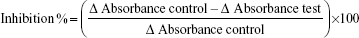Back to Journals » Drug Design, Development and Therapy » Volume 9
HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia
Authors Baskaran G, Salvamani S, Ahmad SA, Shaharuddin NA, Pattiram PD, Shukor MY
Received 27 September 2014
Accepted for publication 8 November 2014
Published 14 January 2015 Volume 2015:9 Pages 509—517
DOI https://doi.org/10.2147/DDDT.S75056
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Gunasekaran Baskaran,1 Shamala Salvamani,1 Siti Aqlima Ahmad,1 Noor Azmi Shaharuddin,1 Parveen Devi Pattiram,2 Mohd Yunus Shukor1
1Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, 2Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, Selangor, Malaysia
Abstract: The enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase is the key enzyme of the mevalonate pathway that produces cholesterol. Inhibition of HMG-CoA reductase reduces cholesterol biosynthesis in the liver. Synthetic drugs, statins, are commonly used for the treatment of hypercholesterolemia. Due to the side effects of statins, natural HMG-CoA reductase inhibitors of plant origin are needed. In this study, 25 medicinal plant methanol extracts were screened for anti-HMG-CoA reductase activity. Basella alba leaf extract showed the highest inhibitory effect at about 74%. Thus, B. alba was examined in order to investigate its phytochemical components. Gas chromatography with tandem mass spectrometry and reversed phase high-performance liquid chromatography analysis revealed the presence of phenol 2,6-bis(1,1-dimethylethyl), 1-heptatriacotanol, oleic acid, eicosyl ester, naringin, apigenin, luteolin, ascorbic acid, and a-tocopherol, which have been reported to possess antihypercholesterolemic effects. Further investigation of in vivo models should be performed in order to confirm its potential as an alternative treatment for hypercholesterolemia and related cardiovascular diseases.
Keywords: HMG-CoA reductase, Basella alba, phytochemical, GC-MS/MS, RP-HPLC, hypercholesterolemia
Introduction
Atherosclerosis, which is caused by hypercholesterolemia, is a major cause of heart diseases such as myocardial infarction. Elevated levels of plasma cholesterol, particularly low-density lipoprotein (LDL) and triglyceride levels, are mainly responsible for hypercholesterolemia, which can also lead to other diseases such as obesity, diabetes, and cancer.1,2 The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is the rate-limiting enzyme in cholesterol biosynthesis that catalyzes the conversion of HMG-CoA to mevalonate. The inhibition of HMG-CoA reductase effectively lowers the level of cholesterol in humans and most animals by the activation of sterol regulatory element-binding protein-2, which upregulates the HMG-CoA reductase and LDL receptor that lead to the reduction of cholesterol levels.3 Although statins are well-known HMG-CoA reductase inhibitors, long-term consumption of statins cause severe adverse effects such as muscle and liver damage, rhabdomyolysis, and acute renal failure.4
Due to the side effects of synthetic drugs, attention is now directed to alternative medicines of plant origin.5 Over the decades, the use of medicinal plants represents the interaction between humans and the environment.6 According to the World Health Organization,7 about 80% of the human population depend on alternative medicine for the primary treatment of various diseases. Medicinal plants have been widely reported to have medicinal properties, nutritional value, and pharmacological activities such as antioxidant, antithrombotic, anti-inflammatory, antiartherogenic, and cardioprotective effects.8,9
Phytochemicals in medicinal plants have gained much interest among researchers and the pharmaceutical and food manufacturing industries. Basically, phytochemicals are bioactive compounds that naturally exist in plants and are known as potent effectors of biological processes capable of decreasing disease risk via complementary as well as overlapping mechanisms. Plant flavonoids offer significant protection against the development of chronic illnesses such as diabetes,10 tumors,11 cancer,12 and cardiovascular diseases.13,14 Flavonoids have been reported to reduce LDL oxidation,15 suppress lipid peroxidation,16 and decrease the progression of atherosclerotic lesions in cardiovascular diseases.17,18
The potential of medicinal plants for the treatment of hypercholesterolemia is still largely unexplored and could be an alternative strategy for the progression of effective and safe antihypercholesterolemia drugs. Thus, in this study, the HMG-CoA reductase inhibitory activity of 25 medicinal plant extracts was tested.
