Back to Journals » Clinical Interventions in Aging » Volume 12
Excessive anterior cervical muscle tone affects hyoid bone kinetics during swallowing in healthy individuals
Authors Yamazaki Y, Tohara H , Hara K , Nakane A, Wakasugi Y, Yamaguchi K , Minakuchi S
Received 3 June 2017
Accepted for publication 16 September 2017
Published 8 November 2017 Volume 2017:12 Pages 1903—1910
DOI https://doi.org/10.2147/CIA.S143175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Yasuhiro Yamazaki, Haruka Tohara, Koji Hara, Ayako Nakane, Yoko Wakasugi, Kohei Yamaguchi, Shunsuke Minakuchi
Department of Gerodontology, Tokyo Medical and Dental University, Tokyo, Japan
Purpose: This study aimed to determine whether excessive neck muscle tone affects hyoid bone kinetics during swallowing using videofluorography (VF) in an unnatural posture in healthy individuals.
Subjects and methods: Subjects were 28 healthy adults (12 men, 16 women; mean age, 39.75±9.50 years) without any history or present complaints of swallowing disorders. We first established the participant’s posture a reclining wheelchair that was adjusted to a 30-degree angle with the headrest (without excessive neck muscle tone) or without headrest (with excessive neck muscle tone), used an electromyogram above the mylohyoid muscle to represent the suprahyoid muscles and above the sternohyoid muscle to represent the infrahyoid muscles to confirm neck muscle tone, and then conducted VF of swallowing measurements. Videofluorographic images were obtained when 5 mL of 50% (w/v) barium sulfate was being swallowed, and hyoid bone coordinate (the resting position and the elevated position), extent of horizontal and vertical hyoid bone elevation, as well as duration and velocity of hyoid bone elevation were evaluated (x-axis and y-axis coordinates for the resting position of hyoid bone are referred to as Xr and Yr, respectively; those for the elevated hyoid bone position induced during swallowing are referred to as Xs and Ys, respectively).
Results: In the resting position of the hyoid bone, the Yr coordinates in those with excessive neck muscle tone were significantly lower than in those without excessive neck muscle tone. Vertical hyoid bone elevation and hyoid bone elevation velocity were significantly higher with excessive neck muscle tone than without excessive neck muscle tone, whereas horizontal elevation showed no significant differences.
Conclusion: Our findings suggest that the generation of neck muscle tone due to inappropriate posture may encourage hyoid depression and increase the extent of hyoid bone elevation, thereby increasing the risk of aspiration.
Keywords: dysphagia, swallow, muscle tone, hyoid bone, infrahyoid muscles, suprahyoid muscles
Corrigendum for this paper has been published
Introduction
Head and neck posture, as well as muscle tone in the anterior cervical region, can negatively affect swallowing function.1,2 For example, elderly individuals with low activities of daily living (ADL) who require care or patients with neurodegenerative disorders3 can experience difficulty in maintaining a seated posture during meals due to a decline in physical function or from restricted joint range of motion or postural instability. This can ultimately lead to a higher risk of aspiration, which is thought to be associated with the difficulty of maintaining a given posture.1,2 However, the association between difficulties in maintaining posture and neck muscle tone, and that between anterior cervical muscle tone and swallowing function remain unclear. Several studies have examined patients with particular illnesses in regard to the association between neck muscle tone and swallowing function. One study that evaluated patients with Parkinson’s disease divided them into two groups according to symptom severity on the Hoehn and Yahr scale (mild/moderate symptoms versus severe symptoms, including issues such as neck muscle hypertonia).4 They found complaints of swallowing disorders were more frequent in the mild/moderate patient group, although a marked decline in swallowing function was more prevalent in the group with severe symptoms (including neck muscle hypertonia).5 Another study evaluated activity in the suprahyoid muscles during swallowing and found that patients with spasmodic torticollis, which caused hypertonia in the neck, lacked coordinated movement between the laryngeal, submandibular, and suprahyoid muscles.6 In our previous surveys of patients with swallowing disorders receiving in-home care, a comparison of the presence of cervical muscle hypertonia and severity of swallowing disorder7 revealed that patients with high muscle tone in the suprahyoid muscles (corresponding to the mylohyoid muscle) and the infrahyoid muscles had significantly lower swallowing function than patients who lacked such high muscle tone.
