Back to Journals » Clinical Interventions in Aging » Volume 14
Exercise restores impaired endothelium-derived hyperpolarizing factor–mediated vasodilation in aged rat aortic arteries via the TRPV4-KCa2.3 signaling complex
Authors Huang J , Zhang H, Tan X, Hu M, Shen B
Received 21 June 2019
Accepted for publication 25 August 2019
Published 2 September 2019 Volume 2019:14 Pages 1579—1587
DOI https://doi.org/10.2147/CIA.S220283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Junhao Huang,1 Hai Zhang,2 Xianming Tan,1 Min Hu,1 Bing Shen3
1Guangdong Provincial Key Laboratory of Sports and Health Promotion, Scientific Research Center, Department of Sports and Health, Guangzhou Sport University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Physical Education, Guangdong University of Petrochemical Technology, Maoming, Guangdong, People’s Republic of China; 3Department of Physiology, School of Basic Medical Sciences, Anhui Medical University, Hefei, Anhui, People’s Republic of China
Correspondence: Min Hu
Scientific Research Center, Guangzhou Sport University, 1268 Middle Guangzhou Avenue, Guangzhou 510500, People’s Republic of China
Tel +86 0 203 802 7669
Fax +86 0 203 802 7669
Email [email protected]
Bing Shen
Department of Physiology, Anhui Medical University, 81 Meishan Road, Hefei 230032, People’s Republic of China
Tel +86 05 516 516 1132
Fax +86 0 5516 516 1126
Email [email protected]
Background: Aging leads to structural and functional changes in the vasculature characterized by arterial endothelial dysfunction and stiffening of large elastic arteries and is a predominant risk factor for cardiovascular disease, the leading cause of morbidity and mortality in modern societies. Although exercise reduces the risk of many age-related diseases, including cardiovascular disease, the mechanisms underlying the beneficial effects of exercise on age-related endothelial function fully elucidated.
Purpose: The present study explored the effects of exercise on the impaired endothelium-derived hyperpolarizing factor (EDHF)–mediated vasodilation in aged arteries and on the involvement of the transient receptor potential vanilloid 4 (TRPV4) channel and the small-conductance calcium-activated potassium (KCa2.3) channel signaling in this process.
Methods: Male Sprague-Dawley rats aged 19–21 months were randomly assigned to a sedentary group or to an exercise group. Two-month-old rats were used as young controls.
Results: We found that TRPV4 and KCa2.3 isolated from primary cultured rat aortic endothelial cells pulled each other down in co-immunoprecipitation assays, indicating that the two channels could physically interact. Using ex vivo functional arterial tension assays, we found that EDHF-mediated relaxation induced by acetylcholine or by the TRPV4 activator GSK1016790A was markedly decreased in aged rats compared with that in young rats and was significantly inhibited by TRPV4 or KCa2.3 blockers in both young and aged rats. However, exercise restored both the age-related and the TRPV4-mediated and KCa2.3-mediated EDHF responses.
Conclusion: These results suggest an important role for the TRPV4-KCa2.3 signaling undergirding the beneficial effect of exercise to ameliorate age-related arterial dysfunction.
Keywords: endothelium, EDHF, exercise, TRPV4, KCa2.3, aging
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in modern societies, and aging is the dominant risk factor for the development of CVD.1,2 Currently, aging is an irreversible biological process and leads to structural and functional changes in the vasculature characterized by stiffening of large elastic arteries and arterial endothelial dysfunction.3 In contrast to the effects of aging, regular exercise has been shown to greatly improve vascular structure and function.4–6 Although it is well recognized that exercise reduces the risk of many age-related diseases, including CVD, the mechanisms underlying the beneficial effects of exercise on age-related endothelial dysfunction are not completely understood.
