Back to Journals » Drug Design, Development and Therapy » Volume 10
Fenretinide inhibits macrophage inflammatory mediators and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway
Authors Lin CH, Lee SY, Zhang CC, Du YF, Hung HC, Wu HT, Ou HY
Received 15 June 2016
Accepted for publication 8 September 2016
Published 1 November 2016 Volume 2016:10 Pages 3591—3597
DOI https://doi.org/10.2147/DDDT.S114879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr James Janetka
Ching-Han Lin,1,* Shang-Yu Lee,2,* Chun-Cheng Zhang,3 Ye-Fong Du,1 Hao-Chang Hung,1 Hung-Tsung Wu,4 Horng-Yih Ou1
1Department of Internal Medicine, Division of Endocrinology and Metabolism, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, 2Department of Internal Medicine, Division of Endocrinology and Metabolism, Chi-Mei Medical Center, 3Department of Internal Medicine, Division of Holistic Care, Chi-Mei Medical Center, 4Research Center of Clinical Medicine, National Cheng Kung University Hospital, Tainan, Taiwan
*These authors contributed equally to this work
Abstract: Fenretinide is a novel anticancer agent reported to exhibit anti-invasive and antimetastatic activities. It has also been shown to improve obesity and diabetes, although the effects of fenretinide on hypertension are still unknown, and the detailed mechanisms remain unclear. In this study, we have shown that treatment with lipopolysaccharide (LPS) decreased the expression of peroxisome proliferator-activated receptor γ (PPARγ) in RAW264.7 macrophages, and pretreatment with fenretinide reversed the effect of LPS on PPARγ expression. In addition, LPS-induced pro-inflammatory cytokine production, including tumor necrosis factor-α, interleukin 6, and monocyte chemoattractant protein 1 were dose-dependently reversed by fenretinide, and the effects of fenretinide on LPS-induced pro-inflammatory cytokine production were blocked by treatment with PPARγ antagonist. Moreover, fenretinide decreased LPS-induced inducible nitric oxide synthase expression and nitrogen oxide production. These effects were blocked by the pretreatment with PPARγ antagonist in a dose-dependent manner, indicating fenretinide activated PPARγ to exert anti-inflammation activity. In view of the role of inflammation in hypertension and the anti-inflammatory action of fenretinide, we found that administration of fenretinide in spontaneously hypertensive rats significantly decreased blood pressure. Taken together, these results indicate that fenretinide might be a potent antihypertensive agent that works by suppressing inflammation via activating PPARγ.
Keywords: fenretinide, hypertension, inflammation, macrophage, peroxisome proliferator-activated receptor γ
Introduction
All-trans retinoic acid is extensively used for the treatment of acute promyelocytic leukemia. However, in order to reduce liver toxicity and any other side effects of the treatment, several analogues have been synthesized, such as fenretinide.1 Fenretinide induces tumor cell apoptosis by mitochondrial depolarization, caspase-9 activation, and caspase-3 activation.2 In addition, fenretinide corrects a phospholipid-bound fatty acid imbalance that impacts the phosphorylation of extracellular signal-regulated kinase (ERK)1/2 to modulate inflammatory cytokine expression in macrophages.3 A recent study also demonstrated that fenretinide prevents obesity and hepatic steatosis in high-fat diet-induced obese mice.4 Furthermore, fenretinide ameliorates insulin resistance through an increase in the clearance of retinol binding protein-4 in spontaneously hypertensive rats (SHR).5 Although the role of fenretinide in the improvement of insulin resistance is known, the effects of fenretinide on blood pressure remain unclear.
