD) were calculated in all appropriate experiments. The statistical analysis was performed applying double-sided, unpaired Student’s t-test and analysis of variance. The IBM SPSS 22.0 Statstics Genericom (IBM Corporation, Armonk, NY, USA) program was used. P<0.05 and P<0.01 was acknowledged as statistically significant.
Results
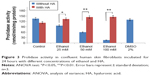
In order to evaluate the activity of ethanol on collagen synthesis in human skin fibroblasts, the cells were incubated for 24 hours in 25, 50, and 100 mM of ethanol as well as in ethanol with HA at concentration of 500 μg/mL. It has been shown that ethanol induced decrease in collagen synthesis in dose-dependent manner (Figures 1 and 2). Exposure of the fibroblasts to different concentrations of ethanol contributed to inhibition of collagen biosynthesis by 58.08% (2.39%±SD; n=3), 76.23% (3.52%±SD; n=3), 83.15% (4.03%±SD; n=3) P<0.05, P<0.01, respectively, compared to the control value. However, in the presence of HA at 500 μg/mL, the potency of ethanol to inhibit collagen biosynthesis was much lower. HA added to cells alone did not have any significant effect on collagen biosynthesis in control cells. The results presented provide evidence that ethanol has an inhibitory effect on collagen synthesis in confluent human skin fibroblasts and HA counteracted this process (Figures 1 and 2).
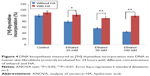
The enzyme responsible for recycling of proline for collagen resynthesis are prolidase and MMPs. The addition of ethanol at concentrations of 25, 50, and 100 mM to cell cultures contributed to decrease in prolidase activity by 29.05% (4.55%±SD; n=3), 56.15% (3.82%±SD; n=3), and 88.18% (1.16%±SD; n=3). The addition of HA had a significant protective effect on prolidase activity in fibroblasts incubated with ethanol. HA restored prolidase activity in cells incubated with ethanol to control level (Figure 3).
The conducted research proves that ethanol impairs collagen metabolism in human skin fibroblasts, leading to a significant decrease in DNA synthesis. The disintegrations per minute of [3H] thymidine incorporated into the DNA of these cells was taken as 100%. This was a reference value. We evaluated the effect of ethanol in the presence or in the absence of HA (500 mg/mL). Exposure of the fibroblasts to different concentrations of ethanol contributed to inhibition of DNA synthesis by 21.06% (7.08%±SD; n=3), 44.15% (5.06%±SD; n=3), 56.27% (4.26%±SD; n=3), 50.16% (4.56%±SD; n=3) P<0.05, P<0.01, respectively, compared to the control value. HA has protective influence on the processes induced by ethanol (Figure 4).
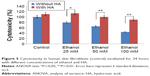
The efficacy of HA in reducing the cytotoxicity of 25, 50, and 100 mM ethanol is shown in Figure 5. It has been shown that ethanol induced cytotoxicity in dose-dependent manner. The presence of ethanol inhibition of cell survival 20.16% (4.05%±SD; n=3), 35.18% (3.63%±SD; n=3), 56.03% (2.53%±SD; n=3) P<0.01, respectively, compared to the control value. The β1 integrin receptor plays a pivotal role in the control of collagen biosynthesis and prolidase activity. In this study, we found that ethanol decreased expression of β1 integrin receptor in dose-dependent manner. In cultured cells incubated with HA and ethanol, this effect was abrogated. The expression of integrin receptor in the cells treated with different concentration of ethanol and HA was found to be similar as in control cells (Figure 6A).
It is known that cross-talk between β1 integrin receptor and IGF-IR receptor plays key role on activation of expression of collagen gene and prolidase activity. Ethanol, similarly as in case of integrin receptor, induced decrease in the expression of IGF-IR (in dose-dependent manner). HA also had protective effect on the level of expression of this receptor (Figure 6B). In this case, expression of IGF-IR receptor in cells incubated with ethanol and HA was close to control.
The signal transmitted by stimulated IGF-IR and β1 integrin receptor leads to the activation of MAPKs (ERK1/2) and Akt.14,15 As can be seen in Figure 6C in fibroblasts treated with ethanol, the expression of MAPK (phospho-ERK1/2) decreased in dose-dependent manner. An addition of HA to cells treated with ethanol had protective effect on the ethanol-dependent decrease in the expression of phospho-ERK1/2. Ethanol and ethanol with HA added to cultured fibroblasts had no effect on the expression of phospho-AKT kinases (Figure 6D). Various concentrations of ethanol have a cytotoxic effect on human skin fibroblasts, leading to induction of apoptosis, which results in the observed increase of caspase 9 expression (Figure 6E). What is more, HA application has a protective effect on cell proliferation disorders caused by ethanol. Moreover, expression of β-actin was not affected in the cells when treated with ethanol in the presence or in absence of HA (Figure 6F).