Normal endothelial function is essential to maintain a healthy homeostasis of the vascular wall, and endothelial dysfunction is associated with a number of pathological conditions, including aging, diabetes, and coronary heart disease.7–9 Vasoactive substances, such as acetylcholine (ACh) and bradykinin, induce vasodilation by activating endothelium to release nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF). EDHF plays an important role in the regulation of vascular tone, and impaired EDHF has been associated with endothelial dysfunction.10 Increasing evidence has shown that EDHF-mediated vasodilation decreases with aging, hypertension, and diabetes.9,11,12
EDHF-mediated relaxation and the associated hyperpolarization involve intermediate-conductance and small-conductance calcium-activated potassium channels (KCa2.3) in the endothelium.10 Recently, an important role for the transient receptor potential vanilloid 4 (TRPV4) channel in EDHF-associated functions has been shown. TRPV4 physically and functionally interacts with the KCa2.3 in endothelial cells.9 This channel coupling enables Ca2+ influx via TRPV4 to activate KCa2.3, stimulating EDHF-mediated signaling and subsequent vasodilation. However, in pathological conditions, including diabetes and hypertension, the functional role of the endothelial TRPV4-KCa2.3 signaling is impaired, which may be a mechanism underlying decreased EDHF-mediated vasodilation.9,11 But whether the function of TRPV4-KCa2.3 coupled channels is altered with aging has not been addressed.
Therefore, the present study was designed to explore the functional role of TRPV4-KCa2.3 signaling in the regulation of EDHF-mediated relaxation and to examine the effects of exercise on the response of this channel coupling in aged arteries.
Materials and methods
Materials
Phenylephrine,9 ACh,9 RN1734,7 GSK1016790A,13 NG-nitro-L-arginine (L-NNA),8 indomethacin,8 and apamin8 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-KCa2.3 (catalog No. APC-025) and anti-TRPV4 (catalog No. ACC-034) antibodies were purchased from Alomone Labs.
Animals and the exercise training protocol
Male Sprague-Dawley rats were purchased from the Guangdong Medical Laboratory Animal Center (Guangzhou, Guangdong, China). The rats (aged 19–21 months) were randomly assigned to a sedentary group (Old) and an exercise-trained group (Old-ET). Two-month-old rats were used as young controls. The Old-ET rats were subjected to a motor-driven treadmill training protocol (15 m/min without inclination, 60 min/day, 5 days/week for 12 weeks) as previously described.14 All animal experiments were conducted in accordance with the guidelines of the US National Institutes of Health (NIH publication No. 8523) and were approved by the Animal Experimentation Ethics Committee of Guangzhou Sport University (Guangzhou, Guangdong, China).
Vessel tension measurement
Vessel tension measurement was performed as previously reported.15–18 Briefly, we dissected vessel segments of the rat thoracic aorta in a petri dish, which was filled with ice-cold Krebs solution (118 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4 • 7 H2O, 25.2 mmol/L NaHCO3, and 11.1 mmol/L glucose, pH 7.4) that was continuously bubbled with 95% oxygen (O2) and 5% carbon dioxide (CO2) mixed gas. The vessel segments were cut into approximately 2 mm rings and were then transferred to an organ bath. One end of the vessel ring was fastened to a hook on the bottom of the organ bath. The other end was connected to an isometric force transducer that was connected to an amplifier and recorder. The isometric tension was recorded and analyzed by a data acquisition and analysis system (BL-420S, Chengdu Taimeng Technology). In each experiment, the vessel rings were given a preload tension of 1 g and allowed to equilibrate for 1 h. The Krebs solution was changed every 15 min, and the tension was adjusted as needed to maintain the same preload tension. After equilibration, the vessel rings were first precontracted with phenylephrine (1 μmol/L) and then relaxed in response to the addition of ACh (10 μmol/L). The EDHF-mediated relaxation was studied in the presence of the cyclooxygenase inhibitor indomethacin (7 μmol/L) and the NO synthase inhibitor L-NNA (300 μmol/L).
Cell preparation and culture
The isolation and primary culture of rat aortic endothelial cells has been published elsewhere.9,11 Briefly, we dissected the rat thoracic aorta and then digested the endothelial cells with 0.02% collagenase type I (Sigma-Aldrich) in phosphate-buffered saline (PBS) at 37 °C for 45 min. The digested endothelial cells were collected and centrifuged at 250×g for 5 min. Cell pellets were then resuspended in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. After incubation at 37 °C for 1 h, the culture medium was replaced to remove nonadherent cells. The remaining adherent endothelial cells were continuously cultured at 37 °C with 5% CO2 for 3–5 days before experiments.