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor that is involved in many functions, such as regulation of vascular tone,6,7 inflammation,8 and energy homeostasis.9 Therefore, activation of PPARγ has therapeutic effects on hypertension, obesity, inflammation, and metabolic diseases.10 In addition, PPARγ directly regulates the transcription of pro-inflammatory genes to influence the inflammatory responses.11
Although it is known that fenretinide is a ligand for PPARγ,12 the role of PPARγ in fenretinide-induced anti-inflammatory activity remains unknown. In addition, the effect of fenretinide on blood pressure is unclear. We thus used a PPARγ antagonist, GW9662, to evaluate the role of PPARγ in fenretinide-induced anti-inflammatory effect on lipopolysaccharide (LPS)-induced pro-inflammatory cytokines release. In addition, the antihypertensive effect of fenretinide was also evaluated.
Materials and methods
Cell culture
The murine RAW264.7 macrophage cell line was a gift from Prof CL Wu (Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University Tainan, Taiwan). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone) at 37°C in a 5% CO2 incubator.
Animals
Eight-week-old Wistar-Kyoto rats (WKY) and SHR (Bio-LASCO Taiwan Co. Ltd, Taipei, Taiwan) were housed in the Animal Center of National Cheng Kung University Medical College in a temperature- (25°C±1°C) and humidity-controlled (60%±5%) room, kept on a 12:12 light–dark cycle (light on at 06.00 am), and randomly divided into four groups (n=7–10/group). WKY and SHR control groups were administered normal saline of the same volume of tested drugs. The valsartan-treated SHR group was administered 15 mg/kg valsartan (Diovan®; Novartis AG, Basel, Switzerland) orally twice a day. The fenretinide-treated SHR group was fed with standard diet supplemented with 737 μmol/kg diet of fenretinide (Taizhou Hikong Chemical Co., Ltd, Zhejiang, People’s Republic of China) for 1 week, following our previous study.5 A noninvasive tail-cuff method with a computer-assisted detection device was used to measure blood pressure in conscious animals (MK-2000; Muromachi, Tokyo, Japan). At the end of the experiment, all the rats were sacrificed under well-anesthetized conditions. The animal procedures were approved by the Institutional Animal Care and Use Committee of the National Cheng Kung University, and were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institute of Health in Taiwan.
Western blot analysis
The cells or the aorta of the rats with different treatments were harvested and lysed with a radioimmunoprecipitation assay buffer (Abcam, Cambridge, MA, USA) containing protease inhibitors (Sigma-Aldrich, St Louis, MO, USA). The protein lysates were quantified and analyzed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting. The membrane was probed with 1:1,000 primary antibodies, including PPARγ, and inducible nitric oxide synthase (iNOS; Abcam) at 4°C overnight. A 1:5,000 dilution of horseradish peroxidase-conjugated secondary antibodies was added and incubated on the membrane at room temperature for 1 hour. The protein bands were visualized using an enhanced chemiluminescence kit (PerkinElmer, Waltham, MA, USA), and the optical densities were clarified using VisionWorks LS software (Upland, CA, USA).
Measurements of the pro-inflammatory cytokines and nitrate/nitrite levels
A nitrate/nitrite fluorometric assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) was used to measure NO concentrations. The levels of pro-inflammatory cytokines were determined using commercial enzyme-linked immunosorbent assay kits (BioLegend, San Diego, CA, USA).
Statistical analysis
Student’s t-test or one-way analysis of variance was used to test the differences in Western blots, pro-inflammatory cytokines and NO release between control group and LPS-treated group, or between LPS-treated group and fenretinide-treated groups, using MS Excel version 2007, Taipei, Taiwan and SPSS version 18, Chicago, IL, USA. The statistical significance was set at P<0.05.
Results
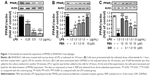
Fenretinide increased the expression of PPARγ in RAW264.3 macrophages
Consistent with one previous study,13 treatment with LPS dose dependently decreased the expression of PPARγ in RAW264.3 macrophages (Figure 1A). Pretreatment with fenretinide significantly reversed the effects of LPS on PPARγ expression in a dose-dependent manner (Figure 1B). Moreover, blockade of PPARγ by GW9662 pretreatment partially inhibited the effects of fenretinide on the LPS-induced decrease in PPARγ (Figure 1C).
