Back to Journals » Drug Design, Development and Therapy » Volume 13
Ginsenoside Rb1 pretreatment reverses hippocampal changes in BDNF/TrkB mRNA and protein in rats subjected to acute immobilization stress
Authors Kang X , Hong W , Xie K, Tang H, Tang J, Luo S, Geng W, Jia D
Received 12 January 2019
Accepted for publication 14 May 2019
Published 1 July 2019 Volume 2019:13 Pages 2127—2134
DOI https://doi.org/10.2147/DDDT.S201135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Xianhui Kang,1,2,* Wandong Hong3,*, Kangjie Xie,4 Hongli Tang,1 Jingjing Tang,1 Shan Luo,1 Wujun Geng,1 Danyun Jia1
1Department of Anesthesiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325000, People’s Republic of China; 2Department of Anesthesiology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China; 3Department of Gastroenterology and Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325000, People’s Republic of China; 4Department of Anesthesiology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang 310022, People’s Republic of China
*These authors contributed equally to this work
Purpose: Episodes of acute emotional or physical stress can have significant adverse effects on the hippocampus. Ginsenoside Rb1, the most predominant ginsenoside present in Panax species, has been reported to show a neuroprotective effect. The purpose of this study was to investigate the influence of ginsenoside Rb1 on plasma corticosterone (CORT) and adrenocorticotropic hormone (ACTH) levels and hippocampal brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) levels in rats subjected to acute immobilization stress.
Methods: Wistar rats were divided into controls treated with saline only (N), rats exposed to stress only (M), and rats pretreated with Rb1 (40 mg.kg (−1)) thirty minutes prior to stress exposure (R). In the model, animals were restrained in a plastic immobilizer for 2 h of acute immobilization stress at room temperature. ELISA was used to determine plasma levels of CORT and ACTH. The effect of Rb1 pretreatment on the expression of BDNF and TrkB was determined by immunofluorescence, real-time PCR, and Western blotting analysis.
Results: The R group showed significantly increased plasma CORT and ACTH levels compared to the N and M groups. Acute stress stimulation suppressed BDNF and TrkB protein and mRNA expression in the hippocampus; otherwise, Rb1 pretreatment reversed the decreases.
Conclusion: The results from this study demonstrate that Rb1 pretreatment reverses the decreases in hippocampal BDNF/TrkB and increases the plasma levels of CORT and ACTH, indicating a potential neuroprotective effect of Rb1 against acute stress.
Keywords: ginsenoside Rb1, CORT; ACTH, acute immobilization stress, BDNF, TrkB
Introduction
The response of an animal or human to acute stress is characterized by neuronal and hormonal changes. Brain-derived neurotrophic factor (BDNF) belongs to the neurotrophin family and plays an important role in neurons and neurogliocytes. Most of the neural effects of BDNF are mediated by binding to its specific high-affinity receptor, tyrosine kinase B (TrkB), through ligand-induced dimerization, phosphorylation, and activation.1 The up/downregulation of BDNF and TrkB were affected by the stimulus paradigm, which might reflect important mechanisms by which the hippocampus copes with stressful stimuli.2 The expression of BDNF in the hippocampus is dramatically downregulated in response to acute immobilization stress.2,3 Our previous studies have shown that the expression of TrkB mRNA in the hippocampus decreased significantly under acute stress conditions. The changes in BDNF and TrkB affected the outgrowth, survival, differentiation, maintenance, and protection of function in different neuronal populations.1,4
The hormonal changes are triggered by the adverse stimulus and enable the organism to cope more effectively with the stressful situation before returning to normal homeostasis.5 Activating the hypothalamic-pituitary-adrenal (HPA) axis promotes a major neuroendocrine response during stress.6 Corticotropin-releasing hormone (CRH) is secreted by the paraventricular nucleus (PVN) of the hypothalamus, and a population of anterior pituitary cells subsequently release ACTH.7 Furthermore, ACTH stimulates the adrenal glands to release CORT, which targets the hippocampus, a region of the brain that is intrinsically linked to learning and memory, and interacts BDNF, a neurotrophic factor expressed at high levels in the hippocampus.6,7
Ginseng refers to the roots of species of the genus Panax, which has been used as a typical tonic for 2000 years in Far East countries and has also gained popularity in the West during the past decade.8 Researchers have found that ginseng has a wide range of therapeutic and pharmacological uses and now focus on using purified individual ginsenosides to reveal the mechanism underlying its function.9 Numerous studies have demonstrated that ginsenoside Rb1, one of the main ingredients of ginseng root, exerts diverse neuromodulatory effects.10
In this study, we therefore examined whether pretreatment with Rb1 affects the expression of BDNF and its receptor TrkB within the rat hippocampus under conditions of acute immobilization stress. Moreover, to analyze the levels of the hormones CORT and ACTH in plasma, we investigated their concentrations before, immediately after and 30 min after modeling.
