Back to Journals » Drug Design, Development and Therapy » Volume 14
HIF-1α is a Potential Molecular Target for Herbal Medicine to Treat Diseases
Authors Li RL, He LY, Zhang Q, Liu J, Lu F, Duan HXY, Fan LH, Peng W, Huang YL, Wu CJ
Received 31 July 2020
Accepted for publication 22 October 2020
Published 16 November 2020 Volume 2020:14 Pages 4915—4949
DOI https://doi.org/10.2147/DDDT.S274980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Ruo-Lan Li,1 Li-Ying He,1 Qing Zhang,1 Jia Liu,1 Feng Lu,1 Hu-Xin-Yue Duan,1 Lin-Hong Fan,1 Wei Peng,1 Yong-Liang Huang,2 Chun-Jie Wu1
1School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan 611137, People’s Republic of China; 2Pharmacy Department, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan 610072, People’s Republic of China
Correspondence: Yong-Liang Huang; Chun-Jie Wu Tel +86-28-6180-1001
Email [email protected]; [email protected]
Abstract: HIF-1α is an important factor regulating oxygen balance in mammals, and its expression is closely related to various physiological and pathological conditions of the body. Because HIF-1α plays an important role in the occurrence and development of cancer and other diseases, it has become an enduring research hotspot. At the same time, natural medicines and traditional Chinese medicine compounds have amazing curative effects in various diseases related to HIF-1 subtype due to their unique pharmacological effects and more effective ingredients. Therefore, in this article, we first outline the structure of HIF-1α and the regulation related to its expression, then introduce various diseases closely related to HIF-1α, and finally focus on the regulation of natural medicines and compound Chinese medicines through various pathways. This will help us understand HIF-1α systematically, and use HIF-1α as a target to discover more natural medicines and traditional Chinese medicines that can treat related diseases.
Keywords: HIF-1α, disease, natural medicine, traditional Chinese medicine
Introduction
Mammalian cells depend on oxygen, while hypoxia is also a common physiological and pathological phenomenon in organisms and a common microenvironment for solid tumors.1 Therefore, the mechanism in the body that can adapt to hypoxia is particularly important to the survival of human beings. Hypoxia-inducible factors (HIFs), a family of transcription factors involved in hypoxia response, is one of the key regulatory mechanisms of hypoxia stress at the cellular level.2,3 Although there are many mechanisms for hypoxic adaptation in mammals, including those with a faster response time than the HIF system, the HIF system’s unique extent of the effects makes it to be a more important regulator of hypoxic responses.4 Among them, HIF-1α is the most important regulator of oxygen balance in mammals.
HIF-1α, widely existed in mammalian cells, participates in multiple signaling pathways and is a transduction center that mediates hypoxia signals. Increasing studies have found that HIF-1α is closely related to the formation of animal cardiovascular system, the development of cartilage system, the formation of neural embryos and so on. HIF-1α regulated target gene involves erythropoiesis, angiogenesis, cell proliferation, metabolism and apoptosis. For example, HIF-1α can regulate the metabolism, apoptosis and autophagy of tumor cell to suppress tumor cells survival.5–7 In addition, the new blood vessels generation, epithelial–mesenchymal transition (EMT), transfer invasion, radiation and chemotherapy resistance, pH steady-state of cells are also related to HIF-1α.8–11 Therefore, HIF-1α is closely related to a variety of physiological and pathological processes in humans.
Abnormally increased or decreased of HIF-1α may cause damage to the body. Up to now, many active ingredients of traditional Chinese medicine (TCM) and natural products have been found to regulate HIF-1α content. For example, curcumin, mainly extracted from Curcumae longae Rhizoma, can inhibit the transcription of HIF-1α in vitro, and then produce a certain therapeutic effect on liver cancer.12 Traditional Chinese formulas, such as Wenshen Yangxue Decoction, can promote endometrial repair by increasing HIF-1α.13 Natural medicines that can influence HIF-1α are rich in variety and resources, and they are worthy of development and utilization.
The Protein Structure and Regulation of HIF-1α
HIF-1α Protein Structure
HIF-1α is a 120–130 kD protein. It is the most widely expressed HIF-α subunit in mammalian tissues and is also highly conserved in many other species. The N-terminal of HIF-1α subunits contains basic helix-loop-helix (bHLH) domains, Per-ARNT-Sim-A (PAS-A) and Per-ARNT-Sim-B (PAS-B) domains. The bHLH domain defines a large superfamily of dimeric eukaryotic transcription factors, mediates combination of HIF-1α and DNA.14 The bHLH domain can be dimerized with the PAS domain to form bHLH-PAS proteins, which are only found in multicellular animals and are a relatively small family of bHLH proteins.15 The bHLH-PAS domains mediate α, β-dimerization of HIFs and binding of HIFs to hypoxic response elements (HRE) on target genes.4 The C-terminal of HIF-1α subunits contain oxygen-dependent degradation domains (ODD) and two transcriptional activation domains (TAD), N-TAD and C-TAD (N/C terminal activation domains). In the ODD, the LAPYIXMD motif plays a key role in the binding of HIF-1α to von Hippel-Lindau E3 ubiquitin ligase complex (pVHL), while N-TAD contributes to HIF-1α stabilization against proteasomal degradation, and the C-TAD has the role of recruiting coactivator proteins, CREB-binding protein (CBP)/p300, to form active transcriptional complexes on DNA (Figure 1).16–18
The Regulation of HIF-1α
The Synthesis of HIF-1α
The synthesis of HIF-1α under normoxic conditions is regulated by phosphatidyl inositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. The PI3K pathway can mediate the translation of HIF-1α through protein kinase B (Akt) and mammalian target of rapamycin (mTOR), while the MAPK pathway can initiate the translation of HIF-1α through extracellular regulated protein kinases (ERK). mTOR and ERK can activate the translation of specific mRNA sequences by inactivating the inhibitor eukaryotic initiation factor 4E-binding protein (4E-BP) or activating ribosomal protein S6 kinase (S6K).19,20
Eukaryotic initiation factor 4E (eIF4E) is a cap-binding protein that can be combined with eIF4G and eIF4A to form eukaryotic initiation factor 4F (eIF4F), which binds to the 5ʹ cap structure of mRNA to initiate HIF-1α translation. It is generally believed that eIF4E plays a central role in the initial stage of cap-dependent translation since its minimum content and is present in the cell in limiting molar amounts.21,22 The combination of 4E-BP and eIF4E inhibited the formation of eIF4F. Low phosphorylated 4E-BP has a high affinity with eIF4E and can compete with eIF4G for the binding site of eIF4E, thus preventing the formation of eIF4F and the initiation of HIF-1α translation. When 4E-BP is fully phosphorylated, eIF4E is released and can be combined with eIF4G and eIF4A to form eIF4F, which improves the efficiency of translation.23,24 Activation of mTOR and ERK phosphorylates 4E-BP at multiple sites, thereby releasing eIF4E, allowing eIF4F formation and subsequent cap-dependent translation. What is more, MAPK pathways can also activate the MAPK signal integrating kinase (MNK), which plays a role of eIF4E kinase, so as to promote the formation of eIF4F (Figure 2).25
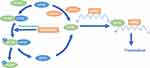 |
Figure 2 MTOR-mediated synthesis of HIF-1α. |
The HIF-1α translation mechanism that S6K mediated is still controversial, but the viewpoint that activation of S6K can increase HIF-1α translation is widely accepted. At first, it was speculated that S6K could stimulate HIF-1α translation by increasing the affinity of ribosomes for the 5ʹ-terminal oligopyrimidine tract (5ʹ TOP) motif in certain mRNAs.26 But the new study found that the 5ʹ TOP motif was not present in HIF-1α mRNA. Furthermore, S6K is dispensable for the translational activation of TOP mRNAs by growth factors.27 Therefore, the mechanism remains to be further studied.
Although the synthesis of most proteins declines under hypoxia, the translation of a few protein critical to cell survival does not stop, including HIF-1α. However, it is unclear that how HIF-1α is selectively translated during periods of global translation inhibition remains incompletely understood. One possible mechanism is related to the internal-ribosome-entry-site (IRES). IRES do not require the presence of the elF4F cap-binding complex to initiate translation. It is a short RNA sequence (150~250bp) located at the 5ʹ untranslated region (UTR) of HIF-1α. IRES can fold into a structure similar to the initiation tRNA, which then mediates the binding of ribosomes to HIF-1α mRNA and initiates its translation in a cap-independent manner.28–30 However, whether an IRES-dependent mechanism occupies a dominant position in hypoxia conditions is debatable. When researchers evaluated IRES-mediated HIF-1α translation under hypoxia, they found that only <1% of the transcriptional activity was mediated by IRES, suggesting that the role of IRES was not primary.31 Another mechanism is related to the RNA-binding proteins polypyrimidine tract-binding protein (PTB) and HuR. It was found that in the COCl2-induced hypoxia model, PTB and HuR could combine with the 3ʹ UTR and 5ʹ UTR of HIF-1α to jointly promote the translation of HIF-1α.30,32
The role of microRNAs (miRNAs) in regulating HIF-1α translation has also received increasing attention. MiRNAs are small endogenous non-coding RNAs consisting of 21 to 25 nucleotides. These miRNAs can be base-paired with the 3ʹ UTR of mRNA. After forming an RNA-induced silencing complex (RISC), it can degrade the mRNA or hinder its translation.33 Currently, it has been found that miRNA-17-92 clusters, −155, −199 and −519c can be directly base-paired with HIF-1α mRNA to inhibit translation. MiRNA-21 can indirectly enhance the expression of HIF-1α, mainly by targeting the phosphatase and tension homolog (PTEN) to reduce HIF-1α expression. Moreover, the reduction of PTEN can activate AKT and ERK, thereby increasing the expression of HIF-1α.34
Degradation and Stabilization of HIF-1α
Located in the cytoplasm, HIF-1α is easily degraded under normal oxygen with a half-life of less than five minutes, but its stability and transcriptional activity are significantly increased under hypoxic conditions.35–37 HIF-1α regulates its stability mainly through multiple ways.
Prolyl hydroxylase domain proteins (PHD) is an oxygen-dependent enzyme. The oxygen-dependent degradation pathway is called the O2/PHDs/pVHL pathway (Figure 3). When oxygen levels are normal, it can make two of HIF-1α proline residues (P402 and P564 of HIF-1α) in the ODD (NODD and CODD, respectively) hydroxylation, with oxygen, α-Ketoglutaric acid as substrate, Fe2+ and ascorbate as coenzyme. And then pVHL combined with HIF-1α, recruited a variety of ubiquitin protein, to form the E3 ubiquitin ligases proteasome, make HIF-1α subunit ubiquitination, and then degradation by the proteasome.38–41 PVHL is the substrate recognition component of the complex and can bind directly to HIF-1α. A single hydroxylation at any one site (NODD or CODD) is sufficient for HIF-1α to target the pVHL for degradation.42 In some cancers, particularly kidney cancer, pVHL is inactivated, and the resultant upregulation of HIF-1α may serve to promote tumor growth.43 The E3 ubiquitin ligases consist of Elongin-C, Elongin-B and lysine residues catalyzed by pVHL, which degrade HIF-1α by protease. PHD is a key speed limiting enzyme of this pathway. There are 4 PHDs in mammals, namely PHD1-4, among which PHD2 is mainly responsible for regulating the degradation of HIF-1α. PHDs are expressed differently in different tissues and have different affinities with different HIF proteins, which may lead to the diversity of hypoxic responses.44 Under hypoxic conditions, intracellular accumulation of succinic acid, fumaric acid, reactive oxygen species (ROS) and other chemicals such as CoCl2, dimethyloxalylglycine (DMOG) and iron ion chelator can inhibit the hydroxylation activity of PHD, thereby blocking the O2/PHDs/pVHL degradation pathway and stabilizing HIF-1α. Among them, DMOG is a structural analogue of α-ketoglutaric acid and a competitive inhibitor of PHD.45 PHD’s catalytic center contains Fe2+, so iron chelators can also inhibit its activity.