Immunoprecipitation and immunoblots
Immunoprecipitation and immunoblotting were conducted as previously described.9,15,16 Briefly, TRPV4 or KCa2.3 proteins were immunoprecipitated by incubating 800 µg of the extracted proteins with 5 µg of anti-TRPV4 or anti-KCa2.3 antibody at 4 °C overnight. Protein A magnetic beads were then added, followed by an additional incubation at 4 °C overnight. The immunoprecipitates were washed with saline three times and then resolved via sodium dodecyl sulfate polyacrylamide gel electrophoresis using an 8% gel. The resulting proteins were transferred to a polyvinylidene difluoride membrane using a wet transfer system (Bio-Rad Laboratories). The membrane containing the transferred proteins was incubated at 4 °C overnight with the primary antibody (at a dilution of 1:200) in PBS containing 0.1% Tween 20 and 5% nonfat dry milk. The next day, immunodetection was performed using horseradish peroxidase–conjugated secondary antibodies. Protein binding was detected using an enhanced chemiluminescence system and recorded with imaging capture equipment.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analyses were conducted using one-way analysis of variance followed by the Tukey post hoc test or by Student’s unpaired t tests when appropriate. A two-sided value of P<0.05 was considered statistically significant.
Results
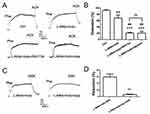
TRPV4 physically associates with Kca2.3 in endothelial cells of rat aortic arteries
In a co-immunoprecipitation experiment using lysates freshly prepared from rat aortic endothelial cells, an anti-KCa2.3 antibody pulled down TRPV4 proteins (Figure 1A) and an anti-TRPV4 antibody pulled down KCa2.3 (Figure 1B). In the control pull-down experiments conducted using preimmune IgG, no bands were detected (Figure 1). Taken together, these results indicate that TRPV4 can physically associate with KCa2.3 and suggest that they may form a signaling complex in endothelial cells of rat aortic arteries.
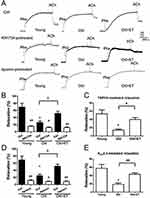
Role of TRPV4-Kca2.3 signaling in EDHF-mediated vasodilation in rat aortic arteries
In an ex vivo arterial tension study, ACh induced significant relaxation in rat aortic arteries (Figure 2A and B). In the presence of the cyclooxygenase inhibitor indomethacin and the NO synthase inhibitor L-NNA, ACh-induced EDHF-mediated relaxation was significantly decreased by approximately 20% of the control value (Figure 2A and B). However, the EDHF-mediated response was markedly inhibited by approximately 70% of the control response by L-NNA plus indomethacin combined with RN-1734 (a selective blocker of TRPV4; 20 µmol/L) or apamin (a KCa2.3 selective inhibitor; 200 nmol/L). Moreover, no significant difference was found in the degree of inhibition by RN-1734 and apamin (Figure 2A and B). To further explore the role of TRPV4 in EDHF-mediated relaxation, GSK1016790A, a selective activator of TRPV4, was used. The results showed that GSK1016790A (300 nmol/L) induced relaxation even when indomethacin and L-NNA were present. However, the GSK1016790A-induced EDHF-mediated relaxation was significantly inhibited by apamin in the presence of indomethacin and L-NNA compared with that in the control, as expected (Figure 2C and D). Taken together, these results indicate that TRPV4 functionally interacts with KCa2.3 and suggest that this interaction plays a critical role in regulating EDHF-mediated vasodilation.