Basella alba, locally known as remayung, belongs to the family of Basellaceae and is a wild vegetable that has been employed for the benefit of human health from ancient times. The leaves and stems of B. alba are used for medicinal purposes and it has been proven to have analgesic, antifungal, and antiulcer activities.19 The hypocholesterolemic effects of B. alba have not been investigated to date. In the present study, phytochemicals present in B. alba leaf extract were determined using gas chromatography with tandem mass spectrometry (GC-MS/MS) and reversed phase high-performance liquid chromatography (RP-HPLC). The phytochemicals of B. alba against hypercholesterolemia and its related cardiovascular diseases have been highlighted based on previous reports.
Materials and methods
Preparation of plant extract
The fresh leaves of the plants were collected from various regions of Selangor, Malaysia. The plants were botanically identified, and the plant voucher specimens were deposited at the Institute of Bioscience, Universiti Putra Malaysia. The leaves were air dried and the sample (500 g) was ground using a blender (Panasonic MX 8967) and subjected to methanol 50% (v/v) distillation for 48 hours. After filtration, the extract was isolated using a separatory funnel. The crude methanol extract of the plants was then concentrated using a rotary evaporator (Heidolph) under reduced pressure at 40°C and freeze dried at −40°C.20,21
Enzyme assay
HMG-CoA reductase inhibitory activity of the plants was determined based on spectrophotometric measurements. The HMG-CoA reductase assay kit was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The concentration of the HMG-CoA reductase stock solution was 0.5–0.75 mg/mL. Each crude extract (50 μg) was mixed with a reaction mixture containing nicotinamide adenine dinucleotide phosphate (400 μM), HMG-CoA substrate (400 μM), and potassium phosphate buffer (100 mM, pH 7.4) containing potassium chloride (120 mM), ethylenediaminetetraacetic acid (1 mM), and dithiothreitol (5 mM), followed by the addition of HMG-CoA reductase (2 μL). The reaction was incubated at 37°C, and absorbance was measured at 340 nm after 10 minutes. Simvastatin (Sigma-Aldrich Co.) was used as a positive control, and distilled water as a negative control.2 The HMG-Co A reductase inhibition (%) was calculated using the following formula:22
|
|
Phytochemical screening
The phytochemical constitutes of B. alba extract were evaluated qualitatively for flavonoids, phenolics, saponins, tannins, alkaloids, triterpenes, and steroids. The phytochemical tests were carried out using freeze–dried B. alba extract.
Test for flavonoids
Ethyl acetate (10 mL) was added to B. alba extract (0.5 mg) and heated for 3 minutes over a steam bath. After filtration, the filtrate (4 mL) was shaken with 10% ammonia solution (1 mL). The formation of yellow color indicates the presence of flavonoids.23
Test for phenolic content
B. alba extract (200 μL, 0.5 mg/mL) was mixed with Folin–Ciocalteu reagent (tenfold dilution, 0.75 mL). After incubation for 5 minutes, 6% sodium carbonate solution (0.75 mL) was added, and the mixture was further incubated at room temperature for 90 minutes. A brown coloration indicates the presence of phenolic compounds.24
Test for saponins
Distilled water (5 mL) was mixed with B. alba extract (0.5 g) and shaken vigorously. The formation of froth for 15 minutes determines the presence of saponins.25
Test for tannins
B. alba extract (0.5 g) was boiled in water (10 mL) and filtered. A few drops of 1% ferric chloride solution were mixed with the filtrate. Blue–black color formation indicates the presence of hydrolysable tannins, while brownish green precipitate shows the presence of condensed tannins.26
Test for alkaloids
B. alba extract (0.5 g) was partitioned with chloroform followed by ammoniacal chloroform. The mixture was treated with 10% sulfuric acid and tested with Mayer’s reagent. The formation of white precipitate indicates the presence of alkaloids.25
Test for steroids and triterpenes
