Back to Journals » Drug Design, Development and Therapy » Volume 14
Film-Forming Sprays for Topical Drug Delivery
Authors Umar AK , Butarbutar M , Sriwidodo S , Wathoni N
Received 8 April 2020
Accepted for publication 16 May 2020
Published 22 July 2020 Volume 2020:14 Pages 2909—2925
DOI https://doi.org/10.2147/DDDT.S256666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Abd Kakhar Umar, Maria Butarbutar, Sriwidodo Sriwidodo, Nasrul Wathoni
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor 45363, Indonesia
Correspondence: Nasrul Wathoni; Sriwidodo Sriwidodo
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor 45363, Indonesia
Tel +6222 842 888888
Email [email protected]; [email protected]
Abstract: Film-forming sprays offer many advantages compared to conventional topical preparations because they can provide uniform drug distribution and dose, increased bioavailability, lower incidence of irritation, continuous drug release, and accelerated wound healing through moisture control. Film-forming sprays consist of polymers and excipients that improve the characteristics of preparations and enhance the stability of active substances. Each type of polymer and excipient will produce films with different features. Therefore, the various types of polymers and excipients and their evaluation standards need to be examined for the development of a more optimal form of film-forming spray. The selected literature included research on polymers as film-forming matrices and the application of these sprays for medical purposes or for potential medical use. This article discusses the types and concentrations of polymers and excipients, sprayer types, evaluations, and critical parameters in determining the sprayability and film characteristics. The review concludes that both natural and synthetic polymers that have in situ film or viscoelastic properties can be used to optimise topical drug delivery.
Keywords: film-forming solution, film-forming polymer, topical drug delivery
Introduction
Topical routes of drug delivery aim for systemic or local effects and offer various advantages, including avoiding first-pass metabolism and the effect of low pH and enzymes in the gastrointestinal tract, as well as a large available surface area.1–7 To improve therapeutic efficiency or pharmacokinetic profiles, drugs administered via the topical route are generally made in a dosage system, such as a patch, gel, lotion, cream, ointment, or spray.8–10 However, the concern is that patch preparations still leave drug residues after use and can be deliberately abused.11 Patch preparations are also often associated with hypersensitivity, irritation, and blistering.12 Problems in the scale-up of production are also often found where drugs are difficult to stabilise and can crystallise during storage.13 Other semisolid preparations also have the disadvantage of being easily attached to clothing while on the move and can cause cross-infection of wounds because it is applied using the fingers.14 Compared to other topical dosages, sprays offer several advantages such as practical use, low incidence of irritation, sterility of the dosage, excellent coverage of the skin or wound, even distribution of the drug when applied, and adjustable dosage.14–20
In recent decades, various innovations have continued to be developed to obtain efficient and effective spray preparations. One of them is a film-forming spray (FFS) which has been applied in multiple fields, such as the food industry, cosmetics, pharmaceuticals, plantations, etc.19,21-26 FFS generally consists of active substances, enhancers, and polymers that are dissolved in organic solvents.24 A thin, non-sticky film forms that can increase the contact time and permeability of the drug, resulting in continuous drug release, and can prevent crystallisation so that more drug is available to provide therapeutic effects compared to other conventional topical preparations.27
The type of nozzle, the size of the aperture, the pressure of spray applied, and the nature of the liquid strongly influence the sprayability of FFS.28,29 The viscoelastic, in situ gel, pH and thermal-sensitive properties of FFS are essential to study to determine what aspects need to be considered in selecting polymers, solvents, and other excipients.30 Therefore, this review explores the types of polymers, excipients, and sprayers commonly used in FFS and the evaluation standards needed to determine the quality of FFS for better development.
Methodology
This review employed literature originating from Scopus, PubMed, and Google Scholar by using the keywords ‘film-forming spray‘, ‘spray of film-forming solution‘, ‘polymer in film-forming spray‘, and ‘spray of polymer‘. The selected literature included research on polymers as film-forming matrices that are applied using a spray for medical purposes or for potential medical use. We excluded reviews and literature in which the application was not appropriate for medical use such as spray drying or spray pyrolysis methods. A flowchart of the methodology can be seen in Figure 1.
 |
Figure 1 Flowchart of the methodology. |
Definition and Mechanism of a Film-Forming Spray
An FFS is a drug delivery system in the form of a sprayed solution that will form a film when it contacts the target therapeutic site by utilising the polymer as a matrix for film formation.20,25,30 After forming the film, the drug release process is similar to a patch, in which the polymer matrix containing the drug will release it in a sustained fashion.21 However, in contrast to topical patches and other topical preparations, films form following the pattern of the skin or wound since deep indentations can be exposed to small droplets of the film-forming solution (see Figure 2). Of course, this greatly facilitates drug access to the target tissue. In a film-forming spray, drug dosages can also be adjusted based on the volume of solution per spray so that systemic or local effects can be controlled. An FFS also provides an even distribution of drugs and spreads well. Ease of use can also increase patient compliance.20,30–33
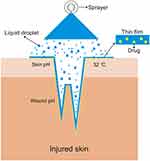 |
Figure 2 Mechanism of film-forming spray. |
The thin film is easy to wash away with water.20,34 This thin and non-sticky film also increases patient comfort during activities compared to using patches, ointments, gels, etc., because these have a rough and sticky texture when applied.35,36 The thin film also facilitates the permeation of wound moisture so that the balance can be maintained. Inappropriate wound humidity can cause infection or irritation, as happens with the use of patch preparations.37–39
In formation of droplets, the film-forming solution is sprayed using any kind of sprayer. Each sprayer has different specifications and intended uses, but has specific potential in medical applications. The following is an explanation of several types of sprayers that have the potential to be used as drug delivery devices in film-forming systems.
Film-Forming Sprayers
Ordinal Spray
The ordinal spray is a type of spray that does not use unique technology in the spraying process, generally employing a plastic or aluminium container with a dip tube diameter of 1.2 mm and an aperture size of 0.3 mm.40 The average spray angle produced is 78.69–87.39°.40–42 The average amount of film-forming solution that can be sprayed is 0.11–0.35 g or mL.40,41,43 The average leakage rate of an ordinal spray container is 0.01–0.03 %.34 An ordinal spray can be either horizontal or vertical. The 3 K® Horizontal Spray Nozzle (Ursatec, St. Wendel, Germany) has been reported to be able to maintain the sterility of the film-forming solution during storage and use. The spray force of the ordinal spray also varies depending on the type and concentration of the polymer used.31 The ordinal spray can also be used for extract preparations.43,44
Metered Dose Spray
The metered dose spray (MDS) is a spray device that can adjust the amount of spray. This tool is generally used to deliver preparations to the systemic compartment via the transdermal or transmucosal route. Therefore, in evaluating a film-forming spray, the spray volume needs to be considered because it is related to the dose of the drug. The spray volume of the MDS can be influenced by the volume available in the bottle, the homogeneity of the particle dispersion, and the position of the container during use.45 The average amount of FFS that can be sprayed is 90–102 mL.30,32,33 The average spray angle of MDS is 83.51°.20 The average leakage rate of an MDS container is 0.01–0.02 %.30
Electrostatic Spray
Electrostatic spray (ES) is used extensively in the agricultural field of pesticide application. ES can improve the deposition efficiency, speed of droplet formation, uniformity of coverage, and reduce the loss to drift.46 The performance of ES is influenced by the viscosity, surface tension, and electrical resistivity of the solution.47 A solution cannot be sprayed with ES if the conductivity is not within 10−8-10−5 S/m.48 The size of the droplets produced by ES ranges from 4–26 µm with an average diameter of 6.3–12 µm.25,26
Ultrasonic Spray
The ultrasonic spray has excellent potential to deliver film-forming solutions. The resulting droplet can reach the nano size with thin-film characteristics. The ultrasonic spray nozzle can function at low and high pressures, producing uniform droplets with diameter sizes of less than 10 µm. The nozzle of the ultrasonic spray is 0.5 mm in diameter with a droplet diameter of 1–10 µm. The resonant frequency of the electrode used is 10 MHz. For applications in the medical industry, an ultrasonic spray can produce layer-by-layer (LBL) coating films with better particle size uniformity compared to ordinary LBL spray.49
Each type of sprayer has specifications that match certain polymers. Multiple types of polymers, both natural and synthetic, have been used in the FFS system.