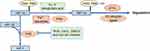 |
Figure 3 Degradation pathway of HIF-1α by O2/PHDs/pVHL. |
The oxygen-dependent O2/PHDs/pVHL pathway is not the only pathway that leads to the degradation of HIF-1α. There are also some proteins that can affect the stability of HIF-1α. The most typical ones are small ubiquitin-like modifier (SUMO), receptor for activated protein C kinase 1 (RACK1), heat shock protein 90 (HSP90) and proteins related to the PI3K/Akt signaling pathway. SUMOs are small proteins (12 kD) with low sequence identity to ubiquitin, but their 3D structures are very similar. It can bind substrate proteins and carry on the modification after translation, the process is called the SUMOylation.46 Initially, it was found that SUMO-l can cause SUMOylation of HIF-1α. Overexpression of SUMO-1 under normal and hypoxia can increase the stability and transcriptional activity of HIF-1α, because SUMOylation may compete with ubiquitination for the same modification site of HIF-1α. But new evidence suggests that SUMOylation of HIF-1α can also lead to degradation. Hypoxia induces SUMOylation of HIF-1α, which promotes its binding to the β-subunit of pVHL to form an oxygen-independent HIF-1α-pVHL-E3 ubiquitin ligases complex, and result in ubiquitination and degradation of HIF-1α.47 Therefore, when PHD is absent, pVHL can also bind to HIF-1α through this pathway. The effect of SUMO on HIF-1α is controversial and needs further research. In addition, the RACK1 and HSP90 can also regulate the stability of HIF-1α in a manner similar to pVHL pathway. After the homodimerization of RACK1, it can bind to the PAS region of HIF-1α, and then recruit Elongin-C and other components of the E3 ubiquitin ligase, resulting in ubiquitination and degradation of HIF-1α. HSP90 can compete with RACK1 to bind to the PAS region of HIF-1α, preventing the ubiquitination of HIF-1α.48
The PI3K/Akt signaling pathway is not only involved in the synthesis of HIF-1α but also can regulate the degradation of HIF-1α through different mechanisms. One mechanism is mediated by glycogen synthase kinase-3 (GSK-3). GSK-3 exists in two forms: serine-9 (Ser-9) phosphorylation and tyrosine-216 (Tyr-216) phosphorylation, while Ser-9 phosphorylation is its inactive form, and Tyr-216 phosphorylation is its active form.49 Active GSK-3 can phosphorylate serine 551 (Ser551), threonine 555 (Thr555), and serine 589 (Ser589) at HIF-1α. After that, HIF-1α is ubiquitinated and degraded. Transient hypoxia can activate the PI3K-Akt pathway, which can make GSK-3 exist in an inactive Ser-9 phosphorylated form, thereby preventing the degradation of HIF-1α. In contrast, chronic hypoxia can inhibit the PI3K/Akt pathway, which can reduce the phosphorylation of Ser-9 and increase the phosphorylation of Try-216. So that more GSK-3 exist in active form which induces degradation of HIF-1α.50 Another mechanism is achieved by fork-headed box O4 (FOXO4). PI3K-Akt can phosphorylate FOXO4 and prevent it from entering the nucleus, and FOXO4 can induce the ubiquitination and degradation of HIF-1α.51
In summary, the degradation of HIF-1α is very complicated, including many pathways and regulators, some of which have not been fully proven and require further research.
Nuclear Transport of HIF-1α
The amount of protein accumulated in the nucleus depends on the relative proportion of its nuclear import and export. Classical HIF-1α nuclear import is mediated by nuclear transport receptors importin. Importin is a receptor of nuclear localization signal (NLS), which can bind to NLS and help nuclear proteins enter the nucleus. HIF-1α in the cytoplasm can enter the nucleus just because its C-terminal NLS (CNLS) can bind to importin α/β, while N-terminal NLS (NNLS) does not have this function.52 Moreover, subsequent research found that in addition to the classical importin α/β NLS receptor, importins 4 and 7 can also mediate nuclear import of HIF-1α. They can bind directly to HIF-1α and promote its entry into the nucleus. The difference between the two pathways is that importin a/β may release HIF-1α around the inner nuclear envelope, while importins 4 and 7 release HIF-1α in the nucleus.53,54
The nuclear export of HIF-1α involves the MAPK pathway. At first, some scholars found that the HIF-1α protein always appeared on the SDS-PAGE gel as a band with a molecular weight of 20 kDa more than expected. Subsequently, it was found that phosphorylation can change the migration rate of HIF-1α on the gel under normal or hypoxic conditions. Besides, it was confirmed that MAPK played a major role in this change. It can phosphorylate HIF-1α in vivo and in vitro and increase its transcriptional activity. In most research, MAPK does not change HIF-1α protein expression, stability and DNA-binding activity, but promotes HIF-1α transcriptional activity. MAPK increases HIF-1α transcriptional activity through different mechanisms, one of which involves the nuclear export of HIF-1α. The main component of HIF-1α nuclear export is located at the C-terminal nuclear export signal (NES) of HIF-1α, which is an atypical hydrophobic nuclear exporting factor I (CRM1)-dependent signal. HIF-1α in the nucleus can bind to CRM1 through its NES, allowing it to be exported from the nucleus. But when the Ser641 or Ser643 adjacent to NES is phosphorylated by MAPK, NES will be masked and unable to bind to CRMI, so that phosphorylated HIF-1α is accumulated in the nucleus55,56 (Figure 4).
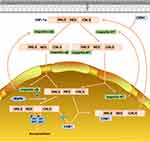 |
Figure 4 Nuclear transport of HIF-1α. |
Heterodimerization, Transcriptional Activation of HIF-1α and Interaction with Other Proteins
The stabilization and nuclear transport of HIF-1α are essential for its function, but these are not enough. Phosphorylated HIF-1α must be heterodimerized with HIF-1β to form HIF-1. After its active regions N-TAD and C-TAD bind to co-activating factors such as P300/CBP (CREB-binding protein), an active complex is formed. The complex then binds to the HRE on the target gene to exerting its activity.
Under normoxic conditions, the N-terminal of aspartic acid hydroxylase (also known as factor-inhibiting HIF-1α, FIH) can bind to the HIF-1α region, while the C-terminal cannot bind to HIF-1α. What is more, FIH-1 can hydroxylate Asn803 in the C-TAD domain of HIF-1α subunit, blocking the binding of HIF-1α to p300/CBP, thereby inhibiting the transcriptional activation of HIF-1. Like PHDs, FIH-1 is inhibited when in the hypoxia or the presence of CoCl2, DMOG, Fe2+, etc., and HIF-1α subunits that have not been hydroxylated were successfully combined with p300/CBP, thus activating target gene transcription (Figure 5).57 FIH-1 and PHDs have different requirements for oxygen to maintain the activity. In vitro, the Km of FIH for O2 was about 40% of its atmospheric concentration, being about one-third of those of the PHDs.58 Therefore, PHDs can be inactivated under mild hypoxia (1% −5% O2), while FIH-1 can be inactivated only under severe hypoxia. In addition, researchers have found that pVHL can also regulate FIH-1, and FIH-1 can bind to the β-domain of pVHL.59 In aerobic conditions, Asn803 in C-TAD of HIF-1α is hydroxylated by FIH-1, and the combination of pVHL and FIH-1 can enhance this effect. In moderate hypoxia conditions, PHDs become inactive, which causes the accumulation of HIF-1α. Moreover, the FIH-pVHL complex depolymerized, and it can weaken the binding of FIH-I to C-TAD, thereby partially activating C-TAD. On the other hand, when in severe hypoxic conditions, FIH-1 and PHDs were completely inactivated, so C-TAD is fully activated. This makes us speculate that when the oxygen concentration begins to decrease, the cells first express a series of genes regulated by N-TAD, and when the oxygen concentration is further lower, the genes regulated by C-TAD can be expressed.60
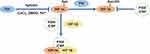 |
Figure 5 Transcriptional activation of HIF-1α. |
Previous studies suggested that only C-TAD needs to recruit the transcription co-activator p300/CBP, so that C-TAD can play its role. However, the subsequent research found that N-TAD mediated transcriptional activities also require the participation of CBP. The difference is that C-TAD is bound to the CH1 region of CBP, while N-TAD is bound to its CH3 region.61 Moreover, MAPK can increase the recruitment of P300/CBP to HIF-1α, because P300 and CBP are direct phosphorylation substrates of MAPK, and MAPK can cause phosphorylation of P300 and CBP. This phosphorylation can have specific effects on the affinity of P300/CBP and different protein factors, resulting in the redistribution of P300/CBP in the complex of interacting factors. In particular, because MAPK signals can stimulate the transcriptional activity of P300/CBP, so it is likely to increase the interaction between P300/CBP and basic transcriptional mechanisms.62 In addition to MAPK, the PI3K/Akt pathway can also indirectly regulate the binding of HIF-1α to p300/CBP. PI3K/Akt can phosphorylate fork-head box O3a (FOXO3a), thereby preventing FOXO3a from entering the nucleus. FOXO3a can interfere with the recruitment of p300 to HIF-1α after entering the nucleus, thereby negatively regulating its transcriptional activation activity.63
Role of HIF-1α in Different Physiological or Pathological States
HIF-1α and Angiogenesis
Angiogenesis is one of the basic events of many physiological processes (eg, embryonic development, and menstrual cycle) and pathological processes (eg, tumor, ischemic disease, chronic inflammation, diabetic retinopathy, endometrial hyperplasia, psoriasis, obesity, and wound healing). HIF-1α plays an important role in ischemia-induced angiogenesis by upregulating gene expression of proteins associated with the vascular system, while the most typical of which is vascular endothelial growth factor (VEGF) and its receptor. HIF-1α can regulate the angiogenic factor angiopoietin-1, angiopoietin-2, placental growth factor (PLGF), platelet-derived growth factor B (PDGF-B) and VEGF, to participate in the whole process of angiogenesis.64 Related studies have confirmed that removing HIF-1α gene or blocking HIF-1α transcription can prevent tumor cells from secreting VEGF and inhibit the formation of tumor neovascularization. With the continuous growth and volume increase of the tumor, hypoxic necrosis occurred due to insufficient blood supply, which induced the overexpression of HIF-1α. Meanwhile, HIF-1α can enhance the expression of its downstream target gene VEGF, and then act on VEGF-R1 and VEGF-R2 on the surface of vascular endothelial cells, thereby promoting angiogenesis and accelerating tumor metastasis.65 In the femoral artery ligation model with HIF-1α gene knockout, it was found that vascular growth factors such as VEGF were not activated and the reperfusion capacity decreased after ischemia.66 In addition, HIF-1α also had a role in promoting angiogenesis in models of myocardial hypertrophy, myocardial infarction, wound healing and retinal angiogenesis.67–69
HIF-1α and Cancer
The rapid proliferation of tumor cells leads to insufficient blood supply, so that tumor cells are often in a hypoxic environment. A large number of literatures have reported that HIF-1α is overexpressed in breast cancer, ovarian cancer, esophageal cancer, colon cancer, lung cancer and other tumor tissues. In addition, HIF-1α expression was significantly higher in multiple metastatic tumors than in primary tumors.70–73 Current studies have found that HIF-1α is involved in transcriptional regulation of more than 70 target genes in various tumor growth processes. Among them, there are four types of gene that are closely related to tumor, which are factors related to glucose transport and glycolysis, factors related to angiogenesis, factors related to tumor proliferation and apoptosis, and factors related to tumor invasion and metastasis.74 They induce a series of responses in cells and tissues to adapt to the hypoxic environment, promoting tumor angiogenesis, and also increasing the invasiveness of the tumor itself and its resistance to chemoradiotherapy.