Chloroform (1 mL) was added to B. alba extract (0.5 g) followed by few drops of acetic anhydride and concentrated sulfuric acid. The appearance of green or blue indicates the presence of steroids, while the appearance of brown or red color indicates the presence of triterpenes.25
Gas chromatography with tandem mass spectrometry (GC-MS/MS) analysis
B. alba leaf extract (1 μL) was analyzed using gas chromatography (TSQ Quantum XLS; Thermo Fisher Scientific, Waltham, MA, USA), which is equipped with a flame ionization detector and a TG-5 MS capillary column (30 m length ×0.25 mm ID ×0.25 μm thickness). Helium was used as the carrier gas at a constant flow rate of 0.8 mL/minute. The oven temperature was held 5 minutes at 40°C and raised 2°C/minute gradually up to 280°C. The injector and flame ionization detector temperature were maintained at 200°C and 250°C, respectively. The mass spectrometer was operated in scan mode from m/z 40–450 Da and the mass spectra were taken at 70 eV with a scan interval of 0.7 seconds. Identification of individual compounds was made by comparing the obtained mass spectra with internal references in the mass spectra library, the National Institute of Standards and Technology (Gaithersburg, MD, USA).21,27
RP-HPLC analysis of flavonoids and ascorbic acid
Standard stock solution of eight flavonoid standards (rutin, luteolin, catechin, quercetin, apigenin, naringin, myricetin, and histidine) and ascorbic acid (Sigma-Aldrich Co.) were prepared in methanol at concentrations of 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, and 1.0 mg/mL and filtered through a membrane filter (0.45 mm) (EMD Millipore, Billerica, MA, USA). The standards were subjected to RP-HPLC separately. The linear calibration curve was plotted at the absorbance of 280 nm as the peak area against standard concentration (mg/mL).29
Gradient RP-HPLC
The flavonoids and ascorbic acid compounds in the sample were analyzed using an RP-HPLC method, as described by Wang and Helliwell,28 with some modifications. The RP-HPLC analyses were performed with a Waters 600 pump controller and 9,486 tunable absorbance ultraviolet detector, and equipped with an Eclipse XDB-C18 reversed phase column (25 cm ×4.6 mm ID ×5 μm) (Supelco; Sigma-Aldrich Co.) at room temperature. The compounds were eluted with a gradient elution of mobile phase solvent A (deionized water, pH adjusted to 2.5 with trifluoroacetic acid) and solvent B (HPLC-grade methanol). The gradient elution program was begun with 100% solvent A at 0 minutes, followed by 70% solvent A and 30% solvent B for the next 10 minutes, 50% solvent A and 50% solvent B for 30 minutes and, finally, with 100% solvent A for 40 minutes. The flow rate was 1.0 mL/minute and the injection volume was 20 μL with a post-time of 15 minutes before the next injection. The detection of flavonoid wavelength was set at 280 nm. Flavonoids in the sample were identified based on comparison with standard retention times of chromatographic peaks.
RP-HPLC analysis of α-tocopherol
Standard stock solution of α-tocopherol with the mobile phase composed of methanol:deionized water (92:8) were prepared at concentrations of 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, and 1.0 mg/mL and subjected to HPLC separately. The detection wavelength was set at absorbance 292 nm. The linear calibration curve was obtained by fitting the peak area against the standard concentration (mg/mL).29
Isocratic RP-HPLC
An isocratic RP-HPLC method was carried out with some modifications.29 The method was performed using an Eclipse XDB-C18 reversed phase column (25 cm ×4.6 mm ID ×5 μm) (Supelco; Sigma-Aldrich Co.). The α-tocopherol compound in the sample was eluted using an elution solvent composed of methanol: deionized water (92:8) with a total run time of 40 min. The sample injection volume was 20 μL with a flow rate of 1.0 mL/mintue. The detection of α-tocopherol wavelength was set at 292 nm. The α-tocopherol compound was identified by matching the retention time against the standard. The laboratory methods performed in this study are summarized in Table 1.