In general, when we engage with patients, a common practice is to adjust food texture and patient posture, including that of the head and neck,8 to help them swallow safely.1,2,6 In patients with a high degree of neck muscle hypertonia, head and neck muscles with high tone, such as the sternocleidomastoid muscle, the scalene muscle, the trapezius, and the oblique muscles of the head, can be targeted with botox treatment. Alleviation of hypertonia in the neck can also be attempted through neck relaxation, which also helps to address swallowing disorders or voice disorders in healthy individuals as well as in patients with post-laryngectomy aphonia.9,10
In summary, a number of studies have been published on neck muscle hypertonia as it correlates with the effects of certain illnesses, but none have examined how head and neck hypertonia caused by difficulties in maintaining posture can influence swallowing kinetics.
Prior to examining patients with swallowing disorders, we conducted this preliminary study to figure out whether the excessive neck muscle tone generated by inappropriate posture affects hyoid bone movement during swallowing using videofluorography (VF) in healthy individuals.
Methods
Study participants
The present study evaluated 28 healthy adults (12 men, 16 women; mean age, 39.75±9.50 years) without any history or present complaints of swallowing disorders. Thorough written and verbal explanations of the study were provided to all participants, and written consent was obtained from each (Approval No 1143, Tokyo Medical and Dental University, Dentistry Department Ethics Committee). The present study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent for publication of images has been obtained.
Experimental design
We first established the participant’s posture, used an electromyogram (EMG) to confirm neck muscle tone, and then undertook VF of swallowing for measurement.
Establishing posture
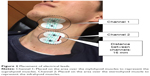
First, we had participants sit in a reclining wheelchair (GF-Uni_sp, Miki, Japan) that was adjusted to a 30-degree angle. Participants were asked to sit in a posture with the headrest behind their head that did not create increased muscle tone in the neck (hereafter, “without excessive neck muscle tone”; Figure 1).
  | Figure 1 Postures used in the present study. |
Using an electromyogram to measure neck muscle tone
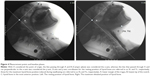
In order to measure neck muscle tone, we placed electrodes (F-120M, NIHON KOHDEN, Japan) above the mylohyoid muscle to represent the suprahyoid muscles (Channel 1) and above the sternohyoid muscle to represent the infrahyoid muscles (Channel 2; Figure 2). Next, using a surface EMG (MyoTrac Infiniti Encoder SA9800, Thought Technology, Canada), we visually monitored waveforms indicating neck muscle tonality (Figure 3). Waveforms were observed in a resting position and during an air swallow. EMG waveforms with no excessive neck tone comprised two potentials, including the stable EMG waveform with the participant in a resting position, and the trimodal active potential that is generated only during an air swallow. If one of the above two waveforms could not be obtained, we re-established posture. The distance between electrodes placed on muscles was set at 16 mm. As a prior study showed no significant differences in muscle activity on the left side versus the right, the leads were attached to the left side in all participants, and bipolar leads were used for surface electrode recordings.
VF measurements
The VF (Winscope 4000, Toshiba Medical Systems, Japan) was performed after increased muscle tone was confirmed in the neck region. We attached metal spheres (9.2 mm diameter) on the bottom tip of the nostril, the lower margin of the tragus, and over cervical vertebrae 3–4 as markers for VF image analysis. We used 50 w/v% barium sulfate (Baritop, Kaigen Pharma, Japan) as the contrast agent, 5 mL per swallow. Participants were asked to use the syringe to keep the contrast agent in the floor of their mouths, and then to swallow all at once. The swallow was timed such that, after the researcher gave a signal, the participant could swallow at will (Dipper-type swallow).11 VF images were taken at a velocity of 30 frames/sec, twice for each posture, and mean values were calculated for each participant.