In tumor cells, HIF-1α can not only promote angiogenesis but also help tumor cells obtain energy by reconstructing the metabolic pathway of cells, among which glycolysis is an important means. First, HIF-1α is able to initiate glucose transporter and lactate dehydrogenase A (LDHA) to mediate pathway conversion to non-oxidative carbon metabolism and ATP-producing pathways such as glycolysis. In addition, PDK protein encoded by pyruvate dehydrogenase kinase-1 (PDK1), another HIF-1α target gene, inhibits the production of acetyl CoA, blocks the tricarboxylic acid cycle, and reduces oxygen consumption. It was found that cells lacking HIF-1α reduced ATP production under hypoxic conditions, producing more oxygen-free radicals and promoting apoptosis.75 In addition to these two pathways, activation of HIF-1α affects the pentose phosphate pathway, which converts the intermediate products of glycolysis into 5-phosphoribose, an important raw material for synthesis of nucleotides.76 These results suggest that HIF-1α promotes cell survival under hypoxic conditions by reconstructing cell metabolic pathways, one of the steps required to convert glucose metabolism into RNA and DNA synthesis, which is important for survival and growth of hypoxic tumor cells.
HIF-1α can also promote the proliferation of tumor cells through the regulation of related factors. For example, HIF-1 can induce the production of factors such as insulin-like growth factor-2 (IGF-2) and transforming factor-2 (TGF-2). These factors activate the MAPK and PI3K pathways through binding to cognate receptors, which not only cause cells hyperplasia but also increase the activity of HIF-1α and accelerate the transcriptional activity of HIF-1α-induced genes. This activity plays an important role in the evolution of tumors.77 In terms of tumor cell apoptosis, the mechanism is more complex. First, many scholars believed that HIF-1α could promote the apoptosis of tumor cells. HIF-1α stabilized p53 by inhibiting p53 ubiquitination and blocking p53 translocation outside the nucleus, thus inducing various apoptotic genes to promote apoptosis.78 In other studies, HIF-1α also had an anti-apoptotic effect. Hypoxia or COCl2 can induce the expression of HIF-1α and inhibit the apoptosis of hepatoma cell line HepG2 caused by tert-butyl hydrogen peroxide or serum depletion.79
In addition to the above aspects, HIF-1α can also enhance the invasion and metastasis of tumor, which are closely related to the matrix metallo-proteinases (MMPs). HIF-1α may increase the expression of MMPs, thus promoting the metastasis of malignant tumors. On the other hand, increased HIF-1α lead to the decreased of epithelial cadherin (E-cad) and β-catenin (β-cat), and the destruction of the E-cad/β-cat complex can reduce the adhesion between cells and matrix, which finally cause cell separation and migration. Further studies also found that multidrug resistance gene 1 (MDR1) and its encoded p-glycoprotein could be increased by HIF-1α, and the high expression of both was one of the main mechanisms of drug resistance in tumors.80,81
HIF-1α and Inflammation
HIF-1α can be detected in inflammatory diseases such as immune inflammation, bacterial infection, macrophage metabolism and viral infection. When an inflammatory response occurs, increased vascular permeability will cause more immune cells to reach the site of inflammation. At the same time, the increased oxygen consumption of inflammatory cells and antigens will lead to the formation of a local hypoxic environment in the inflammatory site due to the slow blood flow, which will induce the immune cells to transcribe HIF-1α.82 The response of immune cells to hypoxia is closely related to the nuclear factor-κb (NF-κb). As a key immune regulator, NF-κb can positively regulate the transcription of HIF-1α mRNA under hypoxia, and it can also activate NF-κb in macrophages, neutrophils, and some non-immune cells, which are not only participants in acute and chronic inflammation but also crucial front-line effectors for innate host defense against invading microbial pathogens. Hypoxia inhibits PHD1 activity and makes HIF-1α accumulation, leading to the activation of inhibitor of NF-κb (Iκb) kinase (IKK), which then phosphorylates NF-κb. Dissociated NF-κb activates transcription of corresponding downstream genes, such as inflammatory cytokines.83
HIF-1α plays an important role in the inflammatory response. It can not only promote the secretion of inflammatory factors but also be regulated by inflammatory factors. Take rheumatoid arthritis (RA), a chronic, autoimmune inflammatory disease characterized by synovial inflammation in the joints, especially the hands and feet, and progressive destruction of these and other joints. IL-1 and TNF-α play important roles in the progression of RA as pro-inflammatory and immune regulators. Stimulated by IL-1 and TNF-α, fibroblast synovial cells in RA patients can promote HIF-1α increase in mRNA and protein levels. Among them, IL-1 can increase the binding of the heterodimer HIF-1 to the HIF consensus sequence.84,85 In addition, IL-33 also can promote the expression of HIF-1α in synovial tissue of RA. The study found that the level of IL-33 in the synovial fluid of RA patients was increased, and the expression of HIF-1α upregulated, while the expression of HIF-1α, in turn, could control the expression of IL-33 through the p38 and ERK pathways, thus forming a closed loop of HIF-1α/IL-33, and aggravating the inflammatory response.86 Meanwhile, the role of VEGF, another downstream target gene of HIF-1α, cannot be ignored in RA. The synovial membrane of RA patients can produce a large amount of HIF-1α, which further stimulates the production of VEGF. During the development of RA, VEGF can promote the formation of new blood vessels in synovium and further aggravate synovial tissue hyperplasia.87
HIF-1α and Physiological Hypoxia
In addition to pathological hypoxia, HIF-1α also plays an important role in the physiological hypoxia of the body. After entering plateau or strenuous exercise, hematopoietic organs increase the oxygen-carrying capacity of the blood by increasing the number of red blood cells, a process mediated by erythropoietin (EPO), the target gene of HIF-1α. EPO is an erythrocyte-specific hematopoietic hormone produced mainly by the kidney and liver. The presence of EPO promotes the production of hemoglobin and red blood cells and is a key factor in regulating the body’s balance in response to hypoxia. When the body is stimulated by hypoxia, the activated HIF-1α binds to the HRE located in the promoter region of the EPO gene, which then triggers the transcription of the EPO gene.88,89 Familial polycythemia is a genetic disorder characterized by an abnormal increase in hemoglobin and red blood cells, which has been shown to be caused by a mutation in VHL that prevents HIF-1α from hypoxia and activates EPO.90,91 In the adaptation of plateau and sea level mice to hypoxia, hypoxia-activated HIF-1α expression in sea level mice but had no significant effect on HIF-1α expression in plateau mice for generations. It means that the adaptive strategies of highland mammals to hypoxia seem to differ genetically from those of lowland mammals by a long evolutionally acclimatization to the hypoxic environment.92
Bioactive Components of Natural Medicine or Traditional Chinese Medicine Prescription
Natural medicines and TCM formulas have multi-target and multi-level therapeutic effects on various diseases due to their various components. After a large number of studies on their active components, they can be roughly divided into flavonoids, quinones, terpenes, polysaccharides and glycosides, etc., based on the chemical structure differences. As a central regulator, HIF-1α is involved in many diseases. On the one hand, overexpression of HIF-1α is closely related to diseases such as solid tumors, and on the other hand, inhibition of HIF-1α is involved in hypoxic-ischemic diseases such as anemia. Therefore, by inhibiting or promoting HIF-1α, it can play a therapeutic role in related diseases. Natural medicine and TCM formulas can affect the content of HIF-1α in the body by regulating the synthesis, degradation and nuclear transport of HIF-1α, thereby playing a therapeutic effect on cancer, inflammation and other diseases.
Inhibition of HIF-1α
Bioactive Components
The pathogenesis of many diseases such as solid tumor, endometriosis, renal interstitial fibrosis and intestinal epithelial barrier dysfunction is closely related to the overexpression of HIF-1α, which threatens human health.93–96 Up to now, researchers have found that they can mainly downregulate HIF-1α in the following ways: 1) block HIF-1α/p300 interactions; 2) decrease the expression of HIF-1α mRNA; 3) reduce stability of HIF-1α protein (Figure 6).97 In modern research, a variety of natural medicines and traditional Chinese medicine prescriptions can achieve a therapeutic effect by reducing the content of HIF-1α. However, it is regrettable that the pathway through which many natural medicines or traditional Chinese medicine prescriptions reduce HIF-1α is unknown, and further research is needed.