Several data suggested that prolidase-dependent regulation of collagen biosynthesis may take place at the transcriptional level, depending on NF-κB expressions. NF-κB is the transcription factor that acts as inhibitor of expression of alpha 1 and alpha 2 subunits of type I collagen.19,20 We found increased NF-κB expression in fibroblast treated with ethanol (Figure 7A). An addition of HA to cells treated with ethanol contributed to decrease in the expression of this transcriptional factor to the control level. Moreover, expression of β-actin was not affected in the cells when treated with ethanol in the presence or in absence of HA (Figure 7B). It seems that regulatory mechanism of ethanol-induced collagen aberration take place at the point of collagen biosynthesis, since no effect of ethanol and HA was found on process of collagen degradation by MMP-2 and MMP-9 (Figure 8).
Discussion
Although cytotoxicity of ethanol was well recognized, the mechanism of its effect on collagen metabolism in tissues is not fully understood. It seems that ethanol-induced collagen metabolism abnormalities are tissue specific. In liver it may contribute to stimulation of collagen biosynthesis with consequence of tissue fibrosis, while in the skin an opposite process may occur with consequence on wound healing.
In this study, we provided evidence that ethanol downregulated collagen metabolism in skin fibroblast through several levels of regulations of this protein. At first it affects activity of prolidase, the enzyme that play important role in collagen biosynthesis. The enzyme is responsible for recycling of proline for collagen resynthesis, thus determines production of collagen. Peptide bonds between proline and other amino acids can be cleaved also by another peptidases, prolinase that requires proline at N-terminal position.21 Prolidase is second iminopeptidase that cleaves dipeptides with C-terminal proline in order to harvest free proline. The enzyme digests endogenous as well as dietary Xaa-Pro dipeptides.21
Another level of collagen metabolism regulation in ethanol-treated fibroblast was found at β1 integrin and IGF-IR. Previously, we found that cross-talk between the receptors contribute to upregulation of collagen biosynthesis.22 In the present study, we found that ethanol contributed to suppression of the expression of both receptors. Simultaneously, we found increase in the transcription activity of NF-κB, the known inhibitor of type I collagen that subunits gene expression.20 As a result of the conducted research, it has been concluded that ethanol lead to the impairment of collagen biosynthesis in fibroblast cultures through decreasing prolidase activity and expression of β1 integrin and IGF-IR. This was accompanied by a lowered expression of the signaling pathway proteins induced by β1 integrin and IGF-IR, that is, MAPK (ERK1/2) kinases. The lowered amount of synthesized collagen and prolidase activity disturbance may also be due to the activation of NF-κB transcription factor, which inhibits collagen gene expression. The application of HA in the concentration of 500 μg/mL has a protective effect on disturbances caused by the examined substances. The constellation of processes affected by ethanol explains the mechanism of ethanol-induced downregulation of collagen biosynthesis. Based on our previous studies, we considered HA as an agent counteracting ethanol-induced deregulation of collagen metabolism in fibroblast.11 In fact, we found that HA abolished all studied defect in collagen metabolism, evoked by ethanol. In our previous paper, we have not observed an abolishing effect of HA when caffeine downregulated collagen synthesis.23 Various concentrations of ethanol have a cytotoxic effect on human skin fibroblasts, leading to lowered survivability, DNA biosynthesis inhibition, and induction of apoptosis, which results in the observed increase of caspase 9 expression. What is more, HA application has a protective effect on cell proliferation defect caused by ethanol.
HA plays important role in maintenance of extracellular matrix and evokes protective action on collagen structure.24,25 HA is an art of glycosaminoglycan, where D-glucuronic acid is connected with a particle of N-acetylglucosamine through glycoside bonds.26,27 The cell is embedded around in a layer of hyaluronan that is responsible for shape, adhesion, and migration of the cell.24 Skin contains approximately 50% of all HA in the body. HA improve healing processes in several pathologic conditions like venous ulcers or membrane injuries. Literature data shows that HA play beneficial role in the regenerative processes associated with dysfunction of collagen synthesis.28 We can speculate that HA protects against ethanol-induced disorders. This protective effect may be controlled through interactions of hyaluronan with its receptors CD44 and/or RHAMM.29 At least in endothelial cells, this mechanism contributes to stimulation of collagen production.30 Whether CD44 and RHAMM receptors play important role in the mechanism of HA-dependent counteraction of ethanol toxicity requires further study.
The data presented in this paper may have also some social importance. They suggest that the fibroblasts are particularly sensitive to ethanol and respond to the compound by inhibition of collagen biosynthesis. This process contributes to skin aging, which is of special interest of cosmetology. In view of the above data and fact that ethanol is commonly used in cosmetology, it can be postulated to replace the ethanol in cosmetics by other solvent or preservatives. It can be also considered to include HA to cosmetic preparations containing ethanol.
Acknowledgments
MD is a fellow in the Project Studies, Research, Commercialization: A Support Programme of UMB Doctoral Students, submeasure 8.2.1 Human Capital Operational Programme, cofinanced from the European Union under the European Social Fund. This work was supported in part by grants from the Medical University of Białystok (143-14749F, 143-30730F, 144-30828 F).
Disclosure
The authors report no conflicts of interest in this work.