Next, after 15 minutes of rest, participants switched to a posture where they reclined at 30 degrees without a headrest to generate increased neck muscle tone (hereafter, “with excessive neck muscle tone”; Figure 1), and Steps (1–3) described above were performed. The EMG waveforms with excessive neck muscle tone exhibited the high-frequency discharge and amplitude that begins with the resting position, as well as fewer gradual decreases/increases in discharge frequency (Figure 1), and this was confirmed as increased neck muscle tone. Posture was re-established if these characteristic waveforms could not be obtained.
Data analysis
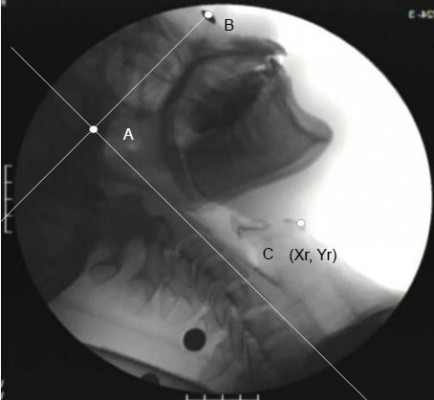
VF images were obtained using a video capture device (GV-USB2 I-O Data Device, Japan), recorded as an MPG file on a computer, and analyzed using two-dimensional data analysis software (ImageJ ver.1.5., National Institutes of Health, USA). The standard plane for analysis was the Camper plane12 that connected the bottom tip of the nostril and the lower margin of the tragus. This created the x-axis, whereas the line that went through the lower margin of the tragus and ran perpendicular to that was the y-axis; the intersection between the two was used as the point of origin (hereafter, “origin”) for analysis (Figure 4). Measurements of the hyoid bone were taken at the lowest anterior point of the body. We analyzed data when participants finished swallowing and had no remaining liquid in their oral cavity13 (hereafter, “the resting position of hyoid bone” and when the hyoid bone) was at the farthest anterior position, when the swallowing reflex is induced from a resting position (hereafter, “the maximum elevated position of hyoid bone”). To determine the standard for the actual length, we adjusted coordinates at the time of analysis according to the 9.2 mm-diameter metal spheres attached to participants during imaging measurements.
Hyoid bone position in a coordinate
In order to determine hyoid bone position in a coordinate with and without excessive neck muscle tone (Figure 1), the X and Y coordinates from the origin to the hyoid bone were determined in both the resting position of the hyoid bone as well as the maximum elevated hyoid bone position. The x and y axes were analyzed separately (hereafter, x-axis and y-axis coordinates for the resting position of hyoid bone are referred to as Xr and Yr, respectively; those for the maximum hyoid bone position induced during swallowing are referred to as Xs and Ys, respectively).
Extent of hyoid bone elevation
We measured hyoid bone displacement (mm) from the resting position of hyoid bone to the maximum elevated position of hyoid bone position, both with and without excessive neck muscle tone (Figure 1). Horizontal and vertical displacements were analyzed separately.
Duration and velocity of the maximum elevated position of hyoid bone
We measured the time (seconds) from when the hyoid bone began to elevate until it reached the elevated hyoid bone position (hereafter, “duration of hyoid bone elevation”). The velocity of hyoid bone elevation was obtained by dividing the extent of hyoid bone elevation by the duration of hyoid bone elevation.
Statistical analyses
The Wilcoxon signed-rank test was used to compare hyoid bone coordinates, hyoid bone elevation, and duration and velocity of hyoid bone elevation using SPSS ver19.0 for Windows (SPSS, Inc., Chicago, IL, USA). Statistical significance was set at p<0.05.
Results
Coordinates for the hyoid bone position with and without excessive neck muscle tone were determined for each participant (Table 1). Table 1 shows that, in the resting position of hyoid bone, the Yr coordinates with excessive neck muscle tone were significantly lower than those without excessive neck muscle tone (p<0.01; Wilcoxon signed-rank test), whereas no significant differences were observed with and without excessive neck muscle tone for Xr, Xs, or Ys coordinates.