Results and discussion
Inhibitory effect of plants on HMG-CoA reductase
Among the 25 plant extracts, B. alba, Amaranthus viridis, and Piper sarmentosum showed inhibitory effects of more than 50% on HMG-CoA reductase activity (Table 2).B. alba showed the highest inhibition of 74.1%. The inhibitory effect of B. alba was higher than Rosa damascene (70%) and Myrtus communis (62%), which have been reported to be potent inhibitors of HMG-CoA reductase.2 The positive control in this study, simvastatin, showed enzyme inhibition of 85.1%. HMG-CoA reductase catalyzes the rate-limiting step in the synthesis of cholesterol. When human and animal models of hypercholesterolemia are given statins (inhibitors of HMG-CoA reductase), the initial reduction in cholesterol synthesis leads to compensatory responses that start with the activation of sterol regulatory element-binding protein-2. As a result, the expression of HMG-CoA reductase and the LDL receptor is upregulated. This results in normal levels of cholesterol synthesis due to the presence of the inhibitor, which compensates for the high levels of the enzyme. However, the increase in LDL receptor expression causes a reduction in cholesterol levels.3,30 Thus, in this study, the inhibition of the enzyme may reflect the potential of B. alba in cholesterol reduction. B. alba is known locally as Indian spinach or remayung and has been used in treating ulcers, hypertension, anemia, digestive disorders, and cancer.19 Further investigation of the phytochemical constitutes of B. alba were performed in order to determine the possible compounds involved in HMG-CoA reductase inhibition.
Phytochemical analysis
The phytochemical screening of B. alba using methanol as an extracting solvent revealed the presence of medically active constituents such as phenolic compounds, flavonoids, condensed tannins, and saponins, while other constituents such as hydrolyzed tannins, alkaloids, steroids, and triterpenes were not detected (Table 3). Phenolic compounds, commonly known as polyphenols, have been shown to possess antioxidant properties,31 raise the antioxidant capacity of human plasma,32 and inhibit LDL oxidation.33,34 Flavonoids and tannins are phenolic compounds; they act as free radical scavengers. Being an antioxidant, flavonoids suppress the oxidation of LDL cholesterol, which is involved in atherosclerotic development.35,36 Flavonoids are also reported to exhibit cardioprotective effects such as improvement in endothelial activity and anti-inflammatory action in both in vitro and in vivo studies.35,37,38 In addition, flavonoids are also claimed to successfully inhibit platelet aggregation in hypercholesterolemic rabbits.39 As with flavonoids, tannins have been proven to have strong antiplatelet38 and antihypercholesterolemic effects by reducing cholesterol absorption in animal studies.41,42 Condensed tannins are preferable in therapeutic treatment since they do not interfere with the absorption of iron compared with hydrolyzed tannins, which inhibit iron absorption that may lead to anemia.43 Several studies on saponins revealed that they inhibit cholesterol absorption in the intestine and decrease the level of plasma cholesterol in various experimental animal models.44–46 Saponins isolated from garlic have shown cholesterol-lowering effects by reducing LDL and total cholesterol concentrations without altering high-density lipoprotein cholesterol levels in hypercholesterolemia-induced rats.47 Furthermore, saponins were also found to reduce the risk of atherosclerosis in rats.48
  | Table 3 Qualitative analysis of phytochemical constitutes |
GC-MS/MS analysis
GC-MS/MS offers enhanced selectivity and sensitivity compared with gas chromatography–mass spectrometry by the elimination of matrix ion interference through selected reaction monitoring. Selected reaction monitoring is highly specific and can provide identification of low levels of compounds even in the presence of a high matrix background.49,50 Compound identification was determined through a comparison of obtained mass spectra with the internal references in the mass spectra library, the National Institute of Standards and Technology. B. alba methanol extract revealed 25 phytocomponents, and their molecular formula, molecular weight, as well as peak area (%) are summarized in Table 4. The major components in the leaves of B. alba were vitamin E (peak area 12.337%); l-(+)-ascorbic acid 2,6-dihexadecanoate (peak area 11.611); phenol, 2,6-bis(1,1-dimethylethyl)- (peak area 11.379); (+)-c-tocopherol, O-methyl- (peak area 9.33); 1-heptatriacotanol (peak area 8.615); and α-tocopherol, O-methyl- (peak area 8.550). The potential effects of the components involved in the treatment of hypercholesterolemia and its related diseases are presented in Table 5.
