Back to Journals » Drug Design, Development and Therapy » Volume 9
Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma
Authors Qiao B , Shui W, Cai L, Guo S, Jiang D
Received 6 November 2014
Accepted for publication 19 December 2014
Published 17 February 2015 Volume 2015:9 Pages 969—976
DOI https://doi.org/10.2147/DDDT.S77116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shu-Feng Zhou
Bo Qiao, Wei Shui, Li Cai, Shuquan Guo, Dianming Jiang
Department of Orthopaedics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Abstract: Biological treatments have been studied extensively and previous studies have proved that osteoprotegerin (OPG) can inhibit the development and progress of human osteosarcoma. However, the utility of biologic agents for cancer therapy has a short half-life, which can hardly deliver to and function in tumor sites efficiently. Mesenchymal stem cells (MSCs) have the potential to migrate to tumor sites. In this study, MSCs transfected with adenoviruses carrying the OPG gene (MSCs-OPG) were used via the tail vein to treat athymic nude mice (nu/nu) bearing osteosarcoma. In vivo and ex vivo images were used to validate the MSCs homing to tumors. The therapeutic effect for osteosarcoma was evaluated by observations on growth of tumors and bone destruction. The results showed that infected MSCs-OPG labeled with red fluorescent protein (RFP) can migrate to tumor sites and express OPG protein. The treatment by MSCs-OPG reduced the tumor growth and inhibited bone destruction in vivo. All these indicated that MSCs can deliver OPG to tumor sites, which could be a new direction of biological treatment for human osteosarcoma.
Keywords: therapy, MSC, OPG, bone tumor
Introduction
Osteosarcoma is the most common primary malignant bone tumor, particularly in adolescence and childhood.1,2 Since multi-agent chemotherapy combined with surgical resection was introduced in the early 1980s, the 5-year survival rate of osteosarcoma patients had increased to 60%–70%.2 However, improvements in osteosarcoma survival have been limited in recent decades.2,3 Therefore, other approaches to treat osteosarcoma have been explored. Given that a vicious cycle between tumor cells and extensive bone destruction leads to progress of malignant bone tumor,4,5 novel bone-targeted strategies for therapy in osteosarcoma have been studied. Recent findings have revealed that the molecular triad RANKL/RANK/osteoprotegerin (OPG) is the key regulator not only for normal but also pathological bone metabolism.6–8 RANKL is absolutely required for osteoclastogenesis, which can be activated by its cognate receptor RANK. OPG is the decoy receptor for RANKL and inhibits osteoclast formation and function. Therefore, RANKL/RANK/OPG have recently become the new therapeutic targets in primary and metastatic bone tumors. Lamoureux et al demonstrated that OPG can prevent tumor-induced osteolysis and indirectly inhibit tumor progression in osteosarcoma.9 However, the utility of biologic agents for cancer therapy has a short half-life, and which can hardly deliver to and function in tumor sites efficiently by systemic treatments.
Mesenchymal stem cells (MSCs) are connective tissue progenitor cells that are easily obtained compared with other stem cells. In addition to multiple differentiation capacity, the ability of MSCs to migrate to injury and inflammatory sites in vivo provokes interest in therapy for tumors. Current studies have revealed that genetically modified MSCs could deliver antitumor proteins directly into tumor tissues as a vehicle.10–16 However, these antitumor agents are limited in non-specific proteins, especially interferon (IFN)-β. Target expression of OPG using MSCs would be a novel approach to treat osteosarcoma, which aimed at bone destruction and also avoided the side effects of systemic OPG administration. In the present study, we expressed OPG in MSCs using an adenovirus-red fluorescent protein (RFP) vector. The genetically modified MSCs carrying the OPG gene permitted direct visualization of the MSCs migrating to the osteosarcoma by an in vivo and ex vivo fluorescence imaging technique. Additionally, we evaluated the therapeutic effect of OPG-expressing MSCs on osteosarcoma using mice bearing 143b human osteosarcoma xenografts.