With regard to hyoid bone elevation, vertical hyoid bone elevation was significantly higher with excessive neck muscle tone than without excessive neck muscle tone (p<0.01; Wilcoxon signed-rank test), while horizontal elevation showed no significant differences (Table 2). Duration of hyoid bone elevation with and without excessive neck muscle tone showed no significant differences (1.19±0.54 seconds and 1.19±0.53 seconds, respectively). Hyoid bone elevation velocity with excessive neck muscle tone (20.06±10.86 mm/sec) was significantly higher than that without excessive neck muscle tone (14.79±5.64 mm/sec; p<0.01; Wilcoxon signed-rank test).
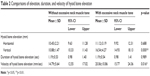  | Table 2 Comparisons of elevation, duration, and velocity of hyoid bone elevation |
Discussion
Neck muscle tone and hyoid bone positioning
The present study used EMG to evaluate muscle tone in regions corresponding to the mylohyoid muscle and sternohyoid muscles, when excessive cervical muscle tone was generated. A decrease in Y coordinates of the hyoid bone was confirmed when the resting position of the hyoid bone is assumed; that is, we confirmed the phenomenon known as hyoid depression. Humbert et al14 found that hyoid depression could be induced by applying surface electrical stimulation (SES) on the anterior region of the neck, thereby contracting the muscles involved in anteversion; this was consistent with our results.
With regard to the suprahyoid muscles, when the mouth is open, the mandible is depressed due to contraction of the mylohyoid muscle, the anterior belly of the digastric muscle, and the geniohyoid muscle.15 During swallowing, contraction of the mylohyoid muscle and digastric muscles pull the hyoid bone upward, whereas contraction of the geniohyoid muscle pulls the hyoid bone forward. The coordinated actions of these muscles lead to the anterosuperior movement of the hyoid bone.16,17 When opening the mouth, the infrahyoid muscles fix the position of the hyoid bone, and assist the suprahyoid muscles to pull the mandible down.18,19 In the present study, when excessive neck muscle tone was generated, we observed the muscle tone in the region of the mylohyoid muscle, which is one of the suprahyoid muscles involved in hyoid bone elevation. Muscle tone was also observed in the region of the sternohyoid muscle, which is one of the infrahyoid muscles involved in hyoid depression. One explanation for the observed hyoid depression may be that the hyoid bone was pulled downward by the excessive contraction of the infrahyoid muscles, including the sternohyoid muscle. Although we also confirmed tension in the suprahyoid muscles involved in hyoid bone elevation, further studies will be needed to understand hyoid depression. Given that neck muscle tone generated by an unnatural posture led to hyoid depression, the resting position of the hyoid bone may be more heavily influenced by infrahyoid muscles than by suprahyoid muscles. Another potential reason why no changes were observed with excessive neck muscle tone along the x-axis of the hyoid bone may have been that muscles that pull the hyoid bone back (ie, the hyoglossus muscle, the posterior belly of the digastric muscle, and the stylohyoid muscle) were not strongly influenced.
Excessive anterior cervical muscle tone effect on the maximum elevated position of hyoid bone
The present study revealed significantly more vertical elevation of the hyoid bone when neck muscle tone was generated. One explanation for this may be that, the resting position of the hyoid bone was depressed with improved neck muscle tone, but as the hyoid bone position did not change during elevation, an increase in vertical elevation was required for swallowing. In other words, in healthy individuals with normal swallowing reflexes, the unnatural posture assumed in the present study did not negatively affect the swallowing reflex.