Polymers Used in Film-Forming Sprays
Polymers play a significant role in the success of FFS preparations. Aside from being a drug release controller, polymers also act as the film-forming base. Polymers can also prevent the transformation of molecules, such as the formation of unexpected crystals.50 General considerations in the selection of polymers are its ease of being washed away by water, stability, biodegradability, and non-irritating properties.10 Polymers used in FFS can be natural or synthetic (see Tables 1 and 2), as long as they have in situ gel or viscoelastic properties.
 |
Table 1 Natural and Semisynthetic Polymers Used in Film-Forming Spray Systems |
 |
Table 2 Synthetic Polymers Used in Film-Forming Spray Systems |
Polymers that have thermo-sensitive properties will form a solution at room temperature and turn into a gel when they are exposed to the body temperature,51–56 while those that have pH-sensitive properties will form a solution at a certain pH and turn into a gel if the pH of the system changes.57–62 Viscoelastic polymers start at a thick consistency but can become elastic when placed under pressure (sprayed) and return to a thick consistency after the pressure is removed.63–67
Natural and Semisynthetic Polymers
Cellulose
Ethylcellulose forms films that are easily washed away with water.34 The concentration of ethyl cellulose that produces films with excellent characteristics is 5.02–5.25% and is generally combined with Eudragit.40,41 Hydroxypropylmethylcellulose (HPMC) is reported to have a slow drying time. The optimal concentration to get excellent film characteristics is 2 %. At these concentrations, HPMC produces clear, thin, and smooth films.43
Na-CMC is known to have a thixotropic flow rate that allows it to become thinner when under pressure so that it is easy to pass through the nozzle and return to initial consistency after being sprayed.31 The maximum limit of Na-CMC concentration that can be sprayed is 2.5 %. The optimal level of Na-CMC that produces films with excellent sticking properties and a constant dose in each spray is 1.5%. Na-CMC has also been reported to maintain stability and release the drug in a controlled manner, thereby increasing its therapeutic efficacy.31
Chitosan
Aside from being a filmmaker, chitosan also has antimicrobial, antioxidant, and mucoadhesive activity, making it suitable for use in topical drug delivery.72 Chitosan has a relatively high surface tension.73 The surface tension of chitosan increases with increasing concentration and molecular weight,26 but these properties make chitosan difficult to dissolve in water. Surfactants are usually used to improve the solubility of chitosan.74
In making FFS from chitosan, Tween 80 can be used to reduce the surface tension.25 The decrease in surface tension of chitosan goes hand in hand with an increase in the degree of deacetylation and its concentration, but the trend is not very significant.25 The use of PEG 400 can also increase the stability and solubility of drugs in chitosan.75
Chitosan also has good conductivity with an increase in molecular weight so that it can be delivered using a electrostatic spray.26 Chitosan viscosity also decreases with increasing degrees of deacetylation. With these properties, chitosan can form films with denser droplet densities with smaller droplet diameters, ranging from 4–27 μm.25 Chitosan is also more hydrophilic at higher degrees of deacetylation. However, its hydrophilic nature does not correlate with its permeability to water vapour. The tensile strength of chitosan will also increase with increasing degrees of deacetylation, contrary to its elongation.25
Cyclodextrin
Cyclodextrin is known to maintain drug stability from crystal deformation.76 Cyclodextrin is also reported to have a small impact on increasing the viscosity of the film-forming solution, so it is easy to spray.68
Gellan Gum
Gellan gum (GG) has viscoelastic properties so that it is easily delivered using a spray system. The viscoelastic nature allows GG to melt in consistency when sprayed and return to its original texture after being on the surface of the skin. In a spray system, GG is also reported to be able to encapsulate cells and deliver them via gel droplets in situ.69,77
GG has thermosensitive properties that are very beneficial in spray systems. Its thermosensitive nature makes it easy to change shape from solution to gel when contacting surfaces with temperatures higher than room temperature (30–40 °C) such as on the skin and eyes.69 Besides, GG is also sensitive to changes in pH.78 The resulting film has excellent mucoadhesive properties. Adding NaCl as a crosslinker can increase its yield stress.70
Low acyl GG is more sensitive to a temperature where a significant increase in viscosity at ± 35 °C, while high acyl GG increases thickness at ± 78 °C in a 0.1% NaCl mixture. Similarly, drug release in low acyl GG is better than in high acyl GG.70
Xanthan Gum
Based on research conducted by Shilin Wang, the sprayability of xanthan gum was strongly influenced by its viscosity. The addition of surfactants in the xanthan gum solution decreased the surface tension and reduced the size of the droplet. Interestingly, the viscosity and flow properties were not significantly changed. In addition, the spray angle and coverage area of the xanthan gum solution decreased with increasing xanthan gum concentration.71
Synthetic Polymers
Carbopol
Carbopol also has thixotropy flow properties.83 Carbopol itself forms an amorphous hydrogel that is good for open wounds because it can donate or absorb wound moisture.79,84 The viscoelastic nature of carbopol allows an increase in the diffusion coefficient of the drug. The combination of Carbopol and Poloxamer is said to be better than using Carbopol alone. At a concentration of 0.05 %, the polymer combination produces a film with good sprayability (spray angle, drying time, and uniform content per spray) with an acceptable release of the drug.42 Carbopol is also known to produce gels with characteristics that are more resistant to heating.79
Eudragit
Eudragit is available in various types with different purposes for use. Generally, these synthetic polymers are used as additives to tablets for modifying drug release.85 However, Eudragit is also known to increase drug permeation in the skin,34,86,87 so that its application in topical preparations is widely developed.
Eudragit EPO, Eudragit E 100, Eudragit S 100, Eudragit RL 100, and Eudragit RS 100 produce transparent and shiny films while Eudragit RSPO and RLPO do not. Films produced by Eudragit EPO, Eudragit E 100, Eudragit RL 100, and Eudragit RS 100 cannot be washed away with water. In contrast, Eudragit S100 provides a film that can be removed with water after being applied to the skin. This is because Eudragit S100 can dissolve above pH 7. Eudragit S100 also does not cause any skin irritation.20
In vitro permeability tests showed that Eudragit RS produced a thick film when sprayed on a silicone membrane. This was likely due to the crystallisation of methylphenidate, which may have reduced permeation.88 However, after being measured, the level of methylphenidate penetration was greater with the Eudragit RS film compared to Eudragit E.80 In addition, films with Eudragit L100 have been reported to be unable to prevent the crystallisation of testosterone.68
Eudragit RS 100 has been reported to have good sprayability, adhesiveness, and flexibility. However, use above a concentration of 15% can reduce the ability to wash with water, whereas Eudragit RLPO produces better films at a level of 10.05% in a mixture with ethyl cellulose 5.02 %.40
Lutrol
Lutrol F-127 has film characteristics and spray patterns similar to Carbopol 940 but produces a more uniform dose of the drug in each spray, with a smaller standard deviation. Lutrol F-127 also produces films with better drug release compared to Carbopol 940. No skin irritation has been reported.30
Plasdone
Research conducted by Lu et al32 shows that Plasdone can increase testosterone permeation better than other polymers. The best testosterone permeation sequence was Plasdone > Eudragit EPO > PVP K30 > Eudragit RL. This was due to the nature of the polymer, which can inhibit the crystallisation of testosterone, thereby increasing its flux.88,89
Kollidon
Kollidon is a synthetic polymer that is also widely used in the pharmaceutical world. There are several types of Kollidon with different characteristics appropriate for solid, semisolid, and liquid preparations. In liquid and semisolid forms, Kollidon can increase solubility and permeability, and control drug release.90
Kollidon® 30 has been reported to form transparent, thin, and well-dispersed films. During 28 days of storage, the pH of Kollidon® 30 remained stable without significant changes.43 Kollidon® 30 and Kollidon® VA64 have the ability to act as antinucleants that can inhibit testosterone crystallisation.68 Drug permeation in the skin has also been reported to be increased with the use of Kollidon® 30 films.81
Excipients Used in Film-Forming Sprays
Besides polymers, other excipients are also added for the purpose of improving the quality of the preparation and its therapeutic efficiency. The following is a list of excipients (Table 3) commonly used in film-forming spray systems.