Materials and methods
Animals
Male Sprague-Dawley rats weighing approximately 240–260 g were used in this study and were obtained from Shanghai Slac Laboratory Animal Co. (Shanghai, China). Four rats were housed in each polycarbonate cage equipped with an SPF (specific pathogen free) barrier system to acclimate to the laboratory conditions for at least 1 week. The system automatically maintained proper temperature (22±2 °C) and humidity (50±15%). Cages were lit by artificial light for 12 h each day. The bedding in the cages was regularly changed once every two days, and sterilized drinking water and standard food were supplied ad libitum. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies and approved by Wenzhou Medical University Animal Ethics Committee.
Chemicals and reagents
Ginsenoside Rb1 was purchased from Shanghai Tauto Biotech Co (purity >98%, Shanghai, China). Anti-BDNF antibody (rabbit monoclonal to BDNF, clone ID: EPR1292) was purchased from Epitomics Co. (Epitomics, USA), and anti-TrkB antibody (rabbit polyclonal to TrkB, Cat No: BS- 1431) was purchased from Bioworld Technology (Bioworld, China). An ACTH and CORT enzyme-linked immunosorbent assay (ELISA) kit was purchased from Shanghai Westang Biotech INC (Westang Biotech, Shanghai, China). TRIzol reagent (Invitrogen, Cat. 15596, USA), a reverse transcription system (Bio-Serve Cat No: BS-PCR005) and a PCR amplification kit (Bio-Serve Cat No: BS-PCR003) were used in the experiment. A BCA Protein Assay Kit was purchased from Biyuntian (p0012, Biyuntian, China). DyLight 488 goat anti-rabbit IgG (H+L) (E03222, USA), DAPI Staining Solution (C1005, Biyuntian, China) and Antifade Mounting Medium (P0126, Biyuntian, China) were used in the immunofluorescence assays.
Immobilization stress and drug administration
For immobilization stress, each rat was held prone in a plastic immobilizer that was large enough to support its entire body.11 After the rat went into the immobilizer, it was placed on a bench top in a quiet procedure room inside the animal facility for 2 h at room temperature. The Rb1 pretreatment was given 30 min before modeling using an intraperitoneal (i.p.) injection (40 mg/kg ginsenoside Rb1 dissolved in physiological saline at 2 grain/ml).12
Experimental protocol
The animals were divided into three groups (n=9). The first group served as the normal control (N). The second group was the acute immobilization stress group, in which the acute stress lasted for 2 h (M). The third group was pretreated with ginsenoside Rb1 (40 mg/kg) 30 min before modeling(R). Blood samples were taken from the angular vein before, immediately after and 30 min after modeling for hormone measurements. After anesthetization with 10% chloral hydrate (3 ml/kg, i.p.), the rats were sacrificed immediately, and their brains were removed for dissection of the hippocampus. These tissues were prepared for Western blotting analysis, reverse transcription-polymerase chain reaction and immunofluorescence.
Enzyme-linked immunosorbent assay (ELISA) for ACTH and CORT
Blood samples were collected, kept on ice and then immediately centrifuged at 3000× g at 4 °C for 15 min. The resulting plasma was kept at −20 °C until analysis. The ACTH and CORT levels were measured using a commercially available radioimmunoassay (RIA) kit.