Studies that targeted elderly individuals or patients with swallowing disorders have reported increased hyoid bone elevation to be a risk factor for aspiration.20 From the perspective of swallowing dynamics, hyoid bone elevation during the pharyngeal stage of swallowing (when the food bolus passes through the pharynx) requires the involvement of the hyoid muscle groups as well as the pharyngeal, laryngeal, maxillofacial, and tongue muscles.21 During this process, pharyngeal muscles, tongue muscles, and suprahyoid muscles contract to induce anterosuperior elevation of the hyoid bone and, together, through the repeated relaxation and contraction of the cricopharyngeal muscles, the food bolus moves through the esophageal opening. Thus, when the resting position of hyoid bone is depressed, hyoid bone elevation must compensate by increasing during swallowing, which breaks down the temporal coordination of laryngeal closure and increases the risk of aspiration.22,23 Furukawa examined elderly people 70 years and older and found marked laryngoptosis when resting, as well as laryngeal elevation that could not keep pace with swallowing; thus, if the lower laryngeal position was not compensated for by increasing laryngeal elevation during swallowing, they were more likely to develop a swallowing disorder.20
Previous studies have reported a higher frequency of sarcopenia – an age-associated decrease in myofiber number and decreased cross-sectional area of muscle that leads to decreased contraction strength in those 70 years or older. This frequency increased to 30% or more in those aged 75–80 years, and to 50% among those 80 years and older.24 Among patients with sarcopenia, muscular atrophy reportedly occurs more readily in fast-twitch muscles than in slow-twitch muscles.25 As suprahyoid muscles are primarily fast-twitch muscles,26 we surmise that age only adds to the influence of sarcopenia on suprahyoid muscles. In the present study of healthy adults, no aspiration occurred, although increased hyoid bone elevation was required. However, if an elderly individual becomes ill with improved neck muscle tone due to an unnatural posture, the combination of the age-related increase in hyoid bone elevation and sarcopenia-related decrease in suprahyoid muscle function would likely increase the risk of aspiration.
Excessive anterior cervical muscle tone effect on duration and velocity of hyoid bone elevation
In the present study, the duration of the maximum elevated position of hyoid bone was not significantly different with and without excessive neck muscle tone; however, when neck muscle tone was generated, hyoid bone elevation velocity increased significantly. While hyoid bone elevation certainly increased with improved anterior cervical muscle tone, this was due to the higher elevation velocity, rather than the transition time, which showed no change. As mentioned above, the posture assumed by our participants did not seem to create any adverse effects on swallowing reflexes. As the participants of this study were healthy adults, even with the excessive neck muscle tone-induced hyoid depression and the concomitant increase in hyoid bone elevation velocity, no aspiration was noted. Because sarcopenia reduces muscle contraction velocity,26,27 it is possible that hyoid bone elevation velocity may also decrease with aging. In other words, if elderly individuals generate high muscle tone on anterior cervical area due to an unnatural posture, the added effects of aging may decrease hyoid bone elevation velocity, which would make it more difficult to compensate for hyoid depression. An increase in aspiration risk may result from this, not so much due to the distance, but rather because of timing.
Limitations
The present study has several limitations worth noting. First, given the difficulties of selectively generating muscle tone in only the suprahyoid or infrahyoid muscles, the specific contribution of one muscle group versus the other was unclear, particularly with regard to hyoid depression. Second, muscle tone was confirmed using an EMG that was simply placed on regions corresponding to mylohyoid and sternohyoid muscles when participants were in the unnatural posture without a headrest. Because of this, contributions of muscles other than suprahyoid and infrahyoid muscles could not be determined. Third, as our experiments were conducted in healthy adults, it remains unclear whether our results could be replicated in healthy elderly individuals or patients with swallowing disorders. In the future, we hope to replicate these tests in elderly individuals and those with swallowing disorders.
Conclusion
In order to figure out whether the excessive neck muscle tone generated by inappropriate posture affects hyoid bone kinetics, we used VF to examine healthy adults, specifically with regard to the resting position of hyoid bone, hyoid bone elevation, and duration of hyoid bone elevation. Our main findings were as follows:
- Coordinates for the resting position of hyoid bone were significantly lower with excessive neck muscle tone than without.
- Vertical elevation of the hyoid bone was significantly greater with excessive neck muscle tone than without, but no significant difference in duration of hyoid bone elevation was noted.
- The generation of high neck muscle tone due to inappropriate posture may encourage hyoid depression and increased the maximum elevated position of hyoid bone, thereby increasing the risk of aspiration.
Disclosure
The authors report no conflicts of interest in this work.