 |
Table 3 Excipient Commonly Used in Film-Forming Sprays |
Crosslinkers
The use of crosslinkers can affect the elasticity, viscosity, solubility, glass transition, and film stiffness of the polymer.91 The use of NaCl as a crosslinker in gellan gum also affects the gel’s sensitivity to temperature, so that film formation is better and faster. NaCl also increases cell encapsulation in gellan gum.69
Permeation Enhancers
Eutectic blends are often used as enhancers to drug permeation.34,40,41,81 One of the most potent eutectic blends is a mixture of camphor and menthol.92–95 Camphor and menthol form a hydrophobic mixture, so it is suitable as a penetration enhancer for drugs that are also hydrophobic. However, camphor and menthol can cause leaching and the formation of pores in the skin.40 A warm feeling followed by a cold feeling that builds slowly is characteristic of a mixture of camphor and menthol.40
The eutectic mixture of camphor and menthol significantly increases the permeation of the antifungals fluconazole, clotrimazole, and voriconazole in a Franz diffusion cell using nylon membranes.34,40,41 Because it has hydrophobic properties, camphor and menthol can increase drug permeation through interactions with the lipids of the stratum corneum.93
Research conducted by Lu et al32 showed that the order of permeation enhancers that is the best for increasing testosterone permeation is azone > isopropyl myristate (IPM) > propylene glycol (PG) > N-methyl-2-pyrrolidone (NMP). Furthermore, the results of a study by Lu et al33 showed that dexketoprofen permeation was better using lauryl lactate (LA) > IPM > azone > PG as permeation enhancers. This indicates that the penetration enhancing ability of these compounds varies for each drug. However, azone is very suitable for highly hydrophilic drugs. The combination between azone and PG improves the penetration ability of azone.96,97
Plasticisers and Stabilising Agents
In the film formation, the plasticiser maintains elasticity and prevents cracking of the film. Plasticisers can also maintain the stability of active substances25,26,79 and increase the permeation of drugs. Polyethylene glycol (PEG) and propylene glycol (PG) are reported to have a role in increasing the permeation of antifungal drugs. Apart from being a plasticiser, PG also has a role as a solubiliser, which is also useful in carrying drugs through the skin.41,98 PG has a significant effect on the viscosity of the film-forming solution, so the concentration needs to be considered (see Table 3).68,99 The use of PG in a mixture with water and ethanol does not have a good effect as a mixed solvent in preventing the crystallisation of testosterone.68 The effective PG concentration for increasing drug permeation is below 5 %.34,100
PEG 400 can also increase the volume per spray of a film-forming solution. The amount per spray increases with increasing PEG 400 concentrations. The covered spray area also increases with increasing PEG 400 levels.82 This is associated with a decrease in vapour pressure due to the presence of PEG as a non-volatile solvent.101
Solvents
The solvents used in the FFS system include both volatile and non-volatile solvents. The aim is to balance the film drying rate. Films that dry out too quickly and form a hard film make it difficult for drugs to escape and penetrate. The active substance is usually dissolved to saturation in the solvent to facilitate the film drying process.40–42
Evaluation of Film-Forming Sprays
pH
The pH value is measured and adjusted to improve the stability of the active substance or make it suitable for the area of application. For skin pH ranging from 4–6,102 the pH of diabetic wounds ranges from 6.5–8,103 whereas faster healing time for burns occurs below pH 7.32.104 The pH adjustment of the preparation aims to prevent irritation and changes in the physiological condition of the wound in the healing process. Besides, the pH value of the dosage can also affect drug permeation through the skin based on the degree of ionisation.105,106
Viscosity
Each type and concentration variation of the polymer will result in a different viscosity. The viscosity of the film-forming solution will affect its sprayability, so this is an important parameter, especially in MDS.107–109 Increasing the concentration of the film-forming solution can reduce the coverage area of the spray.26
Tonicity
The application of film-forming solution to certain parts of the body such as wound and eye mucosa requires the tonicity adjustment of the film-forming solution. Non-isotonic preparations can cause mucosal irritation and eye pain. For this reason, the tonicity of the preparations needs to be calculated and adjusted using the Kahar method. The following equation is used to determine the isotonic concentration of the materials.110
(1)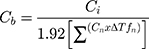
where Cb is the isotonic concentration of the material, Ci is the initial concentration of the material, and ∑ (Cn x ∆Tfn) is the sum of the multiplication between the concentration and the value of the freezing point depression of each ingredient.
Rheological Properties
Flow testing aims to determine whether a compound is thixotropic or not. A mixture can easily pass through the sprayer nozzle repeatedly if it has these flow properties. This flowing property allows thinning of the film-forming solution as it shifts along past the nozzle (stressed) and returns to its original viscosity after being sprayed (stress is lost).31,111,112 The hysteresis circle, ie the area covered by the ascending and descending curves, is characteristic for thixotropic behaviour (see Figure 3).113
 |
Figure 3 Thixotropic in pseudoplastic, plastic, and dilatan systems. Notes: Reprinted from Journal of Controlled Release, 136(2), Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations:88–98, Copyright 2009, with permission from Elsevier.113 |
The nature of this flow can be determined using a rheometer in rotation model with a logarithmic increase in the shear rate of 1–900 s−1 and back again from 900–10 s−1. This test is carried out at room temperature and the storage temperature of the film-forming solution.31
Testing the flow properties using the oscillation time sweep and amplitude sweep method in various variations of excipient concentration and the temperature range can also be done to find out how the effect of excipient and temperature in the change in gel consistency.79
Bioadhesive Strength of the Film
Measurement of the bioadhesive strength of the film can be done by attaching a film to the surface of the mouse skin (2 x 5 cm). Then, the skin is hydrated with 0.5 mL distilled water. The film is allowed to interact with the tissue surface for 5 minutes.114 The total force (F) to detach the film from the surface of the skin is recorded. The bioadhesive strength (Fb) is calculated per unit area (A) of the film.115,116
(2)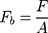
Tensile Strength and Elongation of the Film
Tensile strength (TS) is the ability of the film to resist applied pressure.117 TS testing aims to determine whether the film formed is resistant to abrasion and flexible so that it can follow the movement of the skin without cracking.118 TS can be calculated using the equation below.26
(3)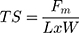
where Fm is the maximum pressure that can be held by the film before tearing, L is the thickness of the film, and W is the initial width of the film.26 After the film is stretched, elongation (EB), which describes its elasticity, can be calculated using the following equation.
(4)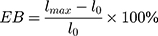
where lmax is the length of the film before the film is torn when pulled and l0 is the initial length of the film.26
Water Washability
The ease of film wetting is assessed in the dried film. The film is washed with water and assessed in ordinal scale, ie easily washed, moderately washed, and poorly washed.34,40 The ease of sprinkling with water will be useful if the film-forming solutions contact with sensitive areas in the body such as eyes and mouth.
Fluid Affinity
This test is carried out to see how the ability of the film formed to absorb moisture from the wound or provide moisture to the wound. An adequate supply of moisture to the injury will speed healing, but excess moisture can cause erosion of the wound tissue.119–121 The testing procedure follows the EU standard EN 13726–1: 2002.79
Occlusion Potential or Water Vapor Permeability of the Film
The permeability of the film to wound fluid is also vital to determine because it affects the moisture of the wound. Excessive humidity will trigger the growth of microorganisms and lead to infection.122 This test is done by covering the mouth of a glass beaker containing 50 mL of water with filter paper. One of the papers is sprayed with a film-forming solution and allowed to form a film. The beaker is then stored at room temperature and humidity. The permeability of the film to water is determined based on the reduced water weight in the beaker.20 The assessment is determined using the following formula.
(5)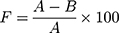
where F is the occlusivity factor and A is the reduction in water weight in the glass beaker covered with the filter paper without a film. In contrast, B value is the reduction in water weight in the glass beaker covered with the filter paper coated by the film. The smaller the occlusivity factor value, the better the film permeability.20
In a study conducted by Zhong et al,26 the water permeability of the film was determined by considering the surface area and thickness of the film. Also, the difference in pressure inside the cup and outside the container was specified. Water vapour permeability (WVP) is calculated using the following equation.