Western blotting analysis
The hippocampus was homogenized in lysis buffer for 30 min. The homogenate was centrifuged, and supernatants were collected. Total protein was estimated by a BCA Protein Assay Kit. Then, the samples were mixed with sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 min. Samples (40 μg protein) were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat-dried milk and hybridized with primary and secondary antibodies against target proteins. Protein bands were visualized by incubating membranes with chemiluminescent solution and exposure to X-ray film.
Real time polymerase chain reaction (qPCR)
Total RNA was isolated from the hippocampus using TRIzol reagent. Then, total RNA (1 μg) was reverse transcribed, and the resulting cDNA was used to detect the transcripts. Then, cDNA was amplified by polymerase chain reaction (PCR) using specific primers (Table 1). The following PCR conditions were used: an amplification reaction was performed for a single 5-min initial denaturation at 94 °C followed by 40 cycles of denaturation at 95 °C for 30 seconds, annealing at 60 °C (BDNF and 18S) or 68 °C (TrkB) for 30 seconds, extension at 72 °C for 30 seconds, and a final extension at 72 °C for 15 min. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
 |
Table 1 Primers used in reverse transcription PCR |
Immunofluorescence
Brains were fixed in 4% paraformaldehyde for 24 h at 4 °C. The brains, including the hippocampus, were cut into blocks that were subsequently dehydrated and embedded in paraffin wax. Then, the hippocampal paraffin sections (4 μm thick) were incubated in phosphate-buffered saline (PBS) containing 3% hydrogen peroxide for 10 min to suppress endogenous peroxidase activity, thoroughly rinsed in PBS, blocked with 10% bovine serum albumin (BSA) for 30 min at room temperature, and incubated overnight in BNDF (1:100) or TrkB (1:150). The sections were washed and then incubated for 1.5 h at room temperature with Dylight 488 goat anti-rabbit IgG(H + L)(1:100) for immunofluorescence. After 3 washes, the sections were incubated with DAPI for 3 min. The immunofluorescence was observed by fluorescence microscopy.
Statistical analysis
All data were expressed as the mean ± SEM. Data were analyzed using either Student’s t-test or one-way ANOVA followed by Dunnett’s test. An α level of P≤0.05 was considered to indicate a statistically significant difference.
Results
Adrenocorticotropic hormone (ACTH) and corticosterone (CORT) in the blood (Figure 1)
The ELISA analysis demonstrated that rats subjected to restraint-stress exposure for 2 h and ginsenoside Rb1 pretreatment showed significantly increased plasma ACTH concentrations of 3.792±0.504 ng/ml (P<0.05) and 5.825±0.614 ng/ml (P<0.01), respectively, compared with rats in the control group [F(2, 15)=12.414; P=0.001] (Figure 1(A)). The ACTH concentration in blood was higher in the medication group than in the model group after modeling (P<0.05) and in the normal group 30 min after modeling (P<0.05). Exposure to both restraint stress and ginsenoside Rb1 pretreatment induced significant increases in blood CORT concentrations of 1.688±0.122 ng/ml (P<0.01) and 4.078±0.700 ng/ml (P<0.05), respectively, compared with the control treatment [F(2, 15)=16.134; P<0.001] (Figure 1(B)).
Effects of ginsenoside Rb1 on the expression of BDNF and TrkB in the hippocampus
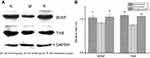
Changes in the protein abundance of BDNF and TrkB were measured by Western blotting analysis (Figure 2(A)). Overall BDNF and TrkB expression in the hippocampus was decreased by 2 h of restraint stress (P<0.05 and P<0.01), respectively. Stress-induced BDNF and TrkB expression were significantly upregulated by ginsenoside Rb1 pretreatment (P<0.01) (Figure 2(B)).