Materials and methods
Cell isolation and culture
Human MSCs were harvested from Wharton’s jelly of umbilical cord as previously described17 under approval of the Ethics Committee of Chongqing Medical University. In brief, umbilical cords were obtained from healthy infants under aseptic conditions and stored in serum-free Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 media (Thermo Fisher Scientific, Waltham, MA, USA) containing 200 U/mL penicillin/streptomycin (Sigma-Aldrich Co., St Louis, MO, USA) for no more than 6 hours. After removal of blood vessels, the Wharton’s jelly was obtained and minced into small pieces, and placed in a 10 cm2 petri dish for 20 minutes at 37°C with 5% CO2 to facilitate tissue attachment. Then, 5 mL DMEM/F12 media containing 10% fetal bovine serum (Thermo Fisher Scientific) and 200 U/mL penicillin/streptomycin were added carefully to cover the Wharton’s jelly pieces and changed every 4 days until visible colonies of MSCs were observed after 10–14 days of incubation. Then, MSCs were trypsinized and passaged by removing the Wharton’s jelly pieces. MSCs were identified by assaying expression of CD34, CD44, CD90, and CD105 surface antigens (Biolegend, San Diego, CA, USA) through flow cytometry and performing adipogenic and osteogenic differentiation. MSCs at passage 3–6 were used in this study.
The human embryonic kidney 293T cells and human osteosarcoma 143b cells were a gift from the Key Laboratory of Diagnostic Medicine Designated by the Chinese Ministry of Education. The 293T and 143b cells were maintained in DMEM-High glucose (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum and 200 U/mL penicillin/streptomycin.
MSCs transduction
Recombinant adenoviruses carrying both OPG and RFP genes (Ad-OPG-RFP) were obtained from the Key Laboratory of Diagnostic Medicine Designated by the Chinese Ministry of Education. MSCs were infected using Ad-OPG-RFP at a multiplicity of infection (MOI) of 3,000; free viral particles were removed from the cultured medium after 24 hours of transfection. After 72 hours, the success of transfection was determined by fluorescent microscopy (Carl Zeiss Meditec AG, Jena, Germany). The supernatants of MSCs infected by Ad-OPG-RFP (MSCs-OPG) were collected and OPG proteins were measured using a competitive human OPG enzyme-linked immunosorbent assay (ELISA) kit (BlueGene Biotechnology, Shanghai, People’s Republic of China).
Transwell migration assay
Migration assays of MSCs in vitro were performed using 6.5 mm transwell chambers containing 8 μm pore membrane (EMD Millipore, Billerica, MA, USA). Briefly, 2×104 143b cells were placed and cultured in the lower wells for 24 hours. The media was then replaced with 400 μL serum-free DMEM/F12. Fifty thousand MSCs or 293T cells in 600 μL serum-free DMEM/F12 were added into the upper well and incubated at 37°C with 5% CO2. After 18 hours, the cells attached to the top side of the transwell plates were carefully removed using a cotton wool swab, and the migrated cells on the bottom were fixed and stained with Giemsa stain. The number of migrated cells were counted from five fields using light microscopy (BX51; Olympus Corporation, Tokyo, Japan) at ×10 magnification. The transwell assays were done in triplicate.
In vivo studies
Animal and cell administration
Four-week-old male athymic nude mice (nu/nu) were purchased from Chongqing Medical University. The mice were used in accordance with institutional guidelines under the approved protocols by the Chongqing Administration Rule of Laboratory Animals. For establishing the orthotopic osteosarcoma model, 5×105 143b cells in 20 μL sterile phosphate-buffered saline (PBS) were transplanted into the proximal tibia of mice in the anesthetized condition. Seven to ten days later, when tumor was macroscopically observable at the tibia, 1×106 MSCs-OPG in 50 μL sterile PBS solution were injected via the tail vein. The MSCs-OPG administration was given twice with an interval of 3 days. PBS solution (50 μL) was administrated to the blank control group.
Tumor measurement
When the osteosarcoma was established, the volume of tumor was measured using a vernier caliper weekly until the 30th day after 143b implantation. The tumor volume was calculated from the measurement of two perpendicular diameters according to the formula V=0.5×L×S2, where V, L, and S, are, the tumor volume and the largest and smallest perpendicular tumor diameters, respectively.18
In vivo and ex vivo fluorescence imaging
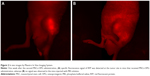
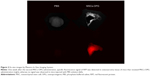
To validate the MSCs homing to the tumor site, the mice were anesthetized 1 week after the second MSCs-OPG administration. Fluorescence image of the tumor was captured by a Maestro In Vivo Imaging System (CRi, Woburn, MA, USA). The excitation wavelength was 560 nm and emission spectra were obtained at 750 nm. After obtaining in vivo images, tumors and other main organs were removed for ex vivo images.
Fluorescence microscope observation
After in vivo and ex vivo observation, tumor tissue was conserved and fixed in 4% paraformaldehyde solution. Then, 5 μm thick paraffin sections were made and observed directly by fluorescence microscope to validate MSCs migrating to tumor tissue.