(6)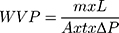
where m is the mass of water lost in the cup (g), L is the thickness of the film (mm), A is the permeation area (m2), t is the time of permeation (days), and ΔP is the difference in water vapour pressure inside and outside the cup.26,123,124
In addition, the release of moisture content from the film can be evaluated by comparing the initial weighed of film (2.0 x 2.0 cm2) (W1) and the weight of the film after a day of storage (W2). The moisture loss and moisture uptake can be calculated by using the following equations.115,125,126
(7) (8)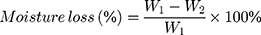
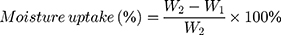
Surface Morphology of the Film
This test is carried out to determine the microscopic shape, surface roughness, and homogeneity of the film using scanning electron microscopy (SEM) or transmission electron microscopy (TEM).26,82,127,128
Film Formation/Drying/Evaporation Time
The drying time of the film is measured to determine how quickly the film forms after the solution is sprayed. Under some conditions, the solution is sprayed on the surface of the glass and then allowed to dry at room temperature.81 In other studies, a mixture of activated silica gel-dye is applied to the surface of the glass plate to provide absorption.30
Drying time can also be observed directly by applying a film-forming solution to the skin. To find out if the film has dried, a glass plate is placed against the film without being pressed. If there is no water adhesion to the glass, the film is said to have dried.34 This method is more representative of actual conditions because the skin has pores and body heat, so the drying time may be different from using glass plates in the film drying time test.
Stickiness
The dry film is pressed gently using cotton wool. The viscosity of the film is assessed by the amount of cotton wool fibres attached to the film. The film adhesiveness is considered high if the attached fibre is thick, medium if the attached fibre is thin, and low if it is little or no attached fibre.20,34 Stickiness is tested to find out whether the film will become easily attached to clothing or other objects, so it needs attention when on the move.
Spraying Force
This test is carried out to find out how much pressure is needed to spray the film-forming solution.31 The tool that can be used is TA.XT Plus texture analyser (Stable Micro Systems).31
Spray Angle, Pattern, and Droplet Size Distribution
Paper soaked with indicator reagents is used, which makes it easy to observe the spray patterns that are formed.81 This depends on the type of solvent and the pH of the film-forming solution. Using a solvent-sensitive paper will clarify the pattern and the spray droplet size distribution. The diameter of the pattern is then measured to determine the area covered and the spray angle.
(9)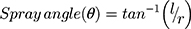
where Ɩ is the distance of the paper surface from the nozzle and r is the radius of the circle. The distance of the nozzle from the paper is generally around 15 cm.81 The higher the spray angle, the more difficult is it for the film-forming solution to spread when sprayed.26 An illustration of the spray pattern measurement can be seen in Figure 4.
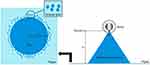 |
Figure 4 Measurement of spray angle and observation of spray patterns. |
To measure the covered area, the spray pattern is scanned at 600x600 dpi (Konica Minolta scanner, bizhub c3350). The image is then converted to a binary image using ImageJ software. Then, the percentage of the covered area is calculated using the following equation.69
(10)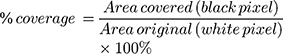
The particle analysis plugin (ImageJ software) is used to determine the size of the droplets.69 Spraytec® by Malvern (Malvern, UK) can also be used, which works on the principle of laser diffraction.20 Droplet diameter is measured in units of mm, then D10 %, D50% and D90% are determined. The relative span factor (RSF) is then calculated to determine the uniformity of the droplet size distribution using the following equation.69
(11)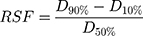
Drug Content per Spray and Uniformity
The dose uniformity of each spray is determined by measuring the weight or volume of each spray, which is then used to obtain the amount of active substance based on its concentration in the film-forming solution. The level of the active substance can also be determined by collecting the sprayed solution, then measuring it instrumentally.81 To determine how much spray volume comes out, the film-forming solution coming out of the nozzle should not be weighed, but rather the weight of the film-forming solution remaining in the sprayer should be determined. Because the droplets that come out of the spray are so small and easily carried by the wind, not all of it will likely be collected to be weighed. The following equation is used for measuring spray volume:20
(12)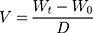
where V is the spray volume, Wt is the weight of the film-forming solution after spraying, W0 is the weight of the film-forming solution beforehand, and D is the specific gravity of the film-forming solution determined using the pycnometer method.20 This test is critical in the use of metered-dose sprays. Drug levels are determined at sprays 5, 10, 20, 30, and 50 by collecting sprays, then measuring them instrumentally.32
Potential Drug Aggregation
Changes in particle size will undoubtedly affect sprayability. Some methods to determine the potential for aggregation of a drug are zeta potential and size exclusion chromatography (SEC).31
In vitro Drug Penetration/Release Study
In this test, cellulose membranes (pore size 0.45 µm), nylon membranes (pore size 0.22 mm) or silicone membranes are generally used as compartment separators using Franz diffusion cells. The medium used is phosphate buffer pH 7.4. After the compartment system is ready, the film-forming solution is put into the donor compartment. The solution that diffuses through cells is taken at certain time intervals and then measured using an instrument. The same volume of fluid is replaced after samples are collected.34,80,81,126
Ex vivo Skin Permeation Study
Drug permeation can be tested on the abdominal skin of mice or rabbits using Franz diffusion cells. The skin is cleaned of all attached fat tissue still using a cotton swab that has been soaked in propanol or isopropanol, then washed with normal saline solution. Diffusion media include phosphate buffer pH 7.4 or acetate pH 6.0. On the receptor compartment side, medium flow is achieved using flow-through cells connected to silica tubes at speeds of 0.3 mL-0.6 mL/hr. After the compartment system is ready, the film-forming solution is placed in the donor compartment. Aliquots are then taken from the receptor compartment at specific time intervals, and then the drug levels are measured using an instrument. New diffusion medium is added at the same time, replacing aliquots that are taken to maintain sink conditions.31,32,34,41,68,81,115 This compartment system can be seen in Figure 5.
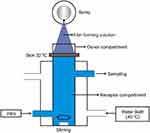 |
Figure 5 Illustration of ex-vivo permeation testing. |
In addition to the methods previously mentioned, the fluorescence method is also useful in observing the extent to which the drug penetrates the skin layer. Samples that contain fluorescence markers can be observed microscopically.31
Permeation Data Analysis
The amount of drug that passes through the membrane per unit area to the receptor compartment per unit time is called flux. Flux is expressed in units of mass/area/time.41,129 If the dose of the drug is a finite dose, the steady-state flux can be calculated using the following equation.
(13)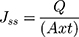
where Q is the number of drugs that penetrate at time t and A is the area of the membrane exposed to the film-forming solution. The units of Jss is quantity/cm2/min. The permeation coefficient (Kp) can also be calculated using the following equation.115,126
(14)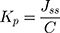
where C is the drug concentration in the film-forming solution placed in the donor compartment. The objective in determining the permeation coefficient is to determine the effectiveness of permeation enhancing agents for increasing the permeation of drugs. This can be determined using the enhancement ratio (ER).41,116
(15)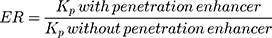
The higher the ER value, the better the effect of penetration enhancers in increasing drug penetration.
In vivo Skin Irritation Test
The film-forming solution is applied to the skin of test animals such as mice and rabbits after being shaved. Irritation, inflammation, erythema, oedema, papule formation, flakiness, and dryness are observed 24 hours – 7 days after application.41,81,115 Scores for rating irritation can be seen in Table 4.
 |
Table 4 Skin Irritation Scale |
 |
Table 5 The Product of FFS |
Stability Study
Characteristics commonly tested are changes in particle size, chemical and 3D structures, levels, and therapeutic activity of active substances after storage under various conditions.31,126,130 In some cases, thermal analysis is also conducted to find out whether or not recrystallisation of metastable active substances occurs. The polymer used is an antinucleant, which can maintain the drug in the initial crystalline form.68 In several studies, the content of the drug per spray and its spray pattern as a metered-dose spray will be tested again. It is essential to guarantee the dose during the storage period.30
Applications of Film-Forming Sprays
Several FFS applications can be found on the market. Most products are intended for the handling and treatment of wounds. Common injuries that can be treated can be incised wounds such as surgical or sharp object contact, burns, and diabetic wounds. Here are some products that use the FFS system.
Author Perspective
In fact, many types of polymers can be used and developed in FFS systems (Table 5). Polymers that have in situ film-forming (pH- and thermo-sensitive) or viscoelastic properties are highly suitable for use. Some polymers that have thermos and/or pH-sensitive properties and are potentially utilised in FFS systems include alginate, carrageenan, prosopis gum, gelatin, Pluronic F127, PLGA, PDMA, poly-caprolactone, poly-(N-isopropyl acrylamide), and β-glycerophosphate. These polymers can control drug release for topical or transdermal purposes for 13 days to 2 months. Some of these polymers can also be used as nanomedicine delivery systems.56,122–134
Among the natural polymers, chitosan has excellent characteristics as a polymer in FFS because, in addition to forming a film in situ, chitosan has been shown to have antimicrobial, antioxidant, anti-inflammatory, antitumor, tissue regeneration inductor, and wound healing properties. Chitosan is also known to be compatible with many drugs and in the body because it is biodegradable. Chitosan derivatives have also been developed to increase the solubility or enhance the effectiveness of drugs.55,131–143
Conclusion
FFS can be a promising drug delivery system with various benefits. Natural or synthetic polymers can be used as drug matrices and film formers following the need for increased stability and therapeutic effectiveness of the active substance. Sprayers help form droplets with better and more uniform distribution and dosage of drugs. Each sprayer also has critical and specific testing specifications and parameters.