Upregulation of hippocampal BDNF and TrkB mRNA expression in ginsenoside Rb1-pretreated rats
The effect of ginsenoside Rb1 pretreatment on BDNF and TrkB mRNA expression levels in rats with restraint-stress-induced hippocampal lesions was investigated by RT-PCR analysis (Figure 3(A)). BDNF and TrkB mRNA expression in the rat hippocampus in the model group was significantly decreased compared to that in the normal group (P<0.001) (Figure 3(B)). The reduced expression of BDNF mRNA in the model group was significantly restored by ginsenoside Rb1 pretreatment in the medication group (P<0.01), and the restored level was similar to that of normal rats in the normal group [F (2, 15)=30.956; P<0.001] (Figure 3(B)). The reduced expression of TrkB mRNA in the model group was also significantly restored by ginsenoside Rb1 pretreatment in the medication group (P<0.05), and the restored level was similar to that of normal rats in the normal group [F (2, 15)=12.928; P=0.001] (Figure 3(B)).
Immunofluorescence staining of BDNF and TrkB
The effect of ginsenoside Rb1 pretreatment on BDNF and TrkB neuronal cell levels in rats with restraint-stress-induced hippocampal lesions was investigated by immunofluorescence (Figure 4(A and B)). The percentages of cells displaying BDNF and TrkB in the rat hippocampus in the model group were significantly decreased compared to those in the normal group (P<0.05) (Figure 4(C)). The reduced percentages of cells displaying BDNF and TrkB in the model group were significantly restored by ginsenoside Rb1 pretreatment in the medication group (P<0.05) (Figure 4(C)).
Discussion
Stress is defined as anything that throws the body out of homeostatic balance. Acute immobilization stress, a relatively mild physiological stress that causes no bodily damage, was used to build the current animal model. Any stressor that activates the HPA axis leads to an increase in the concentrations of the adrenal stress hormone, and the “stress response” is the attempt of the body to counteract the stressor.5 The present study accounts for a close temporal correlation between variations in rat plasma CORT and ACTH concentrations under normal, stressful and ginsenoside Rb1 pretreatment conditions (Figure 1). Exposure to acute immobilization stress produced increases in the plasma CORT and ACTH levels, while ginsenoside Rb1 pretreatment significantly affected the plasma levels of the hormones. Although plasma CORT and ACTH concentrations were increased in both groups, higher increases were seen in the ginsenoside Rb1 pretreatment group.
Ginseng has been used as a representative tonic for 2000 years in Far East countries such as China, Japan, and Korea. Ginsenoside Rb1 is believed to be the main active component in ginseng. Many published research studies have found that Panax ginseng results in euphoria.13 In the present study, we found that ginsenoside Rb1 can increase the levels of plasma hormones. ACTH and CORT are important hormones in the HPA axis, and CORT is a key hormones involved in stress adaptation because of its influence on multiple organs.14 CORT is known to exert feedback regulation on the functioning of the HPA axis and can increase the release of excitatory amino acids, which may explain why Panax ginseng can cause euphoria. Euphoria can have an antistress effect, and ginsenoside Rb1 may take this approach to influence acute, chronic and repeated stress models.10 However, further work is required to clarify this hypothesis and establish the mechanisms.