RP-HPLC analysis
RP-HPLC is a chromatographic technique widely used in the simultaneous separation and quantification of phenolic compounds. The separation of the compounds was performed with a reversed phase column. The chromatographic separations of eight flavonoid standards (rutin, luteolin, catechin, quercetin, apigenin, naringin, myricetin, and histidine) and ascorbic acid at 0.2 mg/mL by gradient elution are shown in Figure 1. The typical HPLC chromatogram of B. alba is presented in Figure 2. The compounds detected in B. alba leaves were ascorbic acid, luteolin, apigenin, and naringin. In addition, α-tocopherol standard (0.2 mg/mL) was separated by isocratic elution chromatography, as presented in Figure 3A, while the detection of α-tocopherol in the methanol extract of B. alba leaves is shown in Figure 3B. The concentration of each flavonoid, ascorbic acid, and α-tocopherol were calculated from the standard calibration curve and presented as the mean of three determinations (Table 6). Ascorbic acid had the highest concentration (0.891 mg/mL), followed by α-tocopherol (0.702 mg/mL), naringin (0.180 mg/mL), apigenin (0.165 mg/mL), and luteolin (0.099 mg/mL).
  | Figure 2 HPLC chromatogram of Basella alba leaves at 280 nm. |
  | Figure 3 HPLC chromatogram for α-ocopherol at 292 nm. |
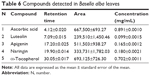  | Table 6 Compounds detected in Basella alba leaves |
Based on previous studies, the compounds detected in RP-HPLC can be associated with the prevention and treatment of hypercholesterolemia. Luteolin and apigenin are flavones, a type of flavonoid, and they were demonstrated to inhibit the adhesion of monocytes on oxidized LDL (in human endothelial cells), which indicates their antiatherogenic properties and effectiveness in treating the initial stage of atherosclerosis.51 In addition, luteolin and apigenin have vasoprotective effects whereby the compounds protect resistance arteries of rats from injuries by superoxide anions, and they are potentially useful as therapeutic treatments for cardiovascular diseases.52 Luteolin has been reported to possess antihypercholesterolemic effects since it reduces the concentration of total cholesterol, triglycerides, and free fatty acid, as well as decreases the levels of cardiac marker enzymes, troponin I and troponin T in rats-enzymes that exist during myocardial injury.53
Naringin is classified as a flavanone, which is a subgroup of flavonoids. Naringin exhibits important properties that can ameliorate hypercholesterolemia and atherosclerosis. Naringin has been proven to inhibit HMG-CoA reductase and decrease plasma cholesterol, LDL, triglycerides, and hepatic lipid levels without altering high-density lipoprotein cholesterol in rats54 and rabbits.55 Naringin also supresses monocyte adhesion on endothelial cells and smooth cell proliferation, as well as decreasing fatty streak formation. In comparison with lovastatin, a synthethic drug for cholesterol lowering, naringin is nontoxic and possesses hepatoprotective action in mice56 and rabbits.57 Ascorbic acid (vitamin C) and α-tocopherol (vitamin E) are known to be effective therapeutics for the treatment of hypercholesterolemia and its related cardiovascular diseases, as mentioned in Table 5.
Conclusion
The present study provides preliminary data that suggest the B. alba leaf extract is capable of lowering cholesterol levels by inhibiting the HMG-CoA reductase activity. In addition, the compounds of B. alba extract (phenol 2,6-bis[1,1-dimethylethyl], 1-heptatriacotanol, oleic acid, eicosyl ester, naringin, apigenin, luteolin, ascorbic acid, and α-tocopherol) have been reported to possess beneficial effects in treating hypercholesterolemia and its related diseases. However, the mechanism of B. alba extract in inhibiting the HMG-CoA reductase is unknown. Studies in in vivo models could give further insights into the effects and roles of B. alba as an alternative therapeutic agent in the prevention and management of hypercholesterolemia.