Abbreviations
API, active pharmaceutical ingredient; Eq, equation; ES, electrostatic spray; FFS, film-forming spray; HPMC, hydroxypropylmethylcellulose; HPC, hydroxypropylcellulose; IPA, isopropyl alcohol; IPM, isopropyl myristate; GG, gellan gum; LA, lauryl lactate; Na-CMC, sodium carboxymethylcellulose; NaCl, sodium chloride; NMP, N-methyl-2-pyrrolidone; MDS, metered dose spray; PDDA, poly(diallyldimethylammonium chloride); PDMA, poly(N, N-dimethylaminoethyl methacrylate); PEO, poly(ethylene oxide); PG, propylene glycol; PVA, polyvinyl alcohol; PVP, polyvinyl pyrrolidone; SiO2, silicon dioxide; VA, vinyl acetate.
Akcnowledgement
The authors thank the Universitas Padjadjaran for the Work For Home grant 2020 (Contract Number : 1733/UN6.3.1/LT/2020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zorec B, Miklavčič D, Pavšelj N, Préat V. Active enhancement methods for intra- and transdermal drug delivery: a review. Zdr Vestn. 2013;82(5):339–356.
2. Cristiano MC, Cilurzo F, Carafa M, Paolino D. Innovative vesicles for dermal and transdermal drug delivery. In: Lipid Nanocarriers for Drug Targeting. Elsevier; 2018:175–197. doi:10.1016/B978-0-12-813687-4.00004-9
3. Sharadha M, Gowda DV, Vishal Gupta N, Akhila AR. An overview on topical drug delivery system – updated review. Int J Res Pharm Sci. 2020;11(1):368–385. doi:10.26452/ijrps.v11i1.1831
4. Kaur J, Kaur J, Jaiswal S, Gupta G. Recent advances in topical drug delivery system. Pharm Res. 2016;6(7).
5. Leppert W, Malec–Milewska M, Zajaczkowska R, Wordliczek J. Transdermal and topical drug administration in the treatment of pain. Molecules. 2018;23(3):681. doi:10.3390/molecules23030681
6. Dayan N. Delivery system design in topically applied formulations: an overview. In: Delivery System Handbook for Personal Care and Cosmetic Products. Elsevier; 2005:101–118. doi:10.1016/B978-081551504-3.50009-2
7. Ruela ALM, Perissinato AG. Evaluation of skin absorption of drugs from topical and transdermal formulations. Brazilian J Pharm Sci. 2016;52(3):527–544. doi:10.1590/s1984-82502016000300018
8. Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. 2015;22(8):969–987. doi:10.3109/10717544.2013.879355
9. Chang R-K, Raw A, Lionberger R, Yu L. Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013;15(1):41–52. doi:10.1208/s12248-012-9411-0
10. Radhakrishnan A, Kuppusamy G, Karri VVSR. Spray bandage strategy in topical drug delivery. J Drug Deliv Sci Technol. 2018;43:113–121. doi:10.1016/j.jddst.2017.09.018
11. Ibrahim SA. Spray-on transdermal drug delivery systems. Expert Opin Drug Deliv. 2015;12(2):195–205. doi:10.1517/17425247.2015.961419
12. Algin-Yapar E, Önal Ö. Transdermal spray in hormone delivery. Trop J Pharm Res. 2014;13(3):469–474. doi:10.4314/tjpr.v13i3.23
13. Variankaval NE, Jacob KI, Dinh SM. Crystallization of ?-estradiol in an acrylic transdermal drug delivery system. J Biomed Mater Res. 1999;44(4):397–406. doi:10.1002/(SICI)1097-4636(19990315)44:4<397::AID-JBM5>3.0.CO;2-Q
14. Mandal UK, Chatterjee B, Husna F, Pauzi B. A review on transdermal spray: formulation aspect mathews journal of pharmaceutical science a review on transdermal spray: formulation aspect. Mathews J Pharm Sci. 2016;1(March):006.
15. Chavan P, Bajaj A, Parab A. Topical sprays: novel drug delivery system. Int J Pharm Chem Res. 2016;2(2):102–111.
16. Parmar K, Patel MB. A review on sublingual spray: novel drug delivery system. Int J Pharm Sci Res. 2017;8(11):4533–4539. doi:10.13040/IJPSR.0975-8232.8(11).4533-39
17. Aravindhanthan V, Anjali PB, Radhakrishnan A. Sublingual spray: a new technology oriented formulation with multiple benefits. Int J Res Pharm Sci. 2019;10(4):2875–2885. doi:10.26452/ijrps.v10i4.1567
18. Algin-Yapar E, Inal Ö. Transdermal spray in hormone delivery. Trop J Pharm Res. 2014;13(3):469. doi:10.4314/tjpr.v13i3.23
19. Kumar Mandal U, Chatterjee B, Husna F, Pauzi B. A review on transdermal spray: formulation aspect. Rev Transdermal Spray Formul Asp M J Pharma. 2016;1(1):6.
20. Ranade S, Bajaj A, Londhe V, Babul N, Kao D. Fabrication of topical metered dose film forming sprays for pain management. Eur J Pharm Sci. 2017;100:132–141. doi:10.1016/j.ejps.2017.01.004
21. Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm Sci. 2017;12(6):487–497. doi:10.1016/j.ajps.2017.07.004
22. Frederiksen K, Guy RH, Petersson K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin Drug Deliv. 2016;13(3):349–360. doi:10.1517/17425247.2016.1124412
23. Kim DS, Kim JS, Lee MC. Thin film forming technique based on hybrid spray coating using electrostatic force and air pressure. Jpn J Appl Phys. 2014;53(5S3):05HC08. doi:10.7567/JJAP.53.05HC08
24. Urkan E, Guler H, Komekci F. A review of electrostatic spraying for agricultural applications. Tarim Makinalari Bilim Derg. 2016;12(4):229–233.
25. Zhuang C, Zhong Y, Zhao Y. Effect of deacetylation degree on properties of Chitosan films using electrostatic spraying technique. Food Control. 2019;97:25–31. doi:10.1016/j.foodcont.2018.10.014
26. Zhong Y, Zhuang C, Gu W, Zhao Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Carbohydr Polym. 2019;212(May2018):197–205. doi:10.1016/j.carbpol.2019.02.048
27. Tran TTD, Tran PHL. Controlled release film forming systems in drug delivery: the potential for efficient drug delivery. Pharmaceutics. 2019;11(6):290. doi:10.3390/pharmaceutics11060290
28. Baio FHR, Antuniassi UR, Castilho BR, Teodoro PE, Silva EE. Correction: factors affecting aerial spray drift in the Brazilian Cerrado. PLoS One. 2019;14(6):e0217957. doi:10.1371/journal.pone.0217957
29. Gaytan I, Nicolas B, Gouriou F, Leru JP, Mallarach J. Effect of working pressure, fluid temperature, nozzle type and nozzle orifice size, on spray characteristics using viscous feed additive DL-2-hydroxy-4-(methylthio)-butanoic-acid. Powder Technol. 2018;336(2017):383–392. doi:10.1016/j.powtec.2018.05.045
30. Bakshi A, Bajaj A, Malhotra G, Madan M, Amrutiya N. A novel metered dose transdermal spray formulation for oxybutynin. Indian J Pharm Sci. 2008;70(6):733–739. doi:10.4103/0250-474X.49094
31. Geh KJ, Stelzl A, Gröne A, Wagner L, Förster B, Winter G. Development of a sprayable hydrogel formulation for the skin application of therapeutic antibodies. Eur J Pharm Biopharm. 2019;142(November2018):123–132. doi:10.1016/j.ejpb.2019.06.015
32. Lu W, Luo H, Wu Y, Zhu Z, Wang H. Preparation and characterization of a metered dose transdermal spray for testosterone. Acta Pharm Sin B. 2013;3(6):392–399. doi:10.1016/j.apsb.2013.10.003
33. Lu W, Luo H, Zhu Z, Wu Y, Luo J, Wang H. Preparation and the biopharmaceutical evaluation for the metered dose transdermal spray of dexketoprofen. J Drug Deliv. 2014;2014:1–12. doi:10.1155/2014/697434
34. Paradkar M, Thakkar V, Soni T, Gandhi T, Gohel M. Formulation and evaluation of clotrimazole transdermal spray. Drug Dev Ind Pharm. 2015;41(10):1718–1725. doi:10.3109/03639045.2014.1002408
35. Tan X, Feldman SR, Chang J, Balkrishnan R. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9(10):1263–1271. doi:10.1517/17425247.2012.711756
36. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatology Venereol. 2012;26:61–67. doi:10.1111/j.1468-3083.2012.04525.x
37. Dhiman S, Singh TG, Rehni AK. Transdermal patches: a recent approch to new drug delivery system. Int J Pharm Pharm Sci. 2011;3(SUPPL. 5):26–34.
38. Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20(1):39–53. doi:10.1097/00129334-200701000-00013
39. Bishop SM, Walker M, Rogers AA, Chen WYJ. Importance of moisture balance at the wound-dressing interface. J Wound Care. 2003;12(4):125–128. doi:10.12968/jowc.2003.12.4.26484
40. Gohel MC, Nagori SA. Fabrication of modified transport fluconazole transdermal spray containing ethyl cellulose and eudragit® RS100 as film formers. AAPS PharmSciTech. 2009;10(2):684–691. doi:10.1208/s12249-009-9256-8
41. Mori NM, Patel P, Sheth NR, Rathod LV, Ashara KC. Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Bull Fac Pharmacy Cairo Univ. 2017;55(1):41–51. doi:10.1016/j.bfopcu.2017.01.001
42. Rajab NA. Preparation and evaluation of ketoprofen as dermal spray film. Kerbala J Pharm Sci. 2013;6:1–8.
43. Saingam W, Chankana N, Madaka F, Sueree L, Homchuam S. Formulation development of topical film formimg spray from Piper nigrum L. Thai J Pharm Sci. 2018;42(supplement):93–97. doi:10.1134/S0965545X11100087
44. Sukhbir K, Navneet K, Sharma AK, Kapil K. Development of modified transdermal spray formulation of psoralen extract. Der Pharm Lett. 2013;5(2):85–94.
45. Hakim M, Walia H, Rafiq M, Grannell T, Cartabuke RS, Tobias JD. Oxymetazoline metered dose spray: factors affecting delivery volume. J Pediatr Pharmacol Ther. 2016;21(3):247–251. doi:10.5863/1551-6776-21.3.247
46. Ru Y, Gan Y, Zheng J, Zhou H. Design and experiments on droplet charging device for high-range electrostatic sprayer. Am Soc Agric Biol Eng Annu Int Meet 2008. 2008;1:561–571. doi:10.5772/18546
47. Barringer SA, Sumonsiri N. Electrostatic coating technologies for food processing. Annu Rev Food Sci Technol. 2015;6(1):157–169. doi:10.1146/annurev-food-022814-015526
48. Abu-Ali J, Barringer SA. Method for electrostatic atomization of emulsions in an EHD system. J Electrostat. 2005;63(5):361–369. doi:10.1016/j.elstat.2004.11.004
49. Kwon S-I, Kyung K-H, Park J-Y, et al. Uniform anti-reflective films fabricated by layer-by-layer ultrasonic spray method. Colloids Surf a Physicochem Eng Asp. 2019;580((August):123785):123785. doi:10.1016/j.colsurfa.2019.123785
50. Oh DW, Kang JH, Lee HJ, et al. Formulation and in vitro/in vivo evaluation of chitosan-based film forming gel containing ketoprofen. Drug Deliv. 2017;24(1):1056–1066. doi:10.1080/10717544.2017.1346001
51. Zarrintaj P, Jouyandeh M, Ganjali MR, et al. Thermo-sensitive polymers in medicine: a review. Eur Polym J. 2019;117:402–423. doi:10.1016/j.eurpolymj.2019.05.024
52. Kim Y-J, Matsunaga YT. Thermo-responsive polymers and their application as smart biomaterials. J Mater Chem B. 2017;5(23):4307–4321. doi:10.1039/C7TB00157F
53. Jun L, Bochu W, Yazhou W. Thermo-sensitive polymers for controlled-release drug delivery systems. Int J Pharmacol. 2006;2(5):513–519. doi:10.3923/ijp.2006.513.519
54. Licea-Claverie A, Schwarz S, Steinbach C, Montserrat Ponce-Vargas S, Genest S. Combination of natural and thermosensitive polymers in flocculation of fine silica dispersions. Int J Carbohydr Chem. 2013;2013:1–8. doi:10.1155/2013/242684
55. Shao P, Wang B, Wang Y, Li J, Zhang Y. The application of thermosensitive nanocarriers in controlled drug delivery. J Nanomater. 2011;2011:1–12. doi:10.1155/2011/389640
56. Sánchez-Moreno P, de Vicente J, Nardecchia S, Marchal J, Boulaiz H. Thermo-sensitive nanomaterials: recent advance in synthesis and biomedical applications. Nanomaterials. 2018;8(11):935. doi:10.3390/nano8110935
57. Kocak G, Tuncer C, Bütün V. pH-Responsive polymers. Polym Chem. 2017;8(1):144–176. doi:10.1039/C6PY01872F
58. Mutalabisin MF, Chatterjee B, Jaffri JM. PH responsive polymers in drug delivery. Res J Pharm Technol. 2018;11(11):5115. doi:10.5958/0974-360X.2018.00934.4
59. Reyes-Ortega F. pH-responsive polymers: properties, synthesis and applications. In: Smart Polymers and Their Applications. Elsevier; 2014:45–92. doi:10.1533/9780857097026.1.45
60. Rizwan M, Yahya R, Hassan A, et al. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers (Basel). 2017;9(12):137. doi:10.3390/polym9040137
61. Yoshida T, Lai TC, Kwon GS, Sako K. pH- and ion-sensitive polymers for drug delivery. Expert Opin Drug Deliv. 2013;10(11):1497–1513. doi:10.1517/17425247.2013.821978
62. Al-Anazi HA, Sharma MM. Use of a pH sensitive polymer for conformance control. In: International Symposium and Exhibition on Formation Damage Control. Society of Petroleum Engineers; 2002. doi:10.2118/73782-MS
63. Grande AM, Martin R, Odriozola I, van der Zwaag S, Garcia SJ. Effect of the polymer structure on the viscoelastic and interfacial healing behaviour of poly(urea-urethane) networks containing aromatic disulphides. Eur Polym J. 2017;97:120–128. doi:10.1016/j.eurpolymj.2017.10.007
64. Gayle AJ, Cook RF. Mapping viscoelastic and plastic properties of polymers and polymer-nanotube composites using instrumented indentation. J Mater Res. 2016;31(15):2347–2360. doi:10.1557/jmr.2016.207
65. Mohamed F, Flämig M, Hofmann M, et al. Scaling analysis of the viscoelastic response of linear polymers. J Chem Phys. 2018;149(4):44902. doi:10.1063/1.5038643
66. Meng R, Yin D, Drapaca CS. A variable order fractional constitutive model of the viscoelastic behavior of polymers. Int J Non Linear Mech. 2019;113:171–177. doi:10.1016/j.ijnonlinmec.2019.04.002
67. Yu C, Kang G, Lu F, Zhu Y, Chen K. Viscoelastic–viscoplastic cyclic deformation of polycarbonate polymer: experiment and constitutive model. J Appl Mech. 2016;83(4). doi:10.1115/1.4032374
68. Leichtnam M-L, Rolland H, Wüthrich P, Guy RH. Impact of antinucleants on transdermal delivery of testosterone from a spray. J Pharm Sci. 2007;96(1):84–92. doi:10.1002/jps.20670
69. Ter Horst B, Moakes RJA, Chouhan G, Williams RL, Moiemen NS, Grover LM. A gellan-based fluid gel carrier to enhance topical spray delivery. Acta Biomater. 2019;89:166–179. doi:10.1016/j.actbio.2019.03.036
70. Mahdi MH, Conway BR, Smith AM. Development of mucoadhesive sprayable gellan gum fluid gels. Int J Pharm. 2015;488(1–2):12–19. doi:10.1016/j.ijpharm.2015.04.011
71. Wang S, He X, Song J, Wang S, Jia X, Ling Y. Effects of xanthan gum on atomization and deposition characteristics in water and Silwet 408 aqueous solution. Int J Agric Biol Eng. 2018;11(3):29–34. doi:10.25165/j.ijabe.20181103.3802
72. Kahya N. Water soluble chitosan derivatives and their biological activities: a review. Polym Sci. 2018; 4(2):1–11. DOI:10.4172/2471-9935.100043