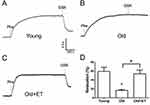
Role of TRPV4-KCa2.3 signaling on the age-related decrease in EDHF-mediated relaxation in rat aortic arteries and the effect of exercise
Aging reduces normal endothelial function. As shown in Figure 3A and B, ACh-induced EDHF-mediated relaxation was markedly attenuated in older rats compared with that in young rats; however, this attenuation was less apparent in older rats after exercise training. In the presence of the TRPV4 blocker RN1734 (Figure 3A and B), EDHF-mediated relaxation was markedly and significantly inhibited in all groups (Figure 3A and B). Subtraction of the relaxation observed in the presence of RN1734 (Figure 3B) from that in the absence of RN1734 revealed the TRPV4-mediated EDHF response (Figure 3C). Our results showed that the TRPV4-mediated EDHF response was significantly decreased with aging compared with that in young rats; however, this response was restored to levels nearer those observed in young rats by exercise training (Figure 3C). Similarly, in the presence of the KCa2.3 blocker apamin, EDHF-mediated relaxation was significantly decreased in all three groups (Figure 3D). Compared with that in the young group, the KCa2.3-mediated EDHF response was significantly attenuated in the aged group, whereas this attenuation was almost completely reversed after exercise training (Figure 3E). Additionally, the GSK1016790A-induced EDHF-mediated relaxation was markedly and significantly decreased with aging compared with that in young rats (Figure 4A–D), indicating that the functional role of TRPV4 in regulating EDHF-mediated vasodilation was decreased in aged rats. However, exercise restored the TRPV4-mediated EDHF response.
Discussion
The main findings of the present study were as follows. (1) Co-immunoprecipitation data demonstrated a physical association between TRPV4 and KCa2.3 in primary cultured rat aortic endothelial cells; (2) ACh and GSK1016790A mainly acted through endothelial TRPV4-KCa2.3 signaling to induce EDHF-mediated vasodilation in rat aortic artery segments; (3) In isolated aged rat arteries, the EDHF response to either ACh or GSK1016790A was impaired; (4) Exercise training in aged rats restored both the age-related TRPV4-mediated and the KCa2.3-mediated EDHF responses. Taken together, our findings suggested that TRPV4-KCa2.3 signaling might play an important role in the beneficial effect of exercise to improve age-related impaired arterial function.
Accumulating evidence suggests that Ca2+-activated potassium channels may interact with Ca2+-permeable transient receptor potential channels to generate signal transduction between these channels in the vasculature.19 For example, TRPC1 is physically associated with BKCa in vascular smooth muscle cells, and Ca2+ entry via TRPC1 activates BKCa to cause membrane hyperpolarization.20 In addition, endothelial TRPA1 forms a signaling complex with KCa channels and KIR channels to mediate endothelium-dependent cerebral artery dilation.21 Recent studies have also shown both physical and functional interactions of TRPV4 with KCa2.3 in rat mesenteric endothelial cells.9 Consistent with the results of previous studies, the present study identified a physical association between TRPV4 and KCa2.3 in rat aortic endothelial cells. A pivotal role for TRPV4-KCa2.3 signaling was observed in regulating the ACh-induced and the GSK1016790A-induced EDHF-mediated vasodilation in rat aortic arteries. Taken together, the results from the present study and previous findings suggest that TRPV4 physically and functionally associates with KCa2.3 to form a signaling complex in vascular endothelial cells that enables Ca2+ influx via TRPV4 to activate KCa2.3, inducing the EDHF-mediated response of vasodilation.
CVD remains the number one cause of death in the world, and age-related vascular endothelial dysfunction is a key risk factor for the development of CVD.3 EDHF plays a central role in the regulation of endothelial function for vasodilation and coordination of blood flow in the vasculature.10 The EDHF-mediated response is altered with aging, hypertension, diabetes, and hypoxia-reoxygenation injury.9,11,12,22 These alterations may either contribute to endothelial dysfunction or compensate for the loss of NO bioavailability, depending on the vascular bed.10 In the present study, we identified an important role of TRPV4-KCa2.3 signaling in regulating EDHF-mediated vasodilation. Decreased expression of TRPV4 and KCa2.3 has been reported in endothelial cells with aging, diabetes, or hypertension.7,9 Therefore, the present results indicate that impaired TRPV4-KCa2.3 signaling might be the mechanism underlying the decreased EDHF responses in aged arteries.