BDNF is a central neurotrophic factor (NAF) that is involved in critical central nervous system (CNS) functions of neurons and neurogliocytes, as well as synaptic transmission and plasticity, and it plays an important role in the survival, maintenance, and growth of neurons.1,4,15 The results of the present study demonstrate that acute stress induced a rapid decrease in the expression of BDNF, BDNF mRNA, TrkB and TrkB mRNA in the hippocampus. A previous study showed that hippocampal BDNF mRNA levels increased after 60 min of immobilization stress and decreased after 2 h of stress.16 However, the expression of TrkB mRNA was not influenced by acute immobilization, which is in contrast to the observations in the present study.17 BDNF and its receptor TrkB play important roles during stress injury. After acute injury, the upregulation of BDNF mRNA, as well as the augmentation of TrkB mRNA, was observed in the cerebral cortex and hippocampus.18,19 However, BDNF protein levels were not significantly decreased under acute immobilization stress for 2 h in mouse hippocampus as determined by ELISA.20 Decreased BDNF content in the hippocampus can play an important role in the pathogenesis of neural disease. Indeed, decreased BDNF expression has been confirmed in the hippocampus in neuropathies such as depression, schizophrenia and Alzheimer’s disease.21,22
In the present study, 2 h of immobilization stress did not influence the protein or mRNA expression of BDNF and TrkB in the ginsenoside Rb1 pretreatment group. The observed upregulation of TrkB might be a further compensatory adaptation to prolonged stress-induced downregulation of BDNF. The present study showed that the expression of BDNF and TrkB enhances mammalian stress coping capacity and was associated with ginsenoside Rb1 pretreatment. This finding suggests that ginsenoside Rb1 pretreatment might, either directly or indirectly, contribute to stress protection. Ginsenosides exert their effects on the central nervous system and peripheral nervous system through the regulation of various types of ion channels, such as voltage-dependent and ligand-gated ion channels, in neuronal and heterologously expressed cells.23 Ginsenoside Rb1 promotes neurotransmitter release by increasing the phosphorylation of synapsin via the protein kinase A (PCA) pathway.24 Moreover, Rb1 was demonstrated to be a functional ligand of glucocorticoid receptor (GR).25 How ginsenosides Rb1 exert this effect needs further study.
In addition to the strong need for exploring the mechanisms underlying our findings, future studies should also address the combined effects of the ginsenoside Rb1 or hormones in stress reactivity. Clearly, further study will also be necessary to confirm the expression of BDNF and its high-affinity receptors and to explore the mechanisms in the up/downregulation of BDNF expression by ginsenoside Rb1 and hormones in acute stress conditions to further understand the complexities of neuronal responses to stress.
Conclusion
In this study, we evaluated the effects of ginsenoside Rb1 pretreatment on acute immobilization stress for the first time. The present study demonstrated that attenuation of the impairments of acute stress by ginsenoside Rb1 pretreatment might be due to restoration of hippocampal BDNF and TrkB levels.
Abbreviations list
CORT, corticosterone; ACTH, adrenocorticotropic hormone; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase B; C, control group; M, model group; R, ginsenoside Rb1 group.
Ethics approval and informed consent
This study is approved by the Wenzhou Medical University Animal Ethics Committee.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81774109), Zhejiang Provincial Department of Education (Y201839270) and Wenzhou Science and Technology Project (Y20180496, Y20180508, Y20170164, and Y20170094).
Disclosure
The authors declare that they have no competing or conflicts of interest in regard to this work.
References
1. Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2(6):1525–1534. doi:10.1016/0896-6273(89)90040-8.
2. Shi SS, Shao SH, Yuan BP, Pan F, Li ZL. Acute stress and chronic stress change brain-derived neurotrophic factor (bdnf) and tyrosine kinase-coupled receptor (trkb) expression in both young and aged rat hippocampus. Yonsei Med J. 2010;51(5):661–671. doi:10.3349/ymj.2010.51.5.661
3. Chen K, Zhang L, Tan M, et al. Treadmill exercise suppressed stress-induced dendritic spine elimination in mouse barrel cortex and improved working memory via BDNF/TrkB pathway. Transl Psychiat. 2017;7(3):e1069. doi:10.1038/tp.2017.41
4. Bengoetxea H, Rico-Barrio I, Ortuzar N, Murueta-Goyena A, Lafuente JV. Environmental enrichment reverses tyrosine kinase inhibitor-mediated impairment through BDNF-TrkB pathway. Mol Neurobiol. 2017;55(1):43–59. doi:10.1007/s12035-017-0716-y
5. Mikics É, Guirado R, Umemori J, et al. Social learning requires plasticity enhanced by fluoxetine through prefrontal Bdnf-TrkB signaling to limit aggression induced by post-weaning social isolation. Neuropsychopharmacology. 2018;43(2):235–245. doi:10.1038/npp.2017.142
6. Alexander N, Osinsky R, Schmitz A, Mueller E, Kuepper Y, Hennig J. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology. 2010;35(6):949–953. doi:10.1016/j.psyneuen.2009.12.008
7. Gerritsen L, Milaneschi Y, Vinkers C, et al. HPA axis genes, and their interaction with childhood maltreatment, are related to cortisol levels and stress-related phenotypes. Neuropsychopharmacology. 2017;42(12):2446–2455. doi:10.1038/npp.2017.118
8. Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9(3):259–274.
9. Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1):183S–185S. doi:10.1093/jn/137.1.183S
10. Jia JM, Wang ZQ, Wu LJ, Wu YL. [Advance of pharmacological study on ginsenoside rb1]. Zhongguo Zhong Yao Za Zhi. 2008;33(12):1371–1377.
11. Theoharides TC, Spanos C, Pang X, et al. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136(12):5745–5750. doi:10.1210/endo.136.12.7588332
12. Shen-Hui LJ. Impact of ginsenoside rbl on brain water content and serum s100β in post–cerebral-wounded sd rats. Jetcm. 2012;21:388–389.
13. Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25(5):323–344. doi:10.2165/00002018-200225050-00003
14. Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25(2):117–142. doi:10.1016/S0149-7634(01)00002-1
15. Shuai Z, Dina Z, Hong L, Zhang H, Feng C, Zhang W. Analyses of mRNA profiling through RNA sequencing on a SAMP8 mouse model in response to ginsenoside Rg1 and Rb1 treatment. Front Pharmacol. 2017;8:88.
16. Park SW, Lee CH, Lee JG, et al. Differential effects of ziprasidone and haloperidol on immobilization stress-induced mRNA BDNF expression in the hippocampus and neocortex of rats. J Psychiatr Res. 2009;43(3):274–281. doi:10.1016/j.jpsychires.2008.05.010
17. Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mrnas in the hippocampus. J Neurosci. 1995;15:1768–1777. doi:10.1523/JNEUROSCI.15-03-01768.1995
18. Tsukinoki K, Saruta J, Sasaguri K, et al. Immobilization stress induces bdnf in rat submandibular glands. J Dent Res. 2006;85(9):844–848. doi:10.1177/154405910608500913
19. Rage F, Givalois L, Marmigere F, Tapia-Arancibia L, Arancibia S. Immobilization stress rapidly modulates bdnf mrna expression in the hypothalamus of adult male rats. Neuroscience. 2002;112(2):309–318. doi:10.1016/S0306-4522(02)00072-6
20. Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor proteinexpression. Neuroscience. 2004;124(4):985–992. doi:10.1016/j.neuroscience.2003.12.039
21. Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (bdnf) levels in serum of depressed patients probably results from lowered platelet bdnf release unrelated to platelet reactivity. Biol Psychiatry. 2005;57(9):1068–1072. doi:10.1016/j.biopsych.2005.01.008
22. Tan YL, Zhou DF, Zhang XY. Decreased plasma brain-derived neurotrophic factor levels in schizophrenic patients with tardive dyskinesia: association with dyskinetic movements. Schizophr Res. 2005;74(2–3):263–270. doi:10.1016/j.schres.2004.08.004
23. Lee JH, Jeong SM, Lee BH, et al. Prevention of ginsenoside-induced desensitization of ca2+-activated cl- current by microinjection of inositol hexakisphosphate in xenopus laevis oocytes: involvement of grk2 and beta-arrestin i. J Biol Chem. 2004;279(11):9912–9921. doi:10.1074/jbc.M310824200
24. Xue JF, Liu ZJ, Hu JF, Chen H, Zhang JT, Chen NH. Ginsenoside rb1 promotes neurotransmitter release by modulating phosphorylation of synapsins through a camp-dependent protein kinase pathway. Brain Res. 2006;1106(1):91–98. doi:10.1016/j.brainres.2006.05.106
25. Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42(2):123. doi:10.1016/j.jgr.2017.01.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.