73. Qun G, Ajun W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr Polym. 2006;64(1):29–36. doi:10.1016/j.carbpol.2005.10.026
74. Zhong Y, Li Y. Effects of surfactants on the functional and structural properties of kudzu (Pueraria lobata) starch/ascorbic acid films. Carbohydr Polym. 2011;85(3):622–628. doi:10.1016/j.carbpol.2011.03.031
75. Sriwidodo, Subroto T, Maksum IP, Subarnas A, et al. Preparation and optimization of chitosan-hegf nanoparticle using ionic gelation method stabilized by polyethylene glycol (PEG) for wound healing therapy. Int J Res Pharm Sci. 2020;11(1):1220–1230. doi:10.26452/ijrps.v11i1.1962
76. Jug M, Bećirević-Laćan M, Bengez S. Novel cyclodextrin-based film formulation intended for buccal delivery of atenolol. Drug Dev Ind Pharm. 2009;35(7):796–807. doi:10.1080/03639040802596212
77. Morales ME, Ruiz MA. Microencapsulation of probiotic cells: applications in nutraceutic and food industry. In: Nutraceuticals. Elsevier; 2016:627–668. doi:10.1016/B978-0-12-804305-9.00016-6
78. Bradbeer JF, Hancocks R, Spyropoulos F, Norton IT. Self-structuring foods based on acid-sensitive low and high acyl mixed gellan systems to impact on satiety. Food Hydrocoll. 2014;35:522–530. doi:10.1016/j.foodhyd.2013.07.014
79. Grip J, Engstad RE, Skjæveland I, Škalko-Basnet N, Holsæter AM. Sprayable Carbopol hydrogel with soluble beta-1,3/1,6-glucan as an active ingredient for wound healing – development and in-vivo evaluation. Eur J Pharm Sci. 2017;107(April):24–31. doi:10.1016/j.ejps.2017.06.029
80. Edwards A, Qi S, Liu F, Brown MB, McAuley WJ. Rationalising polymer selection for supersaturated film forming systems produced by an aerosol spray for the transdermal delivery of methylphenidate. Eur J Pharm Biopharm. 2017;114:164–174. doi:10.1016/j.ejpb.2017.01.013
81. Gohli T, Shah P. Formulation and development of transdermal spray of ibandronate sodium. 2019;1.
82. Reid ML, Benaouda F, Khengar R, Jones SA, Brown MB. Topical corticosteroid delivery into human skin using hydrofluoroalkane metered dose aerosol sprays. Int J Pharm. 2013;452(1–2):157–165. doi:10.1016/j.ijpharm.2013.04.083
83. Varges PR, Costa CM, Fonseca BS, Naccache MF, Mendes PRDS. Rheological characterization of carbopol® dispersions in water and in water/glycerol solutions. Fluids. 2019;4(3):1–20. doi:10.3390/fluids4010003
84. Jones A, Vaughan D. Hydrogel dressings in the management of a variety of wound types: a review. J Orthop Nurs. 2005;9:S1–S11. doi:10.1016/S1361-3111(05)80001-9
85. Patra CN, Priya R, Swain S, Kumar Jena G, Panigrahi KC, Ghose D. Pharmaceutical significance of Eudragit: a review. Futur J Pharm Sci. 2017;3(1):33–45. doi:10.1016/j.fjps.2017.02.001
86. Nandy B, Mazumder B. Formulation and characterizations of delayed release multi-particulates system of indomethacin: optimization by response surface methodology. Curr Drug Deliv. 2014;11(1):72–86. doi:10.2174/15672018113109990041
87. Hasanovic A, Hollick C, Fischinger K, Valenta C. Improvement in physicochemical parameters of DPPC liposomes and increase in skin permeation of aciclovir and minoxidil by the addition of cationic polymers. Eur J Pharm Biopharm. 2010;75(2):148–153. doi:10.1016/j.ejpb.2010.03.014
88. Raghavan S, Trividic A, Davis A, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212(2):213–221. doi:10.1016/S0378-5173(00)00610-4
89. Pellett MA, Roberts MS, Hadgraft J. Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm. 1997;151(1):91–98. doi:10.1016/S0378-5173(97)04897-7
90. Jagtap PS, Tagad RR, Shendge RS. A brief review on Kollidon. J Drug Deliv Ther. 2019;9(2):493–500. doi:10.22270/jddt.v9i2.2539
91. Maitra J, Shukla VK. Cross-linking in hydrogels - a review. Am J Polym Sci. 2014;4(2):25–31. doi:10.5923/j.ajps.20140402.01
92. Kahn M. Bioavailability of vitamin B12 using a small-volume nebulizer ophthalmic drug delivery system. Clin Exp Ophthalmol. 2005;33(4):402–407. doi:10.1111/j.1442-9071.2005.01030.x
93. Ho H-O, Chen L-C, Lin H-M, Sheu M-T. Penetration enhancement by menthol combined with a solubilization effect in a mixed solvent system. J Control Release. 1998;51(2–3):301–311. doi:10.1016/S0168-3659(97)00184-3
94. Aqil M, Ahad A, Sultana Y, Ali A. Status of terpenes as skin penetration enhancers. Drug Discov Today. 2007;12(23–24):1061–1067. doi:10.1016/j.drudis.2007.09.001
95. Rhee Y-S, Choi J-G, Park E-S, Chi S-C. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228(1–2):161–170. doi:10.1016/S0378-5173(01)00827-4
96. Trommer H, Neubert RHH. Overcoming the Stratum Corneum: the Modulation of Skin Penetration. Skin Pharmacol Physiol. 2006;19(2):106–121. doi:10.1159/000091978
97. Haque T, Talukder MMU. Chemical enhancer: a simplistic way to modulate barrier function of the stratum corneum. Adv Pharm Bull. 2018;8(2):169–179. doi:10.15171/apb.2018.021
98. Trottet L, Merly C, Mirza M, Hadgraft J, Davis A. Effect of finite doses of propylene glycol on enhancement of in vitro percutaneous permeation of loperamide hydrochloride. Int J Pharm. 2004;274(1–2):213–219. doi:10.1016/j.ijpharm.2004.01.013
99. Leichtnam M-L, Rolland H, Wüthrich P, Guy RH. Formulation and evaluation of a testosterone transdermal spray. J Pharm Sci. 2006;95(8):1693–1702. doi:10.1002/jps.20641
100. Suyatama NE, Tighzert L, Copinet A. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J Agric Food Chem. 2005;53(10):3950–3957.
101. Vervaet C, Byron PR. Drug–surfactant–propellant interactions in HFA-formulations. Int J Pharm. 1999;186(1):13–30. doi:10.1016/S0378-5173(99)00134-9
102. Ali S, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261–267. doi:10.2340/00015555-1531
103. Mcardle C, Lagan K, Spence S, Mcdowell D. Diabetic foot ulcer wound fluid: the effects of pH on DFU bacteria and infection. J foot ankle res. 2015;8(Suppl 1):1–2. doi:10.1186/1757-1146-8-S1-A8
104. Sharpe JR, Booth S, Jubin K, Jordan NR, Lawrence-Watt DJ, Dheansa BS. Progression of wound pH during the course of healing in burns. J Burn Care Res. 2013;34(3):e201–e208. doi:10.1097/BCR.0b013e31825d5569
105. Vávrová K, Lorencová K, Klimentová J, Novotný J, Holý A, Hrabálek A. Transdermal and dermal delivery of adefovir: effects of pH and permeation enhancers. Eur J Pharm Biopharm. 2008;69(2):597–604. doi:10.1016/j.ejpb.2007.12.005
106. Woodall R, Arnold JJ, McKay D, Asbill CS. Effect of formulation pH on transdermal penetration of antiemetics formulated in poloxamer lecithin organogel. Int J Pharm Compd. 2013;17(3):247–253.