Serum ELISA of OPG protein
Blood was collected from the mice at day 18 and 30 and the serum was conserved at −80°C for detecting the level of OPG protein. To quantitate the amount of OPG protein in serum, a competitive human OPG ELISA kit (BlueGene Biotechnology) was applied according to the manufacturer’s directions, in triplicate. The absorbance was read at 450 nm.
Computed tomography scanning
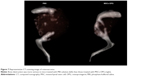
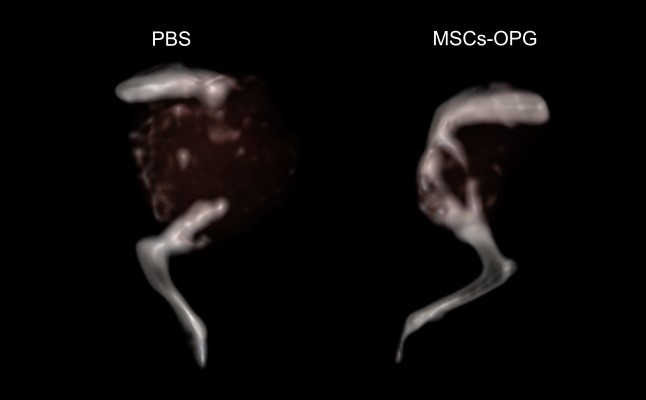
Computed tomography (CT) scanning was used to observe the bone destruction caused by osteosarcoma. Thirty days after injection of the 143b cells, the mice were sacrificed and the posterior leg was collected for CT scanning (SOMATOM Definition; Siemens Healthcare, Erlangen, Germany). The data from the 0.6 mm thin slices were used to reconstruct the images to observe the tumor tissues and bone destruction.
Histology analysis
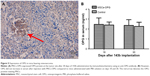
Thirty days after osteosarcoma implantation, 5 μm thick paraffin sections of tumor were made for hematoxylin and eosin (HE) staining, and immunohistochemistry using antibodies against human OPG at a dilution of 1:200 (ab73400; Abcam, Cambridge, MA, USA). For immunohistochemistry, the avidin-biotin-peroxidase complex method (SABC kit; Boster, Wuhan, People’s Republic of China) was applied. A positive reaction was visualized with diaminobenzene and counterstained with hematoxylin.
Statistics
Quantitative data were presented as means ± standard deviation (SD). Student’s t-test or one-way analysis of variance (ANOVA) were performed to determine the statistical significance using SPSS (v17.0; IBM Corporation, Armonk, NY, USA). A value of P<0.05 was defined to be statistically significant.
Results
MSCs transduction and expression of OPG
After 72 hours of infection by Ad-OPG-RFP, MSCs-OPG were observed by fluorescent microscopy. The MSCs were infected by Ad-OPG-RFP successfully with an MOI of 3,000; we used ELISA analysis to detect the amount of OPG in the supernatants from the MSCs-OPG. The results revealed that the content of OPG secreted from the MSCs-OPG was 146±18 ng/mL after 72 hours of transduction, whereas little OPG was detected from uninfected MSCs.
Migration assay in vitro
The result of the transwell experiment showed that there were significantly increased MSCs migrated to 143b and 293T cells compared to serum-free medium (Figure 1). However, no significant difference of migrated MSCs was found when 143b and 293T cells were seeded in the lower well. This result indicates that human osteosarcoma 143b cells did not enhance MSC migration in comparison to 293T cells.
MSCs homing to tumor in vivo
To confirm that MSCs migrated to the tumors, we traced RFP-expressing MSCs-OPG using an in vivo imaging system and histological analysis. The in vivo image showed that a specific fluorescence signal of RFP was detected at the tumor site on the mice that received MSCs-OPG administration (Figure 2), whereas no signal was observed in the mice injected with PBS solution. In addition, the ex vivo images of resected tumor tissues had the same results (Figure 3). We also observed that MSCs-OPG engrafted in tumor tissue by fluorescence microscope using paraffin sections, which showed that a red fluorescence signal accumulated in the tumor tissue resected from the mice injected with MSCs-OPG (Figure 4).
Expression of OPG in osteosarcoma
Immunohistochemistry of the paraffin sections of tumors was performed to detect the expression of OPG protein. As Figure 5A shows, the MSCs-OPG expressed the OPG protein in the tumor tissue after 30 days of 143b administration. However, the OPG level in the serum did not increase after injection of MSCs-OPG compared to the serum from the mice administrated with PBS solution (Figure 5B).