References
Sid B, Verrax J, Calderon PB. Role of AMPK activation in oxidative cell damage: implications for alcohol-induced liver disease. Biochem Pharmacol. 2013;86(2):200–209. | ||
El Hajj EC, El Hajj MC, Voloshenyuk TG, et al. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38(2):448–456. | ||
Xu GF, Li PT, Wang XY, et al. Dynamic changes in the expression of matrix metalloproteinase’s and their inhibitors, TIMPs, during hepatic fibrosis induced by alcohol in rats. World J Gastroenterol. 2004;10(24):3621–3627. | ||
Neuman MG, Nanau RM, Oruña L, Coto G. In vitro anti-inflammatory effects of hyaluronic acid in ethanol-induced damage in skin cells. J Pharm Pharmaceut Sci. 2011;14(3):425–437. | ||
Przylipiak A, Rabe T, Hafner J, Przylipiak M, Runnebaum B. Influence of ethanol on in vitro growth of human mammary carcinoma cell line MCF-7. Arch Gynecol Obstet. 1996;258(3):137–140. | ||
Johnen Ch, Hartmann B, Steffen I, et al. Skin cell isolation and expansion for cell transplantation is limited in patients using tobacco, alcohol, or are exhibiting diabetes mellitus. Burns. 2006;32(2):194–200. | ||
Ranzer MJ, Chen L, DiPietro LA. Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcohol Clin Exp Res. 2011;35(1):83–90. | ||
Boyce JM. Update on hand hygiene. Am J Infect Control. 2013;41(5):94–96. | ||
Kim Y-Ch, Park J-H, Ludovice PJ, Prausnitz MR. Synergistic enhancement of skin permeability by N-lauroylsarcosine and ethanol. Int J Pharmaceutics. 2008;352(1–2):129–138. | ||
Pendlington RU, Whittle E, Robinson JA, Howes D. Fate of ethanol topically applied to skin. Food Chem Toxicol. 2001;39(2):169–174. | ||
Surazynski A, Miltyk W, Czarnomysy R, Grabowska J, Palka J. Hyaluronic acid abrogates nitric oxide-dependent stimulation of collagen degradation in cultured human chondrocytes. Pharmacol Res. 2009;60(1):46–49. | ||
Ghazi K, Deng-Pichon U, Warnet J-M, Rat P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: role of molecular weight. PLoS One. 2012;7(11):e48351. | ||
Nawrat P, Surazynski A, Karna E, Pałka JA. The effect of hyaluronic acid on interleukin-1-induced deregulation of collagen metabolism in cultured human skin fibroblasts. Pharmacol Res. 2005;51(5):473–477. | ||
Peterkofsky B, Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971;10(6):988–994. | ||
Myara I, Charpentier C, Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria. Clin Chim Acta. 1982;125(2):193–205. | ||
Lowry N, Rosenbrough N, Farr L, Randall R. Protein measurement with the Folin fenol reagent. J Biol Chem. 1951;193(1):265–275. | ||
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. | ||
Unemori E, Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986;103(3):1021–1031. | ||
Kouba D, Chung K-Y, Nishiyama T, et al. Nuclear factor-κB mediates TNF-α inhibitory effect on α2(I) collagen (COL1A2) gene transcription in human dermal fibroblasts. J Immunol. 1999;162(7):4226–4234. | ||
Miltyk W, Karna E, Palka J. Prolidase-independent mechanism of camptothecin-induced inhibition of collagen biosynthesis in cultured human skin fibroblasts. J Biochem. 2007;141(2):287–292. | ||
Kitchener RL, Grunden AM. Prolidase function in proline metabolism and its medical and biotechnological applications. J Appl Microbiol. 2012;113(2):233–247. | ||
Surazynski A, Miltyk W, Prokop I, Palka J. Prolidase-dependent regulation of TGF and TGF β receptor expressions in human skin fibroblasts. Eur J Pharmacol. 2010;649(1–3):115–119. | ||
Donejko M, Przylipiak A, Rysiak E, Gluszuk K, Surazynski A. Influence of caffeine and hyaluronic acid on collagen biosynthesis in human skin fibroblasts. Drug Des Devel Ther. 2014;8:1923–1928. | ||
Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59(13):1351–1365. | ||
Karna E, Miltyk W, Surazyński A, Pałka JA. Protective effect of hyaluronic acid on interleukin-1-induced deregulation of beta1-integrin and insulin-like growth factor-I receptor signaling and collagen biosynthesis in cultured human chondrocytes. Mol Cell Biochem. 2008;308(1–2):57–64. | ||
Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80(21):1921–1943. | ||
Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339(1):237–246. | ||
Surazynski A, Miltyk W, Palka J, Phang JM. Prolidase-dependent regulation of collagen biosynthesis. Amino Acids. 2008;35(4):731–738. | ||
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. | ||
Rooney P, Wang M, Kumar P, Kumar S. Angiogenic oligosaccharides of hyaluronan enhance the production of collagens by endothelial cells. J Cell Sci. 1993;105(1):213–218. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.