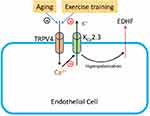
It has been shown that regular exercise has a prominent effect on vascular endothelial function by improving factors such as the vasodilator-to-vasoconstrictor balance, inflammation, and oxidative stress.23 Exercise has a comprehensive effect on the cardiovascular system, including resistance arteries. Thus, the effect of exercise on elastic arteries, including the aorta, may be also important for some diseases, for example, aortic dissection.24 Although exercise can reduce or even reverse the detrimental effects of aging on vascular function, the mechanisms underlying the favorable effects of exercise on age-related endothelial dysfunction have not been fully evaluated. A previous study indicated that exercise restores EDHF responses in aged arteries;25 however, whether TRPV4-KCa2.3 signaling is involved in this effect is not known. To our knowledge, the present study showed for the first time that exercise training restored the function of TRPV4-KCa2.3 signaling in regulating EDHF-mediated relaxation. TRPV4, the mechanosensitive Ca2+-permeable cation channel, is essential for the shear stress–induced intracellular Ca2+ concentration increase.26,27 Our previous study found that an impairment of TRPV4-mediated Ca2+ signaling in endothelial cells contributes to the decreased flow-induced vasodilation in aged mesenteric arteries.7 Interestingly, irisin, an exercise-induced myokine, induces an increase in endothelial intracellular Ca2+ concentration via TRPV4 channels in rat mesenteric arteries, suggesting a critical role of TRPV4 in the vasodilation effect of irisin.28 However, whether exercise exerts a direct effect on TRPV4 channels in endothelial cells needs to be explored in further studies. Calcium-activated potassium channels play a critical role in regulating vascular tone. It has been reported that aerobic exercise restores the age-related reduction in BKCa channel expression on mesenteric arteries.14 Here, we demonstrated that KCa2.3 was involved in the EDHF-mediated aortic artery relaxation and that exercise restored the impaired function of KCa2.3 in aged rat aortic endothelial cells. Taken together, our results suggest that TRPV4-KCa2.3 coupling might play an important role in the beneficial effect of exercise on improving the age-related impairment in arterial function (Schematic 1).
In conclusion, the results of the present study indicated that exercise training significantly ameliorated the impaired TRPV4-KCa2.3 signaling in aged arteries, and this amelioration might underlie the favorable effect of exercise to improve age-related inhibition of EDHF-mediated vasodilation. These findings extended our knowledge of the molecular mechanisms undergirding the beneficial effects of exercise on the vascular function that is impaired with aging.
Abbreviations
Ach, acetylcholine; EDHF, endothelium-derived hyperpolarizing factor; TRPV4, transient receptor potential vanilloid 4; KCa2.3, small-conductance calcium-activated potassium channel; CVD, cardiovascular disease; NO, nitric oxide; L-NNA, NG-nitro-L-arginine.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (grant numbers 31600969, 31771315) and the Natural Science Foundation of Guangdong Province (grant number 2016A030313625).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89(Pt B):122–135. doi:10.1016/j.yjmcc.2015.01.021
2. Yang Y, Zhu J, Wang X, et al. Contrasting patterns of agonist-induced store-operated Ca2+ entry and vasoconstriction in mesenteric arteries and aorta with aging. J Cardiovasc Pharmacol. 2015;65(6):571–578. doi:10.1097/FJC.0000000000000225
3. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011;120(9):357–375. doi:10.1042/CS20100476
4. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122(12):1221–1238. doi:10.1161/CIRCULATIONAHA.110.939959
5. Golbidi S, Laher I. Exercise and the aging endothelium. J Diabetes Res. 2013;2013:789607. doi:10.1155/2013/789607
6. Schuler G, Adams V, Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J. 2013;34(24):1790–1799. doi:10.1093/eurheartj/eht111
7. Du J, Wang X, Li J, et al. Increasing TRPV4 expression restores flow-induced dilation impaired in mesenteric arteries with aging. Sci Rep. 2016;6:22780. doi:10.1038/srep22780