Effect of MSCs-OPG on tumor growth and bone destruction
After 7–10 days, the tumor was established at the tibia and its volume was measured. Over 1 month, all animals treated with MSCs-OPG exhibited a significant decrease of tumor volume compared with animals administrated with PBS solution on days 11, 18, 24, and 30 (Figure 6A); the volume of tumors resected from the mice injected with MSCs-OPG was almost reduced by 65.2% compared to those treated with PBS solution at day 30 (Figures 6A and 7B).
In addition to the volume of tumors, therapy with MSCs-OPG reduced bone destruction caused by osteosarcoma. As CT scanning showed (Figure 7), 30 days after transplantation with 143b cells, the tibia was destroyed with osteolysis in both mice treated with MSCs-OPG or PBS solution. However, tibia damage was less extensive in mice treated with MSCs-OPG compared to mice treated with PBS solution, where almost the entire tibia was destroyed and had disappeared. These results indicate that expression of OPG in osteosarcoma reduces bone destruction with tumor progress.
Discussion
We demonstrated that MSCs transfected with the OPG gene can migrate to osteosarcoma and produce OPG locally at the tumor site via intravenous injection. Importantly, the results in vivo showed that exogenously administered MSCs-OPG reduced the volume of tumor and bone destruction. Many studies have proved that MSCs can be used as delivery vehicles of anticancer genes to malignant tumors, which can inhibit growth or metastases of tumors. Previous studies showed that malignant tumor cells such as breast cancer cells (MDAMB231) and glioma cells (U87) can stimulate migration of MSCs, whereas squamous (H357) and lung (A549) cancer cells did not.11,19 In our test in vitro, osteosarcoma cell lines 143b did not enhance the migration of MSCs compared to 293T cells as shown by transwell assay. However, we have provided evidence by in vivo and ex vivo fluorescence imaging that MSCs can engraft into tumors of mice bearing osteosarcoma. Many studies have convincingly demonstrated that infused MSCs have higher engraftment efficiencies within sites of injury irrespective of the tissue or organ.20–23 Additionally, it is believed that MSCs respond to signals from the sites of injury or inflammation. Many of the same cytokines and chemokines that are secreted by the injury site are found in the tumor microenvironment and are thought to be involved in attracting MSCs to these sites,24,25 so MSCs can home and localize to the tumor site which have been demonstrated by growing number of publications using MSCs to treat malignant tumors.10–16,19,26 In our study, we tracked RFP expressed by MSCs-OPG and demonstrated that intravenously transfused MSCs can localize to osteosarcoma. However, we also found that some MSCs were arrested in the lung tissue in some cases by immunohistochemistry after 7 days of MSCs-OPG administration. Karp and Leng Teo27 and Kean et al28 summarized that MSCs accumulated in the lung is related to the site of MSCs delivery and the large size of MSCs and suggested that intra-arterial injection would reduce engraftment in the lung. MSCs were used as a vehicle not only because they can carry anticancer genes but also engraft in tumors, which can express persistently anticancer agents. In the present study, we found that MSCs expressed OPG in the tumors of mice treated with MSCs-OPG after 30 days without obviously increasing OPG in the serum, which may decrease the side effects of systemic administration.
Previous studies utilized MSCs to carry anticancer genes such as IFN-β, which was mostly used to treat tumors and can directly inhibit or suppress the tumors. Unlike other primary tumors, however, the growth and progress of osteosarcomas disrupts the equilibrium between bone formation and bone resorption, where the destruction of bone facilitates the development of tumor. Due to this specific vicious cycle between tumor proliferation and bone destruction, research has sought a new way to treat osteosarcoma through bone protection instead of antitumor cells.6,9,29 The RANKL/RANK/OPG system is a key signaling pathway in bone remolding, and the interaction between RANK and RANKL can promote osteoclast differentiation and lead to bone resorption. Lee et al30 found that high RANKL expression was related to a poor response to preoperative chemotherapy and lower 5-year event-free survival rate. In addition, it was confirmed that anti-RANKL can block the vicious cycle between osteosarcoma proliferation and bone resorption, and can reduce tumor incidence and inhibit progress of osteosarcoma.9,31 OPG, as the decoy receptor for RANKL, can inhibit osteoclast formation and function, which was used for therapy in osteosarcoma and bone metastases.9,32,33 However, RANKL is essential for lymph node organogenesis and thymocyte development,34,35 which suggests that systemic gene therapy using OPG may weaken and inhibit antitumor immune response. Moreover, the effect of the systemic use of antitumor agents was limited. Therefore, we used MSCs to deliver OPG in tumor sites to function effectively, as well as to avoid side effects on the immune system. We showed that OPG expressed in tumors after MSCs-OPG treatment, whereas serum OPG didn’t increase significantly compared to control group. In addition to the expression of OPG in tumor tissue, MSCs-OPG therapy had an inhibitory effect on osteosarcoma in vivo. Mice bearing osteosarcoma that was treated with MSCs-OPG had slower tumor growth rate after tumor establishment, and the volume of the tumors on day 30 were 34.8% of those in the control group. More importantly, OPG expressed by MSCs in osteosarcomas significantly reduced bone destruction.