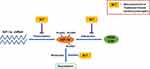 |
Figure 6 Natural medicines and traditional Chinese medicine prescriptions can mainly downregulate HIF-1α in three following ways. |
The known TCMs, such as Curcumae longae Rhizoma (named “Jianghuang” in Chinese), Curcumae Radix (named “Yujin” in Chinese), Curcumae Rhizoma (named “Ezhu” in Chinese), have the effects of promoting blood circulation and promoting qi. In modern pharmacological studies, researchers have found that in addition to the above-mentioned effects, they can also exert anti-inflammatory and anti-tumor effects, all of which are attributed to the curcumin, an active ingredient in these TCMs mentioned above. Curcumin is a diketone compound which is widely used in food industry as a spice and colorant because of its unique aroma and color. In addition, continuous evidences show that anti-inflammatory and anti-tumor efficacy of curcumin is closely related to its inhibitory effect on HIF-1α.98 Curcumin has therapeutic effects on a variety of cancers, in particular for the liver cancer. As mentioned above, local hypoxia, increased angiogenesis, and increased cell adhesions are commonly accompanied by tumors in the body, and interestingly curcumin has a significant effect on these phenomena. First, in the hypoxia-induced HepG2 liver cancer cell model, after co-incubation with curcumin, the transcriptional activity of HIF-1α is inhibited, which in turn reduce the produced HIF-1α. At the same time, in vascular endothelial cells, the expression of HIF-1α and its downstream target gene VEGF will also decrease, inhibiting angiogenesis.12 In thyroid cancer, researchers further found that curcumin can inhibit the mRNA and protein expressions of HIF-1α by reducing the DNA-binding potential of HIF-1α to hypoxia response element under hypoxic conditions. In addition, after co-incubating with K1 papillary thyroid cancer cells, the expression of E-cadherin increased and the activity of metalloproteinase-9 (MMP-9) enzymes decreased. All the above results indicated that curcumin had a strong anti-metastatic effect and could prevent the malignant metastasis of cancer cells.99 With the continuous in-depth study of curcumin’s effects on cancer, researchers have focused their attention on the effect of curcumin on cancer metabolism. After continuous efforts, the researchers were surprised to find that curcumin can also down-regulate pyruvate kinase M2 (PMK2), a key regulator of the Warburg effect, through the mTOR-HIF-1α signaling pathway, and inhibit glucose uptake and lactic acid production in a variety of cancer cell lines.100 In addition to the effective treatment of cancer, curcumin can also exert anti-inflammatory, anti-apoptotic and anti-oxidant effects by inhibiting HIF-1α, which can be used to treat atherosclerosis, hemophilia and other diseases. First, we take atherosclerosis as an example. Atherosclerosis is characterized by accumulation of lipid and fibrous elements and is accompanied by inflammation and immune response in the vascular endometrium. In response to these phenomena, researchers have found that after treatment with curcumin, the total cholesterol and lipid levels in macrophages induced by hypoxia will decrease to varying degrees. At the same time, the expression of HIF-1α will decrease with the inhibition of the ERK signaling pathway, further reducing its downstream target genes such as VEGF and PDGF. In this case, VEGF is responsible for the formation of new blood vessels, while PDGF can cause the proliferation and vasoconstriction of vascular smooth muscle cells and aggravate the disease response. Curcumin can also effectively relieve the inflammation and immune response of the vascular intima during the development of atherosclerosis. For example, curcumin also can inhibit the hypoxia-induced the macrophage apoptosis and the upregulated protein level of inflammation factor, IL-6 and TNF-α. Therefore, all these evidences suggest that curcumin could be a potential drug for treating atherosclerosis.79 Of course, studies have shown that curcumin can also treat hemophilia by inhibiting inflammation and angiogenesis101 (Figure 7). In addition to inhibiting the above factors, it can also inhibit IL-1 and MMP, other mechanisms are shown in Table 1.102 In addition to inhibiting the above factors, it can also inhibit IL-1 and MMP, other mechanisms are shown in Table 1.102 Flavonoids are an important part of natural medicines, and many compounds have the effect of inhibiting HIF-1α. Take isoquercitrin as a simple example. Isoquercitrin is mainly derived from Gossypium herbaceum L. and Apocynum cannabinum Linn. It is a phytoestrogen and a flavonoid. Isoquercitrin has a significant effect on osteoporosis caused by abnormally elevated HIF-1α after menopause. By suppressing NF-κB activation, HIF-1α mRNA levels are reduced, bone histological characteristics are improved, and lumber strength is increased.103 Apigenin, a flavonoid mainly existing in Apium graveolens L., can improve abnormal glucolipid metabolism by reducing the expression of HIF-1α and subsequent up-regulating PPARα-mediated CPT-1 and PDK-4 expressions and down-regulating PPARγ-mediated GPAT and GLUT-4 expressions.104,105
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Table 1 Bioactive Components of Natural Medicine That Can Inhibit HIF-1α |
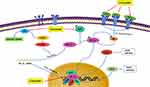 |
Figure 7 Curcumin downregulates the content of HIF-1α. |
In addition to flavonoids, quinones also have inhibitory effect on HIF-1α. Rhei Radix et Rhizoma (named “Dahuang” in Chinese), a known TCM, is mainly used in the treatment of acute intestinal obstruction. Moreover, as a laxative, it has been proven to regulate the contractility of intestinal smooth muscle. However, it has been found in modern studies that the active ingredient emodin, an anthraquinone extracted from Dahuang has the effect of treating intestinal epithelial barrier dysfunction. In the critically ill state, intestinal barrier dysfunction often occurs, involving inflammation and hypoxia damage of intestinal epithelial cells. In certain studies, it has been clearly clarified that NF-κB and HIF-1α signaling pathways will promote the development of this disease. Furthermore, cyclooxygenase (COX) 2 is a key enzyme in the intestinal barrier failure of mouse peritonitis. It is not only controlled by HIF-1α but also depends on the integrity of the NF-κB signaling pathway.106 In order to explore the molecular mechanism of emodin in the treatment of intestinal barrier dysfunction, Caco-2 cells (a human colonic cell line) were co-incubated with lipopolysaccharide (LPS) and treated with hypoxia/reoxygenation to induce barrier dysfunction. In this experiment, the scholars were surprised to find that the paracellular permeability of Caco-2 cells was reduced after emodin treatment, and the levels of NF-κB and HIF-1α protein were significantly reduced, while the damage to cells caused by LPS and hypoxia/reoxygenation has also been alleviated. The above results indicate that emodin may protect the intestinal epithelial barrier dysfunction caused by inflammation and hypoxia by inhibiting HIF-1α and NF-κB signaling pathways.96,107 Subsequent studies on pancreatic cancer cells have shown that in addition to emodin, rhein, another anthraquinone compound in Dahuang, also regulates HIF-1α. In previous studies, we know that Akt and ERK1/2 signaling pathways can induce HIF-1α expression by increasing HIF-1α biosynthesis. In the study on pancreatic cancer, the researchers further clarified that rhein and emodin can reduce the content of HIF-1α regardless of whether it is under normoxia or hypoxia. What is more, this process is achieved by inhibiting Akt and ERK1/2 signaling pathways to reduce the biosynthesis of HIF-1α, rather than by affecting the gene transcription or protein stability of HIF-1α. Besides this, emodin and rhein also can inhibit the growth of cancer cells by inhibiting their proliferation rather than inducing cell death.108 In addition to Dahuang, there are many natural medicines that contain quinones, and they also have inhibitory effects on HIF-1α. For example, Cassiae Semen (named “Juemingzi” in Chinese) is dry, mature seed of the leguminous plant Cassia obtusifoiia L. or Cassia tora L. The quinones extracted from it, obtusifolin can treat choroidal neovascularization by affecting the content of HIF-1α. In previous studies, it has been clarified that hypoxia is an important cause of choroidal neovascularization, and VEGF plays an extremely important role in this disease. In an in vitro hypoxia model of ARPE-19 cells induced by cobalt chloride (CoCl2), the mRNA and protein levels of HIF-1α, VEGF, and VEGFR2 in the cells decreased significantly after obtusifolin treatment, while their transcription and translation processes were inhibited. At the same time, obtusifolin also reduces cell viability under hypoxic conditions and arrests G1 phase cells to treat choroidal neovascularization from many aspects.109
Terpenoids and polysaccharides can be also beneficial for regulation of the content of HIF-1α. Celastrol, also known as tripterine, is a quinine methide triterpenoid compound extracted from the Chinese herb Tripterygium wilfordii HOOK F. It can decrease PPARβ, HIF-1α and elevate p53 in HepG2 cells. At the same time, celastrol inhibits hepatocellular carcinoma survival by inducing cells apoptosis.110 Another diterpene compound, oridonin, derived from the TCM of Rabdosiae rubescentis Herba, has anti-metastatic effect in breast cancer treatment by inhibiting epithelial–mesenchymal transition (EMT) and the HIF-1α/VEGF signaling pathway. After treatment with oridonin, the expression of E-cadherin decreased, while the expression of N-cadherin, Vimentin, Snail, HIF-1 α, VEGF-A and VEGFR2 increased.111 Polysaccharides are a class of macromolecular substances composed of a large number of monosaccharides, some of which have significant inhibitory effects on HIF-1α. For example, fucoidan extracted from Brown seaweed is a kind of sulfated polysaccharide, which has a significant therapeutic effect on mammary cancer. It not only reduces the protein level of HIF-1α but also inhibited the nuclear translocation of HIF-1α. In addition, it inhibits epithelial–mesenchymal transformation by down-regulating N-cadherin and vimentin and up-regulating ZO-1 and E-cadherin.112
Apart from the above compounds, a variety of components that inhibit HIF-1α, including glycosides, peptides, polyphenol and so on. For instance, isoliquiritin apioside, a component isolated from Glycyrrhizae radix et Rhizoma, has been found to exert anti-cancer effects. Isoliquiritin apioside can inhibit matrix metalloproteinase-9 (MMP-9), placental growth factor and VEGF by weakening the HIF-1α pathway, thus playing an anti-angiogenic effect. In addition, it has no significant inhibitory effect on cell proliferation but can suppress cell metastasis by inhibiting the activation of NF- κB.113 Melittin is a kind of polypeptide, which is the main component of bee venom. It can decrease the protein and mRNA levels of HIF-1α, p-Akt, VEGF and MMP-2/9. Besides that, melittin also can restrain EMT and vasculogenic mimicry formation in liver cancer.114 Other bioactive components found at present are shown in Table 1.
Compound Traditional Chinese Medicine Preparation
Compound traditional Chinese medicine (TCM) preparations have been used for thousands of years in Asian countries such as China, Korea, and Japan. With the development of modern medicine, researchers have found that many compound TCM preparations also have an inhibitory effect on the content of HIF-1α. Buyang Huanwu Decoction (BYHW) is a classic prescription for the treatment of stroke. It was first recorded in the literature of Correction on Errors in Medical Works in the Qing Dynasty in 1830. The formula mainly contains seven Chinese herbs, namely: (1) Astragali Radix; (2) Angelicae sinensis Radix; (3) Paeoniaeradix Rubra; (4) Pheretima; (5) Rhizoma Chuanxiong; (6) Carthami Flos; (7) Persicae Semen, while the four components with the highest content in BYHWD are calycosin-7-O-β-D-glucoside, ononin, calycosin and formononetin.115,116 In modern clinical practice, BYHW is widely used in the treatment and prevention of ischemic cardiovascular vascular diseases. Cerebral ischemia and reperfusion may lead to serious consequences such as damage to the blood-brain barrier and brain edema, thereby causing additional loss of brain tissue. Therefore, reducing the permeability of the blood-brain barrier and the severity of cerebral edema is important for the development of cerebral ischemia treatment. In hypoxia or cerebral ischemia, the content of HIF-1α will increase rapidly, promoting the expression of its downstream target genes such as erythropoietin and VEGF, which will increase the vascular permeability and the area of cerebral infarction. In previous studies, it has been known that BYHWD can down-regulate apoptosis-related genes, inhibit inflammation and angiogenesis, and can also up-regulate neurodevelopment-related genes to protect mice from ischemic stroke. But at that time, the specific mechanism was unclear.115 On this basis, after further research, scholars found that after BYHWD was used to treat mice, the HIF-1α/VEGF pathway was inhibited and protein expression decreased. At the same time, the transcription and translation of β-EnaC in the body increase, the blood–brain barrier is effectively protected, the area of brain edema is reduced, and the symptoms of cerebral infarction are alleviated. In summary, we can think that BYHWD can maintain the integrity of the blood-brain barrier and treat ischemic stroke by inhibiting the HIF-1α/VEGF pathway.116
In addition, there are other compound TCM preparations, such as Yi Ai Fang, Shaofu Zhuyu Decoction, and Shengui Sansheng San, which can exert corresponding therapeutic effects by inhibiting HIF-1α. See Table 2 for details.