Acknowledgments
This research is supported by Grant no 9399800 from Universiti Putra Malaysia. Gunasekaran Baskaran is supported by My Brain PhD scholarship from the Ministry of Education of Malaysia.
Disclosure
The authors report no conflicts of interest in this work.
References
Adaramoye OA, Akintayo O, Achem J, Fafunso MA. Lipid-lowering effects of methanolic extract of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vasc Health Risk Manag. 2008;4(1):235–241. | ||
Gholamhoseinian A, Sharifi-Far F, Shahouzehi B. Inhibitory activity of some plant methanol extracts on 3-hydroxy-3-methylglutaryl coenzyme a reductase. Int J Pharmacol. 2010;6:705–711. | ||
Friesen JA, Rodwell VW. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004;5(11):248. | ||
Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101(2):207–213. | ||
Loke WM, Proudfoot JM, Hodgson JM, et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2010;30(4):749–757. | ||
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011; 8(1):1–10. | ||
World Health Organization. WHO Traditional Medicine Strategy 2002–2005. Geneva, Switzerland: World Health Organization; 2002. | ||
Vinson JA, Dabbagh YA, Serry MM, Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J Agric Food Chem. 1995;43(11):2800–2802. | ||
Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16(1):77–84. | ||
Kumar S, Malhotra R, Kumar D. Antidiabetic and free radicals scavenging potential of Euphorbia hirta flower extract. Indian J Pharm Sci. 2010;72(4):533–537. | ||
Feng Z, Hao W, Lin X, Fan D, Zhou J. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. Onco Targets Ther. 2014;7:947–956. | ||
Tahanian E, Sanchez LA, Shiao TC, Roy R, Annabi B. Flavonoids targeting of IκB phosphorylation abrogates carcinogen-induced MMP-9 and COX-2 expression in human brain endothelial cells. Drug Des Devel Ther. 2011;5:299–309. | ||
Hollman PC, Hertog MG, Katan MB. Role of dietary flavonoids in protection against cancer and coronary heart disease. Biochem Soc Trans. 1996;24(3):785–789. | ||
Kris-Etherton PM, Hecker KD, Bonanome A, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113 Suppl 9B:71S–88S. | ||
Tikkanen MJ, Wähälä K, Ojala S, Vihma V, Adlercreutz H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc Natl Acad Sci. 1998;95(6):3106–3110. | ||
McAnlis GT, McEneny J, Pearce J, Young IS. Absorption and antioxidant effects of quercetin from onions, in man. Eur J of Clin Nutr. 1999; 53(2):92–96. | ||
Anthony MS, Clarkson TB, Bullock BC, Wagner JD. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1997;17(11):2524–2531. | ||
Salvamani S, Gunasekaran B, Shaharuddin NA, Ahmad SA, Shukor MY. Antiartherosclerotic effects of plant flavonoids. Biomed Res Int. 2014;2014:480258. | ||
Adhikari R, Naveen Kumar H, Shruthi S. A review on medicinal importance of Basella alba L. Int J Pharma Sci Drug Res. 2012;4(2):110–114. | ||
Lasekan O, Buettner A, Christlbauer M. Investigation of important odorants of palm wine (Elaeis guineensis). Food Chem. 2007;105(1):15–23. | ||
Lee S-J, Umano K, Shibamoto T, Lee K-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91(1):131–137. | ||
Jung K-A, Song T-C, Han D, Kim I-H, Kim Y-E, Lee C-H. Cardiovascular protective properties of kiwifruit extracts in vitro. Biol Pharma Bull. 2005;28(9):1782–1785. | ||
Edeoga H, Okwu D, Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. | ||
Velioglu Y, Mazza G, Gao L, Oomah B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46(10):4113–4117. | ||
Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London, UK: Chapman and Hall; 1984. | ||