References
Rasley A, Logemann JA, Kahrilas PJ, Rademaker AW, Pauloski BR, Dodds WJ. Prevention of barium aspiration during videofluoroscopic swallowing studies: value of change in posture. AJR Am J Roentgenol. 1993;160(5):1005–1009. | ||
Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Effects of postural change on aspiration in head and neck surgical patients. Otolaryngol Head Neck Surg. 1994;110(2):222–227. | ||
Serra A, Maiolino L, Di Mauro P, Licciardello L, Cocuzza S. The senile functional evolution of the larynx after supracricoid reconstructive surgery. Eur Arch Otorhinolaryngol. 2016;273(12):4359–4368. | ||
Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. | ||
Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11(1):14–22. | ||
Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngeal dysphagia. Arch Phys Med Rehabil. 1989;70(10):767–771. | ||
Yamazaki Y. Positional change of the hyoid bone following the change of neck tension. Poster presented at: 21st Meeting of the Japanese Society of Dysphagia Rehabilitation; September 12, 2015; Kyoto. | ||
Logemann JA, Gensler G, Robbins J, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech Lang Hear Res. 2008;51(1):173–183. | ||
Serra A, Di Mauro P, Spataro D, Maiolino L, Cocuzza S. Post-laryngectomy voice rehabilitation with voice prosthesis: 15 years experience of the ENT Clinic of University of Catania. Retrospective data analysis and literature review. Acta Otorhinolaryngol Ital. 2015;35(6):412–419. | ||
Ahsan SF, Meleca RJ, Dworkin JP. Botulinum toxin injection of the cricopharyngeus muscle for the treatment of dysphagia. Otolaryngol Head Neck Surg. 2000;122(5):691–695. | ||
Dodds WJ, Taylor AJ, Stewart ET, Kern MK, Logemann JA, Cook IJ. Tipper and dipper types of oral swallows. AJR Am J Roentgenol. 1989;153(6):1197–1199. | ||
Nakane A, Tohara H, Ouchi Y, Goto S, Uematsu H. Videofluoroscopic kinesiologic analysis of swallowing: defining a standard plane. J Med Dent Sci. 2006;53(1):7–15. | ||
Wintzen AR, Badrising UA, Roos RA, Vielvoye J, Liauw L. Influence of bolus volume on hyoid movements in normal individuals and patients with Parkinson’s disease. Can J Neurol Sci. 1994;21(1):57–59. | ||
Humbert IA, Poletto CJ, Saxon KG, et al. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol (1985). 2006;101(6):1657–1663. | ||
Grant PG. Lateral pterygoid: two muscles? Am J Anat. 1973;138(1):1–9. | ||
Pancherz H, Winnberg A, Westesson PL. Masticatory muscle activity and hyoid bone behavior during cyclic jaw movements in man. A synchronized electromyographic and videofluorographic study. Am J Orthod. 1986;89(2):122–131. | ||
Ahlgren J. Relationship between integrated EMG and tension in opening of the mandible. In: Perryman JH, editor. Oral Physiology and Occlusion: an International Symposium. New York: Pergamon Press; 1978:41–54. | ||
Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal Dysphagia. Dysphagia. 2007;22(1):1–10. | ||
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691–707. | ||
Furukawa K. Cineradiographic analysis of laryngeal movement during deglutition – with special reference to aging. Nihon Jibiinkoka Gakkai Kaiho. 1984;87(2):169–181. Japanese. | ||
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969. | ||
Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43(5):1264–1274. | ||
Shaw DW, Cook IJ, Gabb M, et al. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995;268(3 Pt 1):G389–G396. | ||
Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. | ||
Larsson L, Yu F, Höök P, Ramamurthy B, Marx JO, Pircher P. Effects of aging on regulation of muscle contraction at the motor unit, muscle cell, and molecular levels. Int J Sport Nutr Exerc Metab. 2001;11 Suppl:S28–S43. | ||
Korfage JA, Schueler YT, Brugman P, Van Eijden TM. Differences in myosin heavy-chain composition between human jaw-closing muscles and supra and infrahyoid muscles. Arch Oral Biol. 2001;46(9):821–827. | ||
Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272(2 Pt 1):C638–C649. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.