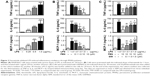
Fenretinide inhibited LPS-induced inflammatory mediators through the PPARγ pathway
A recent study indicated that fenretinide inhibited the levels of inflammatory mediators through the ERK1/2 pathway. In addition, activation of PPARγ by agonists exerts an anti-inflammatory effect.3 We thus further evaluated the role of PPARγ in the fenretinide-induced anti-inflammatory effect (Figure 2). As shown in Figure 2A, LPS significantly increased the release of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin (IL) 6, and monocyte chemoattractant protein-1 (MCP-1), in macrophages. Administration of various doses of fenretinide inhibited LPS-induced release of the pro-inflammatory cytokines (Figure 2B). Furthermore, pretreatment with GW9662 partially reversed the effects of fenretinide on the LPS-induced release of pro-inflammatory cytokines (Figure 2C).
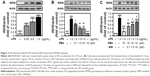
Fenretinide inhibited LPS-induced iNOS expression and NO production through PPARγ pathway
We further examined the effects of fenretinide on the expression of iNOS (Figure 3). As shown in Figure 3A, LPS significantly increased the expression of iNOS. Administration of various doses of fenretinide inhibited LPS-induced iNOS expression (Figure 3B). Furthermore, pretreatment with GW9662 partially reversed the effects of fenretinide on LPS-induced iNOS expression (Figure 3C). Following the increase in iNOS expression, LPS significantly increased the production of NO (Figure 4A). Administration of fenretinide dose dependently inhibited LPS-induced NO production (Figure 4B). Furthermore, pretreatment with GW9662 partially reversed the effects of fenretinide on LPS-induced NO expression (Figure 4C).
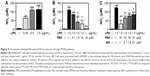
Fenretinide controlled hypertension in SHR
In view of the anti-inflammatory effect, and the ability to inhibit the iNOS-induced NO production of fenretinide, we further evaluated the effects of fenretinide on mean and systolic blood pressure in SHR (Figure 5A and B). Administration of fenretinide significantly decreased the mean and systolic blood pressure of SHR. In addition, the antihypertensive activity showed no significant differences between valsartan and fenretinide. In order to confirm that fenretinide activated PPARγ to regulate blood pressure, we tested the expression of PPARγ in the aorta after treatment with fenretinide. As shown in Figure 5C, we found that the expression of PPARγ was significantly increased in the aorta of SHR, compared with the WKY control group. Administration of fenretinide further enhanced the expression of PPARγ in SHR aorta.
Discussion
In the present study, we provide evidence that fenretinide activated PPARγ to exert an anti-inflammatory effect. Treatment of macrophages with fenretinide inhibited LPS-induced pro-inflammatory cytokines and NO release, and inhibition of PPARγ reversed the pro-inflammatory effects of fenretinide, indicating that fenretinide exhibited anti-inflammatory activity in a PPARγ-dependent manner. In addition, to the best of our knowledge, this is the first report that investigated the antihypertensive activity of fenretinide, in view of its anti-inflammatory activity.
PPARs are ligand-activated transcription factors and key modulators for lipid and glucose homeostasis in adipose tissues.14 A previous study indicated that fenretinide inhibited adipocyte differentiation by blocking CCAAT/enhancer-binding protein α/PPARγ-mediated induction of downstream genes.15 However, Harris et al demonstrated that fenretinide is a ligand for PPARγ and functionally activates PPARγ at clinically achievable doses to potentially be used for antidiabetes and obesity therapies.12 Fenretinide also prevents hepatic steatosis by inducing an increased plasma adiponectin level, increased activation of hepatic AMP-activated protein kinase, and the expression of PPARα and peroxisomal acyl-CoA oxidase, which promote fat oxidation. However, the mechanisms by which fenretinide improves metabolic diseases remain obscure in the published literature. In the present study, we found a novel pathway by which fenretinide activated PPARγ to exert an anti-inflammatory action, in contrast to a previous study indicating that fenretinide modulates inflammatory cytokine expression in macrophages by correcting a phospholipid-bound fatty acid imbalance that impacts the phosphorylation of ERK1/2.3
The expression of PPARγ is found in all relevant cells of the vasculature.16,17 In numerous studies, PPARγ ligands have been found to reduce inflammatory gene expression, including TNF-α, and IL-6 in vascular cells, to protect against vascular inflammation.18–20 PPARγ has also been detected in macrophages of human atherosclerotic lesions,17 and PPARγ agonists reduce the secretion of pro-inflammatory cytokines in human monocytes and inhibit macrophage activation in vitro.21 In our study, we found that fenretinide can inhibit the LPS-induced release of pro-inflammatory cytokines, such as TNF-α, IL-6, and MCP-1 expression in macrophages, which occurs mainly through the activation of PPARγ.