 |
Table 2 Traditional Chinese Medicine Prescription That Can Inhibit HIF-1α |
Promotion of HIF-1α
Bioactive Components
Currently, many researches have focused on the inhibitory effect of medicine on HIF-1α, while there have been far fewer studies on drugs promoting HIF-1α. Rhodiolae Crenulatae Radix et Rhizoma (Rhodiola), the root and rhizome of Rhodiola crenulata (Hook. f. and Thoms.) H. Ohba has been used as a medicine for thousands of years. Rhodiola is mostly used to enhance the body resistance against hypoxia in mountain sickness, but its mechanism has only been gradually revealed in the past decade. First, it was found that the water extract deriving from Rhodiola can induce the expression of erythropoietin (EPO) in liver and kidney cells, increasing the level of mRNA and protein. As we all know, EPO is a key factor in regulating body balance under hypoxic environment, and it is also an erythrocyte-specific hematopoietic hormone that can increase red blood cell production. Application of water extract deriving from Rhodiola promotes the expression of EPO, so that the body can better adapt to the hypoxic environment.89 Subsequently, the research team discovered that salidroside, a glycoside found in Rhodiola, is the main active substance of Rhodiola against hypoxia. Meanwhile, after using salidroside to co-incubate with human embryonic kidney fibroblasts (HEK293T) and human hepatocellular carcinoma HepG2 cells, the HIF-1α mRNA content did not change significantly, but the protein degradation became less, which eventually led to protein accumulation. The high expression of HIF-1α stimulated the expression of EPO mRNA from its transcription regulatory element HRE, located on EPO gene, which in turn increases the level of EPO mRNA and protein, so that cells can better adapt to the hypoxic environment.117 Interestingly, in the study of osteoblasts, it was discovered that salidroside can accelerate the healing of fractures from both cell-autonomous and non-autonomous aspects by regulating the MAPK/ERK and PI3K/Akt signaling pathways. First, salidroside can improve cell viability by changing the cycle of osteoblasts, and induce the expression of RunX2 and osteoblasts to promote cell differentiation and mineralization. In in vitro experiments, salidroside can increase the transcriptional activity of HIF-1α, promote nuclear translocation, thereby increase the expression of VEGF, induce angiogenesis, and accelerate fracture healing.118 Thus, it can be seen that although salidroside can increase the content of HIF-1α in different models, its effects are not the same. The rest are shown in Table 3.
 |
Table 3 Bioactive Components of Natural Medicine That Can Promote HIF-1α |
The same active ingredient can not only increase the content of HIF-1α in different ways but also produce two distinct effects on the content of HIF-1α in different models. As mentioned in Table 3, tanshinone IIA can inhibit the growth and proliferation of HepG2 cells under hypoxic conditions and induce apoptosis. At the same time, the expression of HIF-1α and VEGF is reduced, while p53 is up-regulated, thereby producing a therapeutic effect on hepatocellular carcinoma.119 However, when studying myocardial infarction model rats, it was found that tanshinone IIA can promote angiogenesis by increasing the mRNA expression of HIF-1α and VEGF, improve heart function, and reduce the infarct size.120 Other bioactive ingredients found so far are shown in Table 3.
Compound Traditional Chinese Medicine Preparation
There are also some compound traditional Chinese medicine preparations that can treat related diseases by increasing the level of HIF-1α. Houshihesan (HSHS) is a classic prescription of traditional Chinese medicine for the treatment of stroke, which has been used safely and effectively in clinical treatment for nearly 2000 years. HSHS consists of the following 13 traditional Chinese herbs: Chrysanthemi Flos, Saposhnikoviae Radix, Cinnamomi Ramulus, Chuanxiong Rhizoma, Asari Radix et Rhizoma, Platycodonis Radix, Atractylodis macrocephalae Rhizoma, Poria, Zingiberis Rhizoma, Angelicae sinensis Radix, Ginseng Radix et Rhizoma, Scutellariae Radix and Ostreae Concha. In the experiment, a permanent middle cerebral artery occlusion model was established in rats. After intragastric administration of HSHS with different doses, the symptoms of angioedema in rats were obviously alleviated, and the damage of blood vessels and neurons in the ischemic area was reduced. At the same time, the results of real-time fluorescent quantitative PCR and Western blot showed that HSHS can significantly promote the expression of HIF-1α, and increase the expression of VEGF and VEGFR2 in rats, while the expression of stromal cell-derived factor-1 (SDF-1) decline. Among them, SDF-1 can specifically bind to the CXC chemokine receptor 4 (CXCR4), guide the migration of endothelial cells, and extend the ischemic blood vessels to areas with sufficient blood supply. In addition, the HSHS drug serum was used in vitro test, and the results showed that HSHS significantly promoted the proliferation and migration of HUVECs under hypoxic conditions. The expression trend of related factors was consistent with the in vivo results. HIF-1α, VEGF, SDF-1 and CXCR4 and other related factors have increased expression.121 Other compound traditional Chinese medicine preparations are shown in Table 4.
 |
Table 4 Compound Traditional Chinese Medicine Preparation That Can Promote HIF-1α |
Conclusion and Perspectives
Taken together, all the collected evidences suggested that natural medicines and TCM formulas would be beneficial for treating various diseases (in particular for the tumors) through regulating HIF-1α. However, most of the studies only focus on the inhibitory effects of natural medicines and TCM formulas on HIF-1α, and just few studies go deep into the way through which they affect a certain step in the synthesis of HIF-1α. Besides that, there are few studies on the promotion effect of HIF-1α, and most of the experimental results are only from animal experiments or in vitro cell experiments, which cannot effectively explain the problem. Therefore, it is recommended that in future research, more attention can be devoted to the promotion of HIF-1α by natural products or TCM formulas, and a combination of in vivo and in vitro methods should be adopted to further study its effect. Recent studies have shown that many natural products can not only affect HIF-1α content by themselves but also reduce the side effects of drugs when used in combination with other marketed drugs. We believe that discovering the effect of natural products on HIF-1α from the perspective of combined medicine is also the future research direction. Although the limited clinical trials are not perfect in quality, they still have certain reference value. In the future, we need more scientific and representative clinical trials.
A variety of active ingredients can be extracted from natural medicines, but in the current research, we have only found that some monomer ingredients can affect the content of HIF-1α, and these ingredients are more concentrated in flavonoids, terpenes and glycosides. So there are more active monomers to be discovered. We hope that this review can provide a scientific basis for existing natural medicines and TCM formulas to affect HIF-1α content, further clarify the market positioning, and provide guidance for the development of new products in the future.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (no. 81503254); Sichuan Major Science and Technology Project (no. 2018TZDZX0007); Research Promotion Plan for Xinglin Scholars in Chengdu University of Traditional Chinese Medicine (no. QNXZ2019018; BSH2018006) and Scientific research project of Sichuan Educational Commission (no. 16ZA0455).
Disclosure
The authors declare no conflicts of interest.
References
1. Burroughs SK, Kaluz S, Wang D, Wang K, Van Meir EG, Wang B. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem. 2013;5(5):553–572. doi:10.4155/fmc.13.17
2. Kruschewski M, Foitzik T, Perez-Cantó A, Hübotter A, Buhr HJ. Changes of colonic mucosal microcirculation and histology in two colitis models: an experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci. 2001;46(11):2336–2343. doi:10.1023/a:1012334727509
3. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–5454. doi:10.1128/mcb.12.12.5447
4. Wilkins SE, Abboud MI, Hancock RL, Schofield CJ. Targeting Protein-Protein Interactions in the HIF System. ChemMedChem. 2016;11(8):773–786. doi:10.1002/cmdc.201600012
5. Guru SK, Pathania AS, Kumar S, et al. Secalonic Acid-D Represses HIF1α/VEGF-Mediated Angiogenesis by Regulating the Akt/mTOR/p70S6K Signaling Cascade. Cancer Res. 2015;75(14):2886–2896. doi:10.1158/0008-5472.CAN-14-2312
6. Chen X, Liu J, He B, et al. Vascular endothelial growth factor (VEGF) regulation by hypoxia inducible factor-1 alpha (HIF1A) starts and peaks during endometrial breakdown, not repair, in a mouse menstrual-like model. Hum Reprod. 2015;30(9):2160–2170. doi:10.1093/humrep/dev156
7. Ren QZ, Qian ZH, Jia SH, Xu ZZ. Vascular endothelial growth factor expression up-regulated by endometrial ischemia in secretory phase plays an important role in endometriosis. Fertil Steril. 2011;95(8):2687–2689. doi:10.1016/j.fertnstert.2011.05.001
8. Soeda A, Park M, Lee D, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28(45):3949–3959. doi:10.1038/onc.2009.252
9. Jing SW, Wang YD, Kuroda M, et al. HIF-1α contributes to hypoxia-induced invasion and metastasis of esophageal carcinoma via inhibiting E-cadherin and promoting MMP-2 expression. Acta Med Okayama. 2012;66(5):399–407. doi:10.18926/AMO/48964
10. Xiong Y, Liu Y, Xiong W, et al. Hypoxia-inducible factor 1α-induced epithelial–mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum Reprod. 2016;31(6):1327–1338. doi:10.1093/humrep/dew081
11. Liu H, Zhang Z, Xiong W, et al. Hypoxia-inducible factor-1α promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction. 2017;153(6):809–820. doi:10.1530/REP-16-0643
12. Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15(6):1557–1562.
13. Xin M, He J, Yang W, Yin X, Wang J. Wenshen Yangxue decoction improves endometrial receptivity recovery and promotes endometrial angiogenesis in a rat model. Pharm Biol. 2018;56(1):573–579. doi:10.1080/13880209.2018.1510973
14. Jögi A, Ehinger A, Hartman L, Alkner S. Expression of HIF-1α is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer. PLoS One. 2019;14(12):e0226150. doi:10.1371/journal.pone.0226150
15. Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36(2):189–204. doi:10.1016/s1357-2725(03)00211-5
16. Duan C. Hypoxia-inducible factor 3 biology: complexities and emerging themes. Am J Physiol Cell Physiol. 2016;310(4):C260–C269. doi:10.1152/ajpcell.00315.2015
17. Pezzuto A, Carico E. Role of HIF-1 in Cancer Progression: novel Insights. A Review. Curr Mol Med. 2018;18(6):343–351. doi:10.2174/1566524018666181109121849
18. Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272(31):19253–19260. doi:10.1074/jbc.272.31.19253
19. Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11(3):63–76. doi:10.1016/j.drup.2008.03.001
20. Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25(48):6373–6383. doi:10.1038/sj.onc.1209889
21. Kuroyanagi G, Tokuda H, Yamamoto N, Matsushima-Nishiwaki R, Kozawa O. Unphosphorylated HSP27 (HSPB1) regulates the translation initiation process via a direct association with eIF4E in osteoblasts. Int J Mol Med. 2015;36(3):881–889. doi:10.3892/ijmm.2015.2274
22. Liu M, Peng P, Wang J, et al. RACK1-mediated translation control promotes liver fibrogenesis. Biochem Biophys Res Commun. 2015;463(3):255–261. doi:10.1016/j.bbrc.2015.05.040
23. Zhang C, Liu XR, Cao YC, et al. Mammalian target of rapamycin/eukaryotic initiation factor 4F pathway regulates follicle growth and development of theca cells in mice. Reprod Fertil Dev. 2017;29(4):768–777. doi:10.1071/RD15230
24. Ayuso MI, Hernández-Jiménez M, Martín ME, Salinas M, Alcázar A. New hierarchical phosphorylation pathway of the translational repressor eIF4E-binding protein 1 (4E-BP1) in ischemia-reperfusion stress. J Biol Chem. 2010;285(45):34355–34363. doi:10.1074/jbc.M110.135103
25. Dreas A, Mikulski M, Milik M, Fabritius CH, Brzózka K, Mitogen-activated Protein RT. Kinase (MAPK) Interacting Kinases 1 and 2 (MNK1 and MNK2) as Targets for Cancer Therapy: recent Progress in the Development of MNK Inhibitors. Curr Med Chem. 2017;24(28):3025–3053.