8. Huang JH, He GW, Xue HM, et al. TRPC3 channel contributes to nitric oxide release: significance during normoxia and hypoxia-reoxygenation. Cardiovasc Res. 2011;91(3):472–482. doi:10.1093/cvr/cvr102
9. Ma X, Du J, Zhang P, et al. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension. 2013;62(1):134–139. doi:10.1161/HYPERTENSIONAHA.113.01500
10. Ozkor MA, Quyyumi AA. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol Res Pract. 2011;2011:156146. doi:10.4061/2011/156146
11. He D, Pan Q, Chen Z, et al. Treatment of hypertension by increasing impaired endothelial TRPV4-KCa2.3 interaction. EMBO Mol Med. 2017;9(11):1491–1503. doi:10.15252/emmm.201707725
12. Moreira HS, Lima-Leal GA, Santos-Rocha J, Gomes-Pereira L, Duarte GP, Xavier FE. Phosphodiesterase-3 inhibitor cilostazol reverses endothelial dysfunction with ageing in rat mesenteric resistance arteries. Eur J Pharmacol. 2018;822:59–68. doi:10.1016/j.ejphar.2018.01.019
13. Mendoza SA, Fang J, Gutterman DD, et al. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298(2):H466–H476. doi:10.1152/ajpheart.00854.2009
14. Shi L, Liu B, Zhang Y, Xue Z, Liu Y, Chen Y. Exercise training reverses unparallel downregulation of MaxiK channel alpha- and beta1-subunit to enhance vascular function in aging mesenteric arteries. J Gerontol A Biol Sci Med Sci. 2014;69(12):1462–1473. doi:10.1093/gerona/glt205
15. Chen M, Li J, Jiang F, et al. Orai1 forms a signal complex with BKCa channel in mesenteric artery smooth muscle cells. Physiol Rep. 2016;4(1):e12682. doi:10.14814/phy2.12682
16. Song K, Zhong XG, Xia XM, et al. Orai1 forms a signal complex with SK3 channel in gallbladder smooth muscle. Biochem Biophys Res Commun. 2015;466(3):456–462. doi:10.1016/j.bbrc.2015.09.049
17. Wang XC, Sun WT, Fu J, et al. Impairment of coronary endothelial function by hypoxia-reoxygenation involves TRPC3 inhibition-mediated KCa channel dysfunction: implication in ischemia-reperfusion injury. Sci Rep. 2017;7(1):5895. doi:10.1038/s41598-017-06247-3
18. Yang Q, Huang JH, Yao XQ, Underwood MJ, Yu CM. Activation of canonical transient receptor potential channels preserves Ca2+ entry and endothelium-derived hyperpolarizing factor-mediated function in vitro in porcine coronary endothelial cells and coronary arteries under conditions of hyperkalemia. J Thorac Cardiovasc Surg. 2014;148(4):1665–1673 e1661. doi:10.1016/j.jtcvs.2014.02.026
19. Behringer EJ, Hakim MA. Functional interaction among KCa and TRP channels for cardiovascular physiology: modern perspectives on aging and chronic disease. Int J Mol Sci. 2019;20:6. doi:10.3390/ijms20061380
20. Kwan HY, Shen B, Ma X, et al. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104(5):670–678. doi:10.1161/CIRCRESAHA.108.188748
21. Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104(8):987–994. doi:10.1161/CIRCRESAHA.108.189530
22. Yang Q, Huang JH, Man YB, Yao XQ, He GW. Use of intermediate/small conductance calcium-activated potassium-channel activator for endothelial protection. J Thorac Cardiovasc Surg. 2011;141(2):501–510, 510 e501. doi:10.1016/j.jtcvs.2010.04.005
23. Gliemann L, Nyberg M, Hellsten Y. Effects of exercise training and resveratrol on vascular health in aging. Free Radic Biol Med. 2016;98:165–176. doi:10.1016/j.freeradbiomed.2016.03.037
24. Fuglsang S, Heiberg J, Hjortdal VE, Laustsen S. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J. 2017;51(2):99–105. doi:10.1080/14017431.2016.1257149
25. Minami A, Ishimura N, Harada N, Sakamoto S, Niwa Y, Nakaya Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis. 2002;162(1):85–92. doi:10.1016/s0021-9150(01)00685-2
26. Randhawa PK, Jaggi AS. TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res Cardiol. 2015;110(6):54. doi:10.1007/s00395-015-0467-8
27. Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol. 2013;61(2):113–119. doi:10.1097/FJC.0b013e318279ba42
28. Ye L, Xu M, Hu M, et al. TRPV4 is involved in irisin-induced endothelium-dependent vasodilation. Biochem Biophys Res Commun. 2018;495(1):41–45. doi:10.1016/j.bbrc.2017.10.160
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.