Evans WC, Evans D. Trease and Evans Pharmacognosy, St Louis, MO: Elsevier; 2002. | ||
Lasekan O. Volatile constituents of roasted tigernut oil (Cyperus esculentus L.). J Sci Food Agr. 2013;93(5):1055–1061. | ||
Wang H, Helliwell K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res Int. 2001;34(2–3):223–227. | ||
Alves R, Casal S, Oliveira MBP. Determination of vitamin E in coffee beans by HPLC using a micro-extraction method. Food Sci Technol Int. 2009;15(1):57–63. | ||
Wong J, Quinn C, Brown A. SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. Biochem J. 2006;400:485–491. | ||
Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995;322(2): 339–346. | ||
Duthie GG, Pedersen MW, Gardner PT, et al. The effect of whisky and wine consumption on total phenol content and antioxidant capacity of plasma from healthy volunteers. Eur J Clin Nutr. 1998;52(10):733–736. | ||
Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341(8843):454–457. | ||
Kerry NL, Abbey M. Red wine and fractionated phenolic compounds prepared from red wine inhibit low density lipoprotein oxidation in vitro. Atherosclerosis. 1997;135(1):93–102. | ||
Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol. 2002;13(1):41–49. | ||
O’Byrne DJ, Devaraj S, Grundy SM, Jialal I. Comparison of the antioxidant effects of Concord grape juice flavonoids α-tocopherol on markers of oxidative stress in healthy adults. Am J Clinl Nutr. 2002;76(6):1367–1374. | ||
Rein D, Paglieroni TG, Wun T, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72(1):30–35. | ||
Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005;81(1):292S–297S. | ||
Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–80. | ||
Mekhfi H, ElHaouari M, Bnouham M, Aziz M, Ziyyat A, Legssyer A. Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytother Res. 2006;20(2):135–139. | ||
Tebib K, Besançon P, Rouanet J-M. Dietary grape seed tannins affect lipoproteins, lipoprotein lipases and tissue lipids in rats fed hypercholesterolemic diets. J Nutr. 1994;124(12):2451–2457. | ||
Reed J. Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr. 2002;42(3 suppl):301–316. | ||
Brune M, Rossander L, Hallberg L. Iron absorption and phenolic compounds: importance of different phenolic structures. Eur J Clin Nutr. 1989;43(8):547–557. | ||
Sauvaire Y, Ribes G, Baccou JC, Loubatieères-Mariani MM. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. 1991;26(3):191–197. | ||
Harwood HJ, Chandler CE, Pellarin LD, et al. Pharmacologic consequences of cholesterol absorption inhibition: alteration in cholesterol metabolism and reduction in plasma cholesterol concentration induced by the synthetic saponin beta-tigogenin cellobioside (CP-88818; tiqueside). J Lipid Res. 1993;34(3):377–395. | ||
Hostettmann K, Marston A. Saponins. Cambridge, UK: Cambridge University Press; 2005. | ||
Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131(3):1000S–1005S. | ||
Rodrigues H, Diniz Y, Faine L, et al. Antioxidant effect of saponin: potential action of a soybean flavonoid on glucose tolerance and risk factors for atherosclerosis. Int J Food Sci Nutr. 2005;56(2):79–85. | ||
Garrido Frenich A, Gonzalez-Rodriguez MJ, Arrebola FJ, Martínez Vidal JL. Potentiality of gas chromatography-triple quadrupole mass spectrometry in vanguard and rearguard methods of pesticide residues in vegetables. Anal Chem. 2005;77(14):4640–4648. | ||
Hernández F, Portolés T, Pitarch E, López FJ, Beltrán J, Vázquez C. Potential of gas chromatography coupled to triple quadrupole mass spectrometry for quantification and confirmation of organohalogen xenoestrogen compounds in human breast tissues. Anal Chem. 2005;77(23):7662–7672. | ||