Conclusion
In the present study, we used MSCs-OPG therapy to treat osteosarcomas, which is the first time it has been reported in the literature to our knowledge. The results revealed that MSCs-OPG has an inhibitory effect on osteosarcoma proliferation and bone destruction. However, further research is needed to improve the engraftment efficiency of MSCs in tumors. Additionally, combination delivery of agents against osteosarcoma cell and bone destruction by MSCs may enhance the therapeutic effect for osteosarcoma.
Acknowledgment
This work was supported by the Natural Science Foundation Project of CQ CSTC (2009BB5410). We also thank the Key Laboratory of Diagnostic Medicine Designated by the Chinese Ministry of Education for providing the cells in this research.
Disclosure
The authors report no conflicts of interest in this work.
References
Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. | ||
Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. | ||
Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland – a nationwide population-based study. Acta Oncol. 2011;50(8):1206–1214. | ||
Wittrant Y, Théoleyre S, Chipoy C, et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta. 2004;1704(2):49–57. | ||
Dougall WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2012;18(2):326–335. | ||
Michigami T, Ihara-Watanabe M, Yamazaki M, Ozono K. Receptor activator of nuclear factor kappaB ligand (RANKL) is a key molecule of osteoclast formation for bone metastasis in a newly developed model of human neuroblastoma. Cancer Res. 2001;61(4):1637–1644. | ||
Tat SK, Padrines M, Theoleyre S, et al. OPG/membranous – RANKL complex is internalized via the clathrin pathway before a lysosomal and a proteasomal degradation. Bone. 2006;39(4):706–715. | ||
Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107(2):289–298. | ||
Lamoureux F, Richard P, Wittrant Y, et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67(15):7308–7318. | ||
Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. | ||
Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005; 65(8):3307–3318. | ||
Ren C, Kumar S, Chanda D, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15(21):1446–1453. | ||
Dembinski JL, Wilson SM, Spaeth EL, et al. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013;15(1):20–32. | ||
Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11:20. | ||
Huang X, Zhang F, Wang H, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34(7):1772–1780. | ||
Sage EK, Kolluri KK, McNulty K, et al. Systemic but not topical TRAIL-expressing mesenchymal stem cells reduce tumour growth in malignant mesothelioma. Thorax. 2014;69(7):638–647. | ||
Petsa A, Gargani S, Felesakis A, Grigoriadis N, Grigoriadis I. Effectiveness of protocol for the isolation of Wharton’s Jelly stem cells in large-scale applications. In Vitro Cell Dev Biol Anim. 2009;45(10): 573–576. | ||
Lamoureux F, Picarda G, Garrigue-Antar L, et al. Glycosaminoglycans as potential regulators of osteoprotegerin therapeutic activity in osteosarcoma. Cancer Res. 2009;69(2):526–536. | ||
Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69(10):4134–4142. | ||
Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke. 2002;33(5):1362–1368. | ||
Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8(9):1011–1017. | ||
Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14(6):1035–1041. | ||
Spees JL, Whitney MJ, Sullivan DE, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22(4):1226–1236. | ||
Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5(1):31–36. | ||
Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15(10):730–738. | ||
Reagan MR, Seib FP, McMillin DW, et al. Stem cell implants for cancer therapy: TRAIL-expressing mesenchymal stem cells target cancer cells in situ. J Breast Cancer. 2012;15(3):273–282. | ||
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. | ||
Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. | ||
Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7(11):3461–3469. | ||
Lee JA, Jung JS, Kim DH, et al. RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatr Blood Cancer. 2011;56(5):738–743. | ||
Lamoureux F, Picarda G, Rousseau J, et al. Therapeutic efficacy of soluble receptor activator of nuclear factor-kappa B-Fc delivered by nonviral gene transfer in a mouse model of osteolytic osteosarcoma. Mol Cancer Ther. 2008;7(10):3389–3398. | ||
Zhang J, Dai J, Qi Y, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107(10):1235–1244. | ||
Body JJ, Greipp P, Coleman RE, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003; 97(3 Suppl):887–892. | ||
Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18): 2412–2424. | ||
Kong YY, Feige U, Sarosi I, et al. Activated T-cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–309. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.