26. Ruvinsky I, Sharon N, Lerer T, et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19(18):2199–2211. doi:10.1101/gad.351605
27. Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33(11):526–534. doi:10.1016/j.tibs.2008.08.002
28. Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002;13(5):1792–1801. doi:10.1091/mbc.02-02-0017
29. Zhou J, Callapina M, Goodall GJ, Brüne B. Functional integrity of nuclear factor kappaB, phosphatidylinositol 3ʹ-kinase, and mitogen-activated protein kinase signaling allows tumor necrosis factor alpha-evoked Bcl-2 expression to provoke internal ribosome entry site-dependent translation of hypoxia-inducible factor 1alpha. Cancer Res. 2004;64(24):9041–9048. doi:10.1158/0008-5472.CAN-04-1437
30. Schepens B, Tinton SA, Bruynooghe Y, Beyaert R, Cornelis S. The polypyrimidine tract-binding protein stimulates HIF-1alpha IRES-mediated translation during hypoxia. Nucleic Acids Res. 2005;33(21):6884–6894. doi:10.1093/nar/gki1000
31. Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277(36):32405–32408. doi:10.1074/jbc.C200328200
32. Galbán S, Kuwano Y, Pullmann R, et al. RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2008;28(1):93–107. doi:10.1128/MCB.00973-07
33. Taguchi A, Yanagisawa K, Tanaka M, et al. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008;68(14):5540–5545. doi:10.1158/0008-5472.CAN-07-6460
34. Liu LZ, Li C, Chen Q, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One. 2011;6(4):e19139. doi:10.1371/journal.pone.0019139
35. Haque M, Kousoulas KG. The Kaposi’s sarcoma-associated herpesvirus ORF34 protein binds to HIF-1α and causes its degradation via the proteasome pathway. J Virol. 2013;87(4):2164–2173. doi:10.1128/JVI.02460-12
36. Wu D, Cao W, Xiang D, Hu YP, Luo B, Chen P. Exercise induces tissue hypoxia and HIF-1α redistribution in the small intestine. J Sport Health Sci. 2020;9(1):82–89. doi:10.1016/j.jshs.2019.05.002
37. Haque M, Kousoulas KG. The Kaposi’s Sarcoma-Associated Herpesvirus ORF34 Protein Interacts and Stabilizes HIF-2α via Binding to the HIF-2α bHLH and PAS Domains. J Virol. 2019;93(17):e00764–19. doi:10.1128/JVI.00764-19
38. Zhao J, Du F, Shen G, Zheng F, Xu B. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis. 2015;6(1):e1600.
39. Mikawa T, LLeonart ME, Takaori-Kondo A. Dysregulated glycolysis as an oncogenic event. Cell Mol Life Sci. 2015;72(10):1881–1892. doi:10.1007/s00018-015-1840-3
40. Gao S, Lu L, Bai Y, Zhang P, Song W, Duan C. Structural and functional analysis of amphioxus HIFα reveals ancient features of the HIFα family. FASEB J. 2014;28(4):1880–1890. doi:10.1096/fj.12-220152
41. Henze AT, Acker T. Feedback regulators of hypoxia-inducible factors and their role in cancer biology. Cell Cycle. 2010;9(14):2749–2763. doi:10.4161/cc.9.14.12591
42. Willam C, Masson N, Tian YM, et al. Peptide blockade of HIFalpha degradation modulates cellular metabolism and angiogenesis. Proc Natl Acad Sci U S A. 2002;99(16):10423–10428. doi:10.1073/pnas.162119399
43. Schödel J, Grampp S, Maher ER, et al. Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer. Eur Urol. 2016;69(4):646–657. doi:10.1016/j.eururo.2015.08.007
44. Wang W, Xu B, Xuan H, et al. Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg. 2018;68(5):1538–1550. doi:10.1016/j.jvs.2017.09.030
45. Semenza GL. Targeting hypoxia-inducible factor 1 to stimulate tissue vascularization. J Investig Med. 2016;64(2):361–363. doi:10.1097/JIM.0000000000000206
46. Carbia-Nagashima A, Gerez J, Perez-Castro C, et al. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131(2):309–323. doi:10.1016/j.cell.2007.07.044
47. Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131(3):584–595. doi:10.1016/j.cell.2007.08.045
48. Liu YV, Semenza GL. RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle. 2007;6(6):656–659. doi:10.4161/cc.6.6.3981
49. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88. doi:10.1038/s41568-019-0216-7
50. Flügel D, Görlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27(9):3253–3265. doi:10.1128/MCB.00015-07
51. Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci. 2020;77(4):651–663. doi:10.1007/s00018-019-03297-w
52. Hirota K. Basic Biology of Hypoxic Responses Mediated by the Transcription Factor HIFs and its Implication for Medicine. Biomedicines. 2020;8(2):32.
53. Okada N, Ishigami Y, Suzuki T, et al. Importins and exportins in cellular differentiation. J Cell Mol Med. 2008;12(5B):1863–1871. doi:10.1111/j.1582-4934.2008.00437.x
54. Chachami G, Paraskeva E, Mingot JM, Braliou GG, Görlich D, Simos G. Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem Biophys Res Commun. 2009;390(2):235–240. doi:10.1016/j.bbrc.2009.09.093
55. Albadari N, Deng S, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov. 2019;14(7):667–682. doi:10.1080/17460441.2019.1613370
56. Mylonis I, Chachami G, Samiotaki M, et al. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1alpha. J Biol Chem. 2006;281(44):33095–33106. doi:10.1074/jbc.M605058200
57. Yang G, Xu S, Peng L, Li H, Zhao Y, Hu Y. The hypoxia-mimetic agent CoCl2 induces chemotherapy resistance in LOVO colorectal cancer cells. Mol Med Rep. 2016;13(3):2583–2589. doi:10.3892/mmr.2016.4836
58. Flagg SC, Martin CB, Taabazuing CY, Holmes BE, Knapp MJ. Screening chelating inhibitors of HIF-prolyl hydroxylase domain 2 (PHD2) and factor inhibiting HIF (FIH). J Inorg Biochem. 2012;113:25–30. doi:10.1016/j.jinorgbio.2012.03.002
59. Hwang SH, Bang S, Kim W, Chung J. Von Hippel-Lindau tumor suppressor (VHL) stimulates TOR signaling by interacting with phosphoinositide 3-kinase (PI3K). J Biol Chem. 2020;295(8):2336–2347. doi:10.1074/jbc.RA119.011596
60. Li SH, Chun YS, Lim JH, Huang LE, Park JW. von Hippel-Lindau protein adjusts oxygen sensing of the FIH asparaginyl hydroxylase. Int J Biochem Cell Biol. 2011;43(5):795–804. doi:10.1016/j.biocel.2011.02.004
61. Fauquier L, Azzag K, Parra MAM, et al. CBP and P300 regulate distinct gene networks required for human primary myoblast differentiation and muscle integrity. Sci Rep. 2018;8(1):12629. doi:10.1038/s41598-018-31102-4
62. Braicu C, Buse M, Busuioc C, et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers. 2019;11(10):1618. doi:10.3390/cancers11101618
63. Liu X, Cai X, Hu B, et al. Forkhead Transcription Factor 3a (FOXO3a) Modulates Hypoxia Signaling via Up-regulation of the von Hippel-Lindau Gene (VHL). J Biol Chem. 2016;291(49):25692–25705. doi:10.1074/jbc.M116.745471
64. Baldewijns MM, Thijssen VL, Van den Eynden GG, et al. High-grade clear cell renal cell carcinoma has a higher angiogenic activity than low-grade renal cell carcinoma based on histomorphological quantification and qRT-PCR mRNA expression profile. Br J Cancer. 2007;96(12):1888–1895. doi:10.1038/sj.bjc.6603796
65. Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem. 2011;22(7):634–641. doi:10.1016/j.jnutbio.2010.04.015
66. Bosch-Marce M, Okuyama H, Wesley JB, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101(12):1310–1318. doi:10.1161/CIRCRESAHA.107.153346
67. Veeroju S, Mamazhakypov A, Rai N, et al. Effect of p53 activation on experimental right ventricular hypertrophy. PLoS One. 2020;15(6):e0234872. doi:10.1371/journal.pone.0234872
68. Yoshida T, Zhang H, Iwase T, Shen J, Semenza GL, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24(6):1759–1767. doi:10.1096/fj.09-145664
69. Hadjipanayi E, Schilling AF. Hypoxia-based strategies for angiogenic induction: the dawn of a new era for ischemia therapy and tissue regeneration. Organogenesis. 2013;9(4):261–272. doi:10.4161/org.25970
70. Cha ST, Chen PS, Johansson G, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis [published correction appears in Cancer Res. 2019 Jul 15;79(14):3790]. Cancer Res. 2010;70(7):2675–2685. doi:10.1158/0008-5472.CAN-09-2448
71. Baba Y, Nosho K, Shima K, et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176(5):2292–2301. doi:10.2353/ajpath.2010.090972
72. Ioannou M, Papamichali R, Kouvaras E, et al. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor in biopsies of small cell lung carcinoma. Lung. 2009;187(5):321–329. doi:10.1007/s00408-009-9169-z
73. Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer [published correction appears in Cancer Res. 2009 Jul 15;69(14):6005]. Cancer Res. 2009;69(11):4708–4715. doi:10.1158/0008-5472.CAN-08-4417
74. Dang DT, Chun SY, Burkitt K, et al. Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition. Cancer Res. 2008;68(6):1872–1880. doi:10.1158/0008-5472.CAN-07-1589
75. Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab. 2006;3(3):150–151. doi:10.1016/j.cmet.2006.02.007