Obesity-induced pro-inflammatory cytokines are released in adipose tissue and contribute to the development of insulin resistance.22 In addition, chronic inflammation may promote hypertension by causing endothelial dysfunction and oxidative stress. The form of NOS expressed in macrophages, iNOS, is a high-output enzyme that can produce copious amounts of NO.23 Large quantities of NO could combine with superoxide to interfere with intracellular signaling by blocking the normal cycle of phosphorylation and dephosphorylation.24 It has been suggested that PPARγ activators can inhibit iNOS expression by downregulation of the transcription of iNOS.25 In the present study, we found that fenretinide can inhibit LPS-induced iNOS expression and the excessive production of NO, mainly through the activation of PPARγ. With regard to the anti-inflammatory action of fenretinide, it also exerted an antihypertensive effect in SHR.
There are several factors that are related to the development of hypertension, including impaired autonomic function, kidney diseases, and vascular functional and structural alterations. Inflammation is one of the key factors that is related to endothelial dysfunction and arterial stiffening.26 Blockade of inflammation signaling reduces blood pressure and the augmented vascular stiffness in SHR.27 In addition, administration of anti-inflammatory drugs decreases the main signs of vascular damage in hypertension, such as increased vessel stiffness and extracellular matrix deposition, increased vasoconstrictor responses, endothelial dysfunction, and vascular inflammation.28 Anti-inflammation is thus also a strategy to treat hypertension. Although Diep and Schiffrin found that the expressions of PPARs are significantly increased in SHR, they demonstrated that the changes in PPARγ expression may play a compensatory role in the remodeling of blood vessels in SHR.29 In the present study, we found that fenretinide can increase the expression of PPARγ to exert an anti-inflammatory activity, and this might participate in the antihypertensive effect. On the other hand, fenretinide not only increases PPARγ expression but also acts as a ligand to increase its activity.12 Furthermore, we found that the expression of PPARγ was increased in the aorta of SHR, and administration of fenretinide further enhanced the expression of PPARγ in the aorta of these rats, as shown in Figure 5C. These results are consistent with the results of the cell study.
Conclusion
In summary, we found that fenretinide can enhance the expression of PPARγ in macrophages, and further inhibit LPS-induced proinflammatory cytokine secretion, iNOS expression, and NO production. Fenretinide can also decrease the systolic pressure of SHR, possibly by activating PPARγ. Further investigations are needed to evaluate the anti-inflammatory and blood pressure-lowering effects of fenretinide in vivo.
Acknowledgment
The work was supported by the grants from the Ministry of Science and Technology of Republic of China (MOST 105-2321-B-006-021-, MOST 104-2314-B-006-029-MY2, and MOST 105-2314-B-006-054-MY3), and National Cheng Kung University Hospital (NCKUH-10204025 and NCKUH-10509005).
Disclosure
The authors report no conflicts of interest in this work.