107. Pennington AK, Ratcliffe JH, Wilson CG, Hardy JG. The influence of solution viscosity on nasal spray deposition and clearance. Int J Pharm. 1988;43(3):221–224. doi:10.1016/0378-5173(88)90277-3
108. Trows S, Wuchner K, Spycher R, Steckel H. Analytical challenges and regulatory requirements for nasal drug products in Europe and the U.S. Pharmaceutics. 2014;6(2):195–219. doi:10.3390/pharmaceutics6020195
109. Liu X, Doub WH, Guo C. Assessment of the Influence factors on nasal spray droplet velocity using phase-doppler anemometry (PDA). AAPS PharmSciTech. 2011;12(1):337–343. doi:10.1208/s12249-011-9594-1
110. Umar A, Wathoni N, Hasanah A, Kurniawansyah I, Abdassah M. Kahar method: a novel calculation method of tonicity adjustment. J Pharm Bioallied Sci. 2019;11(8):635. doi:10.4103/jpbs.JPBS_210_19
111. Ovarlez G. Introduction to the rheometry of complex suspensions. In: Understanding the Rheology of Concrete. Elsevier; 2012:23–62. doi:10.1533/9780857095282.1.23
112. Lochhead RY. The Use of Polymers in Cosmetic Products. In: Cosmetic Science and Technology. Elsevier; 2017:171–221. doi:10.1016/B978-0-12-802005-0.00013-6
113. Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Control Release. 2009;136(2):88–98. doi:10.1016/j.jconrel.2009.02.013
114. Rasool B, Aziz U, Sarheed O, Rasool A. Design and evaluation of a bioadhesive film for transdermal delivery of propranolol hydrochloride. Acta Pharm. 2011;61(3):271–282. doi:10.2478/v10007-011-0025-3
115. Nnamani PO, Kenechukwu FC, Dibua EU, et al. Formulation, characterization and ex-vivo permeation studies on gentamicin-loaded transdermal patches based on PURASORB® polymers. 2013;8(22):973–982. doi:10.5897/SRE2013.5379
116. Kenechukwu FC, Attama AA, Ibezim EC, et al. Surface-modified mucoadhesive microgels as a controlled release system for miconazole nitrate to improve localized treatment of vulvovaginal candidiasis. Eur J Pharm Sci. 2018;111:358–375. doi:10.1016/j.ejps.2017.10.002
117. Stevens ES, Poliks MD. Tensile strength measurements on biopolymer films. J Chem Educ. 2003;80(7):810. doi:10.1021/ed080p810
118. Zurdo Schroeder I, Franke P, Schaefer UF, Lehr C-M. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur J Pharm Biopharm. 2007;65(1):111–121. doi:10.1016/j.ejpb.2006.07.015
119. Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923. doi:10.1002/jps.21210
120. Thomas S, Hay P. Fluid handling properties of hydrogel dressings. Ostomy Wound Manage. 1995;41(3):
121. Thomas S, Hay NP. Assessing the hydro-affinity of hydrogel dressings. J Wound Care. 1994;3(2):89–91. doi:10.12968/jowc.1994.3.2.89
122. Koupil J, Brychta P, Horký D, Smola J, Prásek J. The influence of moisture wound healing on the incidence of bacterial infection and histological changes in healthy human skin after treatment of interactive dressings. Acta Chir Plast. 2003;45(3):89–94.
123. Patricia Miranda S, Garnica O, Lara-Sagahon V, Cárdenas G. Water vapor permeability and mechanical properties of chitosan films. J Chil Chem Soc. 2004;49(2):2. doi:10.4067/S0717-97072004000200013
124. Hu Y, Topolkaraev V, Hiltner A, Baer E. Measurement of water vapor transmission rate in highly permeable films. J Appl Polym Sci. 2001;81(7):1624–1633. doi:10.1002/app.1593.abs
125. Ofokansi KC, Kenechukwu FC, Ogwu NN. Design of novel miconazole nitrate transdermal films based on Eudragit RS100 and HPMC hybrids: preparation, physical characterization, in vitro and ex vivo studies. Drug Deliv. 2015;22(8):1078–1085. doi:10.3109/10717544.2013.875604
126. Nnamani O. Characterization and controlled release of gentamicin from novel hydrogels based on Poloxamer 407 and polyacrylic acids. Afr J Pharm Pharmacol. 2013;7(36):2540–2552. doi:10.5897/AJPP2013.3803
127. Joshi M. Role of Eudragit in targeted drug delivery. Int J Curr Pharm Res. 2013;5(2):58–62.
128. Pereira GG, Guterres SS, Balducci AG, Colombo P, Sonvico F. Polymeric films loaded with vitamin e and aloe vera for topical application in the treatment of burn wounds. Biomed Res Int. 2014;2014:1–9. doi:10.1155/2014/641590
129. Sathali AAH, Rajalakshmi G. Evaluation of transdermal targeted niosomal drug delivery of terbinafine hydrochloride. Int J PharmTech Res. 2010;2(3):2081–2089.
130. Nnamani PO, Kenechukwu FC, Dibua EU, Ogbonna CC, Monemeh UL, Attama AA. Transdermal microgels of gentamicin. Eur J Pharm Biopharm. 2013;84(2):345–354. doi:10.1016/j.ejpb.2012.11.015
131. Turabee MH, Jeong TH, Ramalingam P, Kang JH, Ko YT. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr Polym. 2019;203:302–309. doi:10.1016/j.carbpol.2018.09.065
132. Han X, Meng X, Wu Z, Wu Z, Qi X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater Sci Eng C. 2018;93:1064–1072. doi:10.1016/j.msec.2018.08.064
133. Qi X, Qin X, Yang R, et al. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium–loaded alginate microspheres. J Pharm Sci. 2016;105(1):122–130. doi:10.1016/j.xphs.2015.11.019
134. Zhang D, Sun P, Li P, et al. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette–Guérin in the treatment of bladder cancer. Biomaterials. 2013;34(38):10258–10266. doi:10.1016/j.biomaterials.2013.09.027
135. Kim AR, Lee SL, Park SN. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int J Biol Macromol. 2018;118:731–740. doi:10.1016/j.ijbiomac.2018.06.061
136. Fathi M, Alami-Milani M, Geranmayeh MH, Barar J, Erfan-Niya H, Omidi Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int J Biol Macromol. 2019;128:957–964. doi:10.1016/j.ijbiomac.2019.01.122
137. Song K, Li L, Yan X, et al. Characterization of human adipose tissue-derived stem cells in vitro culture and in vivo differentiation in a temperature-sensitive chitosan/β- glycerophosphate/collagen hybrid hydrogel. Mater Sci Eng C. 2017;70:231–240. doi:10.1016/j.msec.2016.08.085
138. Niranjan R, Koushik C, Saravanan S, Moorthi A, Vairamani M, Selvamurugan N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int J Biol Macromol. 2013;54:24–29. doi:10.1016/j.ijbiomac.2012.11.026
139. Niu Y, Chen KC, He T, Yu W, Huang S, Xu K. Scaffolds from block polyurethanes based on poly(ɛ-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials. 2014;35(14):4266–4277. doi:10.1016/j.biomaterials.2014.02.013
140. Osman A, Oner ET, Eroglu MS. Novel levan and pNIPA temperature sensitive hydrogels for 5-ASA controlled release. Carbohydr Polym. 2017;165:61–70. doi:10.1016/j.carbpol.2017.01.097
141. Lei Z, Singh G, Min Z, et al. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for the management of severe skin wound healing. Mater Sci Eng C. 2018;90:159–167. doi:10.1016/j.msec.2018.04.045
142. Tang B, Shan J, Yuan T, et al. Hydroxypropylcellulose enhanced high viscosity endoscopic mucosal dissection intraoperative chitosan thermosensitive hydrogel. Carbohydr Polym. 2019;209:198–206. doi:10.1016/j.carbpol.2018.12.103
143. Paul A, Hasan A, Kindi HA, et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8(8):8050–8062. doi:10.1021/nn5020787
144. Prabaharan M. Bioactivity of Chitosan Derivative. In: Polysaccharides. Cham: Springer International Publishing;2014:1–14. doi:10.1007/978-3-319-03751-6_17-1
145. Laroche C, Delattre C, Mati-Baouche N, et al. Bioactivity of chitosan and its derivatives. Curr Org Chem. 2018;22(7):641–667. doi:10.2174/1385272821666170811114145
146. Xia W, Liu P, Zhang J, Chen J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011;25(2):170–179. doi:10.1016/j.foodhyd.2010.03.003
147. Kim S. Competitive biological activities of chitosan and its derivatives: antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int J Polym Sci. 2018;2018:1–13. doi:10.1155/2018/1708172
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