76. Zhao F, Mancuso A, Bui TV, et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene. 2010;29(20):2962–2972. doi:10.1038/onc.2010.67
77. Griffiths EA, Pritchard SA, McGrath SM, et al. Increasing expression of hypoxia-inducible proteins in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Br J Cancer. 2007;96(9):1377–1383. doi:10.1038/sj.bjc.6603744
78. Patterson DM, Gao D, Trahan DN, et al. Effect of MDM2 and vascular endothelial growth factor inhibition on tumor angiogenesis and metastasis in neuroblastoma. Angiogenesis. 2011;14(3):255–266. doi:10.1007/s10456-011-9210-8
79. Menrad H, Werno C, Schmid T, et al. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology. 2010;51(6):2183–2192. doi:10.1002/hep.23597
80. Liu L, Sun L, Zhang H, et al. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers occurs via hypoxia-inducible-factor 1-dependent mechanism and contributes to drug resistance. Int J Cancer. 2009;124(7):1707–1715. doi:10.1002/ijc.24135
81. Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi:10.1084/jem.20100587
82. Qiu Y, Huang X, He W. The regulatory role of HIF-1 in tubular epithelial cells in response to kidney injury. Histol Histopathol. 2020;35(4):321–330. doi:10.14670/HH-18-182
83. Nakayama K, Kataoka N. Regulation of Gene Expression under Hypoxic Conditions. Int J Mol Sci. 2019;20(13):3278. doi:10.3390/ijms20133278
84. Westra J, Brouwer E, Bos R, et al. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Ann N Y Acad Sci. 2007;1108:340–348. doi:10.1196/annals.1422.035
85. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi:10.1038/nature01661
86. Hu F, Shi L, Mu R, et al. Hypoxia-inducible factor-1α and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis [published correction appears in PLoS One. 2013;8(8). 10.1371/annotation/f61a5d49-25e7-47ce-8509-6478df526886]. PLoS One. 2013;8(8):e72650. doi:10.1371/journal.pone.0072650
87. Konisti S, Kiriakidis S, Paleolog EM. Hypoxia–a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8(3):153–162. doi:10.1038/nrrheum.2011.205
88. Dame C, Kirschner KM, Bartz KV, Wallach T, Hussels CS, Scholz H. Wilms tumor suppressor, Wt1, is a transcriptional activator of the erythropoietin gene. Blood. 2006;107(11):4282–4290. doi:10.1182/blood-2005-07-2889
89. Zheng KY, Guo AJ, Bi CW, et al. The extract of Rhodiolae Crenulatae Radix et Rhizoma induces the accumulation of HIF-1α via blocking the degradation pathway in cultured kidney fibroblasts. Planta Med. 2011;77(9):894–899. doi:10.1055/s-0030-1250627
90. Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R977–R988. doi:10.1152/ajpregu.00577.2003
91. Lee FS. Genetic causes of erythrocytosis and the oxygen-sensing pathway. Blood Rev. 2008;22(6):321–332. doi:10.1016/j.blre.2008.04.003
92. Chen XQ, Wang SJ, Du JZ, Chen XC. Diversities in hepatic HIF-1, IGF-I/IGFBP-1, LDH/ ICD, and their mRNA expressions induced by CoCl(2) in Qinghai-Tibetan plateau mammals and sea level mice. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R516–R526. doi:10.1152/ajpregu.00397.2006
93. An Q, Shi CX, Guo H, et al. Development and characterization of octreotide-modified curcumin plus docetaxel micelles for potential treatment of non-small-cell lung cancer. Pharm Dev Technol. 2019;24(9):1164–1174. doi:10.1080/10837450.2019.1647236
94. Zhu G, Jiang C, Yan X, Zhao S, Xu D, Cao Y. Shaofu Zhuyu Decoction Regresses Endometriotic Lesions in a Rat Model. Evid Based Complement Alternat Med. 2018;2018:3927096. doi:10.1155/2018/3927096
95. Wang Y, Zhang L, Jin H, Wang D. Based on HIF-1α/Wnt/β-Catenin Pathway to Explore the Effect of Qingshen Granules on Chronic Renal Failure Patients: A Randomized Controlled Trial. Evid Based Complement Alternat Med. 2019;2019:7656105. doi:10.1155/2019/7656105
96. Lei Q, Qiang F, Chao D, et al. Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways. Int J Mol Med. 2014;34(6):1629–1639. doi:10.3892/ijmm.2014.1965
97. Li Z, You Q, Zhang X. Small-Molecule Modulators of the Hypoxia-Inducible Factor Pathway: development and Therapeutic Applications. J Med Chem. 2019;62(12):5725–5749. doi:10.1021/acs.jmedchem.8b01596
98. Hu Y, Wang S, Wu X, et al. Chinese herbal medicine-derived compounds for cancer therapy: a focus on hepatocellular carcinoma. J Ethnopharmacol. 2013;149(3):601–612. doi:10.1016/j.jep.2013.07.030
99. Tan C, Zhang L, Cheng X, et al. Curcumin inhibits hypoxia-induced migration in K1 papillary thyroid cancer cells.Experimental Biology and Medicine. 2015;240(7):925–935. doi:10.1177/1535370214555665
100. Siddiqui FA, Prakasam G, Chattopadhyay S. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci Rep. 2018;8(1):8323. doi:10.1038/s41598-018-25524-3
101. Ouyang S, Yao YH, Zhang ZM, Liu JS, Xiang H. Curcumin inhibits hypoxia inducible factor-1α-induced inflammation and apoptosis in macrophages through an ERK dependent pathway. Eur Rev Med Pharmacol Sci. 2019;23(4):1816–1825. doi:10.26355/eurrev_201902_17145
102. Norooznezhad F, Rodriguez-Merchan EC, Asadi S, Norooznezhad AH. Curcumin: hopeful treatment of hemophilic arthropathy via inhibition of inflammation and angiogenesis. Expert Review of Hematology. 2020;13(1):5–11. doi:10.1080/17474086.2020.1685867
103. Fayed HA, Barakat BM, Elshaer SS, Abdel-Naim AB, Menze ET. Antiosteoporotic activities of isoquercitrin in ovariectomized rats: role of inhibiting hypoxia inducible factor-1 alpha. Eur J Pharmacol. 2019;865:172785. doi:10.1016/j.ejphar.2019.172785
104. Wang L, Ma Q. Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacol Ther. 2018;190:105–127. doi:10.1016/j.pharmthera.2018.05.006
105. Zhu Z-Y, Wang F, Jia C-H, Xie M-L. Apigenin-induced HIF-1α inhibitory effect improves abnormal glucolipid metabolism in AngⅡ/hypoxia-stimulated or HIF-1α-overexpressed H9c2 cells. Phytomedicine. 2019;62:152713. doi:10.1016/j.phymed.2018.10.010
106. Fitzpatrick SF, Tambuwala MM, Bruning U, et al. An Intact Canonical NF-κB Pathway Is Required for Inflammatory Gene Expression in Response to Hypoxia. J Immunol. 2011;186(2):
107. Xiao W-D, Chen W, Sun L-H, Wang W-S, Zhou S-W, Yang H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci. 2011;46(2):527–534. doi:10.1016/j.mcn.2010.12.007
108. Hu L, Cui R, Liu H, Wang F. Emodin and rhein decrease levels of hypoxia-inducible factor-1α in human pancreatic cancer cells and attenuate cancer cachexia in athymic mice carrying these cells. Oncotarget. 2017;8(50):88008–88020. doi:10.18632/oncotarget.21330
109. Shen S. Inhibition of Obtusifolin on retinal pigment epithelial cell growth under hypoxia. Int J Ophthalmol. 2019;12(10):1539–1547. doi:10.18240/ijo.2019.10.04
110. Zhang D, Xu L, Cao F, et al. Celastrol regulates multiple nuclear transcription factors belonging to HSP90’s clients in a dose- and cell type-dependent way. Cell Stress Chaperones. 2010;15(6):939–946. doi:10.1007/s12192-010-0202-1
111. Li C, Wang Q, Shen S, Wei X, Li G. Oridonin inhibits VEGF-A-associated angiogenesis and epithelial-mesenchymal transition of breast cancer in vitro and in vivo. Oncol Lett. 2018;16(2):2289–2298. doi:10.3892/ol.2018.8943
112. Li W, Xue D, Xue M, et al. Fucoidan inhibits epithelial-to-mesenchymal transition via regulation of the HIF-1α pathway in mammary cancer cells under hypoxia. Oncol Lett. 2019;18(1):330–338. doi:10.3892/ol.2019.10283
113. Kim A, Ma JY. Isoliquiritin Apioside Suppresses in vitro Invasiveness and Angiogenesis of Cancer Cells and Endothelial Cells. Front Pharmacol. 2018;9:1455. doi:10.3389/fphar.2018.01455
114. Chen Q, Lin W, Yin Z, et al. Melittin Inhibits Hypoxia-Induced Vasculogenic Mimicry Formation and Epithelial-Mesenchymal Transition through Suppression of HIF-1α/Akt Pathway in Liver Cancer. Evid Based Complement Alternat Med. 2019;2019:9602935. doi:10.1155/2019/9602935
115. Wang HW, Liou KT, Wang YH, et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011;138(1):22–33. doi:10.1016/j.jep.2011.06.033
116. Gong X, Guo Q, Zhao H. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 α, VEGF and promotion β-ENaC expression. J Ethnopharmacol. 2019;228:70–81. doi:10.1016/j.jep.2018.09.017
117. Zheng KY, Zhang ZX, Guo AJ, et al. Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol. 2012;679(1–3):34–39. doi:10.1016/j.ejphar.2012.01.027
118. Guo XQ, Qi L, Yang J, et al. Salidroside accelerates fracture healing through cell-autonomous and non-autonomous effects on osteoblasts. Cell Tissue Res. 2017;367(2):197–211. doi:10.1007/s00441-016-2535-2
119. Li L, Wu LF, Wei D. Effects of tanshinone IIA on proliferation, apoptosis and expression of HIF-1α, VEGF and wild-type P53 in human hepatoma Hep G2 cells under hypoxia. Chin j Pathophysiol. 2014;30:2155–2160.