References
Liu J, Li J, Zhang JF, Xin XY. Combination of fenretinide and selenite inhibits proliferation and induces apoptosis in ovarian cancer cells. Int J Mol Sci. 2013;14(11):21790–21804. | ||
Holmes WF, Soprano DR, Soprano KJ. Comparison of the mechanism of induction of apoptosis in ovarian carcinoma cells by the conformationally restricted synthetic retinoids CD437 and 4-HPR. J Cell Biochem. 2003;89(2):262–278. | ||
Lachance C, Wojewodka G, Skinner TA, Guilbault C, De Sanctis JB, Radzioch D. Fenretinide corrects the imbalance between omega-6 to omega-3 polyunsaturated fatty acids and inhibits macrophage inflammatory mediators via the ERK pathway. PLoS One. 2013;8(9):e74875. | ||
Koh IU, Jun HS, Choi JS, et al. Fenretinide ameliorates insulin resistance and fatty liver in obese mice. Biol Pharm Bull. 2012;35(3):369–375. | ||
Ou HY, Wu HT, Yang YC, Wu JS, Cheng JT, Chang CJ. Elevated retinol binding protein 4 contributes to insulin resistance in spontaneously hypertensive rats. Horm Metab Res. 2011;43(5):312–318. | ||
Usuda D, Kanda T. Peroxisome proliferator-activated receptors for hypertension. World J Cardiol. 2014;6(8):744–754. | ||
Ketsawatsomkron P, Sigmund CD. Molecular mechanisms regulating vascular tone by peroxisome proliferator activated receptor gamma. Curr Opin Nephrol Hypertens. 2015;24(2):123–130. | ||
Narala VR, Subramani PA, Narasimha VR, Shaik FB, Panati K. The role of nitrated fatty acids and peroxisome proliferator-activated receptor gamma in modulating inflammation. Int Immunopharmacol. 2014;23(1):283–287. | ||
Ahmadian M, Suh JM, Hah N, et al. PPAR gamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–566. | ||
Choi S-S, Park J, Choi JH. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Reports. 2014;47(11):599–608. | ||
Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771(8):926–935. | ||
Harris G, Ghazallah RA, Nascene D, Wuertz B, Ondrey FG. PPAR activation and decreased proliferation in oral carcinoma cells with 4-HPR. Otolaryngol Head Neck Surg. 2005;133(5):695–701. | ||
Huang C, Yang Y, Li WX, et al. Hyperin attenuates inflammation by activating PPAR-gamma in mice with acute liver injury (ALI) and LPS-induced RAW264.7 cells. Int Immunopharmacol. 2015;29(2):440–447. | ||
Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004;25(6):331–336. | ||
McIlroy GD, Delibegovic M, Owen C, et al. Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes. 2013;62(3):825–836. | ||
Law RE, Goetze S, Xi XP, et al. Expression and function of PPAR gamma in rat and human vascular smooth muscle cells. Circulation. 2000;101(11):1311–1318. | ||
Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARγ: differentiation-dependent peroxisomal proliferator-activated receptor γ (PPARγ) expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153(1):17–23. | ||
Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106(6):679–684. | ||
Leibovitz E, Schiffrin EL. PPAR activation: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2007;50(2):120–125. | ||
Bruemmer D, Blaschke F, Law RE. New targets for PPAR gamma in the vessel wall: implications for restenosis. Int J Obes (Lond). 2005;29(Suppl 1):S26–S30. | ||
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. | ||
Lastra G, Manrique C. Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm Mol Biol Clin Investig. 2015;22(1):19–26. | ||
MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15(1):323–350. | ||
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. | ||
Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res. 2002;941(1–2):1–10. | ||
Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–256. | ||
Avendaño MS, Martínez-Revelles S, Aguado A, et al. Role of COX-2-derived PGE2 on vascular stiffness and function in hypertension. Br J Pharmacol. 2016;173(9):1541–1555. | ||
Bomfim GF, Echem C, Martins CB, et al. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 2015;122:1–7. | ||
Diep QN, Schiffrin EL. Increased expression of peroxisome proliferator-activated receptor-alpha and -gamma in blood vessels of spontaneously hypertensive rats. Hypertension. 2001;38(2):249–254. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.