Jeong YJ, Choi YJ, Choi JS, et al. Attenuation of monocyte adhesion and oxidised LDL uptake in luteolin-treated human endothelial cells exposed to oxidised LDL. Br J Nutr. 2007;97(03):447–457. | ||
Ma X, Li YF, Gao Q, et al. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci. 2008;83(3):110–117. | ||
Madhesh M, Vaiyapuri M. Luteolin a dietary flavonoid attenuates isoproterenol-induced myocardial oxidative stress in rat myocardium: an in vivo study. Biomed Prev Nutr. 2013;3(2):159–164. | ||
Kim SY, Kim HJ, Lee MK, et al. Naringin time-dependently lowers hepatic cholesterol biosynthesis and plasma cholesterol in rats fed high-fat and high-cholesterol diet. J Med Food. Winter 2006;9(4): 582–586. | ||
Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr. 2004;23(5): 1025–1034. | ||
Chanet A, Milenkovic D, Deval C, et al. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J Nutr Biochem. 2012;23(5): 469–477. | ||
Choe SC, Kim HS, Jeong TS, Bok SH, Park YB. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharm. 2001; 38(6):947–955. | ||
Costantino L, Parenti C, Di Bella M, Zanoli P, Baraldi M. Anti-inflammatory activity of newly synthesized 2,6-bis-(1,1-dimethylethyl)phenol derivatives. Pharmacol Res. 1993;27(4):349–358. | ||
Busch SJ, Chen KS, Parker RA, Wright PS, Yates MT, assignees. Substituted phenols and thiophenols useful as antioxidant agents. United States patent US 6,114,572. 2000 Sept 5. | ||
Ogunlesi M, Okiei W, Osibote EA. Analysis of the essential oil from the leaves of Sesamum radiatum, a potential medication for male infertility factor, by gas chromatography-mass spectrometry. Afr J Biotechnol. 2010;9(7):1060–1067. | ||
Vinson JA, Jang J. In vitro and in vivo lipoprotein antioxidant effect of a citrus extract and ascorbic acid on normal and hypercholesterolemic human subjects. J Med Food. 2001;4(4):187–192. | ||
Sakuma N, Yoshikawa M, Hibino A, et al. Ascorbic acid protects against peroxidative modification of low-density lipoprotein, maintaining its recognition by LDL receptors. J Nutr Sci Vitaminol (Tokyo). 2001;47(1): 28–31. | ||
Siefken W, Höppner H, Harris I. Regulation of cholesterol synthesis by oleic and palmitic acid in keratinocytes. Exp Dermatol. 2000;9(2): 138–145. | ||
Natali F, Siculella L, Salvati S, Gnoni GV. Oleic acid is a potent inhibitor of fatty acid and cholesterol synthesis in C6 glioma cells. J Lipid Res. 2007;48(9):1966–1975. | ||
Tan DT, Khor H, Low W, Ali A, Gapor A. Effect of a palm-oil-vitamin E concentrate on the serum and lipoprotein lipids in humans. Am J Clin Nutr. 1991;53(4):1027S–1030S. | ||
Ozer NK, Palozza P, Boscoboinik D, Azzi A. d-alpha-Tocopherol inhibits low density lipoprotein induced proliferation and protein kinase C activity in vascular smooth muscle cells. FEBS Lett. 1993;322(3): 307–310. | ||
Parker RA, Sabrah T, Cap M, Gill BT. Relation of vascular oxidative stress, α-tocopherol, and hypercholesterolemia to early atherosclerosis in hamsters. Arterioscler Thromb Vasc Biol. 1995;15(3):349–358. | ||
Tucker J, Townsend D. Alpha-tocopherol: roles in prevention and therapy of human disease. Biomed Pharmacother. 2005;59(7):380–387. | ||
69.Ozer NK, Negis Y, Aytan N, et al. Vitamin E inhibits CD36 scavenger receptor expression in hypercholesterolemic rabbits. Atherosclerosis. 2006;184(1):15–20. | ||
Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–2098. | ||
Catalgol B, Ozer NK. Protective effects of vitamin E against hypercholesterolemia-induced age-related diseases. Genes Nutr. 2012; 7(1):91–98. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.