120. Wei B, Li WW, Ji J, Hu QH, Ji H. The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis. 2014;235(2):318–327. doi:10.1016/j.atherosclerosis.2014.05.924
121. Xiang Y, Yao X, Wang X, et al. Houshiheisan promotes angiogenesis via HIF-1α/VEGF and SDF-1/CXCR4 pathways: in vivo and in vitro. Biosci Rep. 2019;39(10):BSR20191006. doi:10.1042/BSR20191006
122. Jang H. Hovenia dulcis Thunb. and its active compound ampelopsin inhibit angiogenesis through suppression of VEGFR2 signaling and HIF-1α expression. Oncol Rep. 2017;38(6):3430–3438. doi:10.3892/or.2017.6021
123. Zhang Y, Chen C, Zhang J. Effects and significance of formononetin on expression levels of HIF-1α and VEGF in mouse cervical cancer tissue. Oncol Lett. 2019;18(3):2248–2253. doi:10.3892/ol.2019.10567
124. Xing Y, Mi C, Wang Z, et al. Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1α and STAT3. Pharmacol Res. 2018;135:166–180. doi:10.1016/j.phrs.2018.08.004
125. Li S, Li J, Dai W, et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer. 2017;117(10):1518–1528. doi:10.1038/bjc.2017.323
126. Zhang J, Su H, Li Q, Li J, Zhao Q. Genistein decreases A549 cell viability via inhibition of the PI3K/AKT/HIF-1α/VEGF and NF-κB/COX-2 signaling pathways. Mol Med Rep. 2017;15(4):2296–2302. doi:10.3892/mmr.2017.6260
127. Kim GD. Kaempferol Inhibits Angiogenesis by Suppressing HIF-1α and VEGFR2 Activation via ERK/p38 MAPK and PI3K/Akt/mTOR Signaling Pathways in Endothelial Cells [published correction appears in Prev Nutr Food Sci. 2018 Mar;23 (1): 84–85]. Prev Nutr Food Sci. 2017;22(4):320–326. doi:10.3746/pnf.2017.22.4.320
128. Wang J, Gao T, Wang F, Xue J, Ye H, Xie M. Luteolin improves myocardial cell glucolipid metabolism by inhibiting hypoxia inducible factor-1α expression in angiotensin II/hypoxia-induced hypertrophic H9c2 cells. Nutr Res. 2019;65:63–70. doi:10.1016/j.nutres.2019.02.004
129. Jin H, Lian N, Bian M, et al. Oroxylin A prevents alcohol-induced hepatic steatosis through inhibition of hypoxia inducible factor 1alpha. Chem Biol Interact. 2018;285:14–20. doi:10.1016/j.cbi.2018.02.025
130. Feng J, Zhang XL, Li YY, Cui YY, Chen YH. Pinus massoniana Bark Extract: structure-Activity Relationship and Biomedical Potentials. Am J Chin Med. 2016;44(8):1559–1577. doi:10.1142/S0192415X16500877
131. Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 1999;47(2):490–496. doi:10.1021/jf980760h
132. Feng J, Wang C, Liu T, et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J Cell Mol Med. 2019;23(9):6479–6493. doi:10.1111/jcmm.14543
133. Lee DH, Lee YJ. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1alpha (HIF-1alpha) through inhibiting protein synthesis. J Cell Biochem. 2008;105(2):546–553. doi:10.1002/jcb.21851
134. Ansó E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martínez-Irujo JJ. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol. 2010;79(11):1600–1609. doi:10.1016/j.bcp.2010.02.004
135. Wang K, Liu R, Li J, et al. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy. 2011;7(9):966–978. doi:10.4161/auto.7.9.15863
136. Li W, Cao L, Chen X, Lei J, Ma Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol Rep. 2016;35(3):1718–1726. doi:10.3892/or.2015.4504
137. Wang Y, Tan X, Li S, Yang S. The total flavonoid of Eucommia ulmoides sensitizes human glioblastoma cells to radiotherapy via HIF-α/MMP-2 pathway and activates intrinsic apoptosis pathway. Onco Targets Ther. 2019;12:5515–5524. doi:10.2147/OTT.S210497
138. Xie T, Wang JR, Dai CG, Fu XA, Dong J, Huang Q. Vitexin, an inhibitor of hypoxia-inducible factor-1α, enhances the radiotherapy sensitization of hyperbaric oxygen on glioma. Clin Transl Oncol. 2020;22(7):1086–1093. doi:10.1007/s12094-019-02234-4
139. Wang H, Zhao L, Zhu LT, et al. Wogonin reverses hypoxia resistance of human colon cancer HCT116 cells via downregulation of HIF-1α and glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol Carcinog. 2014;53:E107–E118. doi:10.1002/mc.22052
140. Prangsaengtong O, Jantaree P, Lirdprapamongkol K, Svasti J, Koizumi K. Shikonin Suppresses Lymphangiogenesis via NF-κB/HIF-1α Axis Inhibition. Biol Pharm Bull. 2018;41(11):1659–1666. doi:10.1248/bpb.b18-00329
141. Zhou Y, Xu Q, Shang J, Lu L, Chen G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol. 2019;234(10):17876–17885. doi:10.1002/jcp.28418
142. Wen Z, Huang C, Xu Y, et al. α-Solanine inhibits vascular endothelial growth factor expression by down-regulating the ERK1/2-HIF-1α and STAT3 signaling pathways. Eur J Pharmacol. 2016;771:93–98. doi:10.1016/j.ejphar.2015.12.020
143. Wang H, Zhang C, Xu L, et al. Bufalin suppresses hepatocellular carcinoma invasion and metastasis by targeting HIF-1α via the PI3K/AKT/mTOR pathway. Oncotarget. 2016;7(15):20193–20208. doi:10.18632/oncotarget.7935
144. Li Y, Zheng JY, Liu JQ, et al. Succinate/NLRP3 Inflammasome Induces Synovial Fibroblast Activation: therapeutical Effects of Clematichinenoside AR on Arthritis. Front Immunol. 2016;7:532. doi:10.3389/fimmu.2016.00532
145. Zhao P, Han SN, Arumugam S, et al. Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am J Physiol Gastrointest Liverv. 2019;317(4):G387–G397. doi:10.1152/ajpgi.00054.2019
146. Zhao R, Sun L, Lin S, et al. The saponin monomer of dwarf lilyturf tuber, DT-13, inhibits angiogenesis under hypoxia and normoxia via multi-targeting activity. Oncol Rep. 2013;29(4):1379–1386. doi:10.3892/or.2013.2272
147. Li J, Yang YL, Li LZ, et al. Succinate accumulation impairs cardiac pyruvate dehydrogenase activity through GRP91-dependent and independent signaling pathways: therapeutic effects of ginsenoside Rb1. Biochim Biophys Acta Mol Basis Dis. 2017;1863(11):2835–2847. doi:10.1016/j.bbadis.2017.07.017
148. Liu T, Zhao L, Hou H, Ding L, Chen W, Li X. Ginsenoside 20(S)-Rg3 suppresses ovarian cancer migration via hypoxia-inducible factor 1 alpha and nuclear factor-kappa B signals. Tumour Biol. 2017;39(5):1010428317692225. doi:10.1177/1010428317692225
149. Liu T, Zhao L, Zhang Y, et al. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 2014;9(9):e103887. doi:10.1371/journal.pone.0103887
150. He S, Lu G, Hou H, et al. Saikosaponin-d suppresses the expression of cyclooxygenase-2 through the phospho-signal transducer and activator of transcription 3/hypoxia-inducible factor-1α pathway in hepatocellular carcinoma cells. Mol Med Rep. 2014;10(5):2556–2562. doi:10.3892/mmr.2014.2574
151. Law PC, Auyeung KK, Chan LY, Ko JK. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement Altern Med. 2012;12:160. doi:10.1186/1472-6882-12-160
152. Jia LY, Wu XJ, Gao Y, et al. Inhibitory Effects of Total Triterpenoid Saponins Isolated from the Seeds of the Tea Plant (Camellia sinensis) on Human Ovarian Cancer Cells. Molecules. 2017;22(10):1649. doi:10.3390/molecules22101649
153. Tyszka-Czochara M, Bukowska-Strakova K, Kocemba-Pilarczyk KA, Majka M. Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis while Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines. Nutrients. 2018;10(7):841. doi:10.3390/nu10070841
154. Luo LX, Li Y, Liu ZQ, et al. Honokiol Induces Apoptosis, G1 Arrest, and Autophagy in KRAS Mutant Lung Cancer Cells. Front Pharmacol. 2017;8:199. doi:10.3389/fphar.2017.00199
155. Ma J, Xue M, Zhang S, et al. Resveratrol inhibits the growth of tumor cells under chronic stress via the ADRB-2-HIF-1α axis. Oncol Rep. 2019;41(2):1051–1058. doi:10.3892/or.2018.6894
156. Lin S, Tsai SC, Lee CC, Wang BW, Liou JY, Shyu KG. Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Mol Pharmacol. 2004;66(3):612–619.
157. Mao L, Chen Q, Gong K, et al. Berberine decelerates glucose metabolism via suppression of mTOR-dependent HIF-1α protein synthesis in colon cancer cells. Oncol Rep. 2018;39(5):2436–2442. doi:10.3892/or.2018.6318
158. Carrasco-Pozo C, Tan KN, Rodriguez T, Avery VM. The Molecular Effects of Sulforaphane and Capsaicin on Metabolism upon Androgen and Tip60 Activation of Androgen Receptor. Int J Mol Sci. 2019;20(21):5384. doi:10.3390/ijms20215384
159. Zhang F, Lu S, He J, et al. Ligand Activation of PPARγ by Ligustrazine Suppresses Pericyte Functions of Hepatic Stellate Cells via SMRT-Mediated Transrepression of HIF-1α. Theranostics. 2018;8(3):610–626. doi:10.7150/thno.22237
160. Xia Y, Kang TW, Jung YD, Zhang C, Lian S. Sulforaphane Inhibits Nonmuscle Invasive Bladder Cancer Cells Proliferation through Suppression of HIF-1α-Mediated Glycolysis in Hypoxia. J Agric Food Chem. 2019;67(28):7844–7854. doi:10.1021/acs.jafc.9b03027
161. Chen YL, Zheng YY, Dai YC, Zhang YL, Tang ZP. Systems pharmacology approach reveals protective mechanisms of Jian-Pi Qing-Chang decoction on ulcerative colitis. World J Gastroenterol. 2019;25(21):2603–2622. doi:10.3748/wjg.v25.i21.2603
162. Yang W, Zhang L, Chen S, et al. Longshengzhi Capsules Improve Ischemic Stroke Outcomes and Reperfusion Injury via the Promotion of Anti-Inflammatory and Neuroprotective Effects in MCAO/R Rats. Evid Based Complement Alternat Med. 2020;2020:9654175. doi:10.1155/2020/9654175
163. Luo, Zheng C. Shengui Sansheng San extraction is an angiogenic switch via regulations of AKT/mTOR, ERK1/2 and Notch1 signal pathways after ischemic stroke. Phytomedicine. 2018;44:20–31. doi:10.1016/j.phymed.2018.04.025
164. Li YN, Wang XJ, Li B, et al. Tongxinluo inhibits cyclooxygenase-2, inducible nitric oxide synthase, hypoxia-inducible factor-2α/vascular endothelial growth factor to antagonize injury in hypoxia-stimulated cardiac microvascular endothelial cells. Chin Med J. 2015;128(8):1114–1120. doi:10.4103/0366-6999.155119
165. Zeng J, Yan R, Pan H, et al. Weipixiao attenuate early angiogenesis in rats with gastric precancerous lesions. BMC Complement Altern Med. 2018;18(1):250. doi:10.1186/s12906-018-2309-3
166. Yi J, Yang J, et al. Weiqi Decoction Attenuated Chronic Atrophic Gastritis with Precancerous Lesion through Regulating Microcirculation Disturbance and HIF-1α Signaling Pathway. Evid Based Complement Alternat Med. 2019;2019:2651037. doi:10.1155/2019/2651037
167. Pang HQ, Yue SJ, Tang YP, et al. Integrated Metabolomics and Network Pharmacology Approach to Explain Possible Action Mechanisms of Xin-Sheng-Hua Granule for Treating Anemia. Front Pharmacol. 2018;9:165. doi:10.3389/fphar.2018.00165
168. Shi X, Tang Y, Zhu H, et al. Comparative tissue distribution profiles of five major bio-active components in normal and blood deficiency rats after oral administration of Danggui Buxue Decoction by UPLC-TQ/MS. J Pharm Biomed Anal. 2014;88:207–215. doi:10.1016/j.jpba.2013.08.043
169. Hou F, Li W, Shi Q, et al. Yi Ai Fang, a traditional Chinese herbal formula, impacts the vasculogenic mimicry formation of human colorectal cancer through HIF-1α and epithelial mesenchymal transition. BMC Complement Altern Med. 2016;16(1):428. doi:10.1186/s12906-016-1419-z
170. Zhang LY, Ou M, Huang YZ, Qiao YY, Zhang DJ. Effect of Yifei Huoxue Granule on the proliferation of rat pulmonary artery smooth muscle cells upon exposure to chronic hypoxic conditions in vitro. Chin J Integr Med. 2012;18(7):507–513. doi:10.1007/s11655-012-1150-7
171. Zhang L, Liu Q, Lu L, Zhao X, Gao X. Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1α accumulation via phosphatidylinositol 3-kinase/Akt pathway. J Pharmacol Exp Ther. 2011;338(2):485–491. doi:10.1124/jpet.111.180992
172. Li Q, Liang X, Yang Y, Zeng X, Zhong X, Huang C. Panax notoginseng saponins ameliorate cisplatin-induced mitochondrial injury via the HIF-1α/mitochondria/ROS pathway. FEBS Open Bio. 2020;10(1):118–126. doi:10.1002/2211-5463.12760
173. Zhang JP, Liu AH, Hou RR, et al. Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur J Pharmacol. 2009;607(1):6–14. doi:10.1016/j.ejphar.2009.01.046
174. Wang Z, Zhang Z, Zhao J, Yong C, Mao Y. Polysaccharides from Enteromorpha Prolifera Ameliorate Acute Myocardial Infarction in Vitro and in Vivo via Up-Regulating HIF-1α. Int Heart J. 2019;60(4):964–973. doi:10.1536/ihj.18-519
175. Dai GH, Liu N, Zhu JW, et al. Qi-Shen-Yi-Qi Dripping Pills Promote Angiogenesis of Ischemic Cardiac Microvascular Endothelial Cells by Regulating MicroRNA-223-3p Expression. Evid Based Complement Alternat Med. 2016;2016:5057328. doi:10.1155/2016/5057328
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

