Back to Journals » Clinical Interventions in Aging » Volume 15
Examination of the Correlation Between Physical and Psychological Measures in Community-Dwelling Older Adults
Authors Staples WH , Kays A, Richman R
Received 21 November 2019
Accepted for publication 5 February 2020
Published 2 March 2020 Volume 2020:15 Pages 293—300
DOI https://doi.org/10.2147/CIA.S239053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
William H Staples, Adam Kays, Rachel Richman
Krannert School of Physical Therapy, University of Indianapolis, Indianapolis, IN, USA
Correspondence: William H Staples
Krannert School of Physical Therapy, University of Indianapolis, 1400 East Hanna Avenue, Indianapolis, IN 46227, USA
Tel +1 317-788-2112
Fax +1 317-788-3542
Email [email protected]
Introduction: The purpose of this study was to determine if correlations exist between strength and mobility and psychological measures of anxiety and depression in community-dwelling older adults.
Methods: One hundred and eleven participants randomly completed trials of grip strength (GS), the Timed Up and Go (TUG), the 10-meter walk test (10MWT), the Geriatric Anxiety Scale (GAS), and the Geriatric Depression Scale (GDS) in a prospective, correlational study.
Results: This study found significant correlations between and within physical measures of strength and mobility and psychological measures. Age, GS, GDS, and education were significant predictors of gait speed (10MWT). Age, GS, and GDS were predictors of TUG scores. Grip strength was found to be a significant predictor of fall status; fallers had significantly weaker GS than non-fallers. Symptoms of anxiety (GAS) were predictive of symptoms of depression.
Discussion: Objective measures of physical performance can provide information regarding an individual’s symptoms of anxiety and depression. Health professionals should understand the correlations between mood and physical ability to better treat their patients.
Keywords: mood, mobility, older adults, falls
Introduction
The number of adults over the age of 60 in the US is expected to double by the year 2050 due to a large, aging baby boomer population.1 In this population, 8–16% of older adults experience symptoms of depression,2 with an estimated 5–7% having a diagnosable major depressive disorder.3,4 In regard to anxiety, 10–20% of older adults experience symptoms and often are misdiagnosed.5 In addition, the National Council on Aging states that fewer than one-third of Americans are meeting the physical activity requirement suggested by the Centers for Disease Control and Prevention.6 Given these facts, it would be logical to explore whether mood and physical activity are related.
In a large, prospective study of over 9000 participants, Azevedo Da Silva and colleagues found a bidirectional association between symptoms of anxiety and depression and physical activity.7 Although physical therapists’ education includes biopsychosocial screening and interventions, they are often unable to identify or appropriately screen patients with symptoms of depression.8 This lack of symptom identification may also be common with physicians.9
Low or decreased physical activity, along with unintentional weight loss (>10 lbs within the past year), self-reported exhaustion, weakness in grip strength, and slow walking speed are all features of frailty as defined by Fried and colleagues.10 The authors of that study also note that frailty may have a negative effect on psychological and physical well-being.
Measuring grip strength using hand-held dynamometry has been shown to be a clinically useful and time-sensitive option in the screening of physical activity level or functional decline in geriatric populations.11 Not only have studies shown grip strength to be a strong indicator of upper limb strength, but it may also be successful in predicting recurrent falls.11 Furthermore, studies support the use of hand-held dynamometry to identify individuals at risk of mobility limitations and inability to perform instrumental activities of daily living.12–14
In the geriatric population, additional physical outcome measures that assess gait, mobility, and balance are valuable when determining physical activity, frailty, and function during everyday life.15,16 Studenski and colleagues found gait speed and the Established Populations for Epidemiologic Studies of the Elderly (EPESE) performance battery to be predictors of decline in health and function in community-dwelling older adults.16 LeBrasseur determined that
gait has a symbiotic relationship with health as alterations can negatively or positively influence the function of multiple physiological systems. Gait is a plausible vital sign in older adults as it is a powerful determinant of important outcomes, including falls, the geriatric syndrome of frailty, the loss of independence, and survival. (p. 1414)17
Multiple studies suggest associations between physical function and presence of mood disorders, such as depression and anxiety.18–20 Nevertheless, limited research exists regarding measures of physical function such as grip strength, balance, gait speed, and fall risk, and their relationship to depression and anxiety.
Therefore, the purpose of this study is to determine the relationship between physical measures including grip strength, balance, and walking speed and measures of anxiety and depression (mood) in community-dwelling older adults.
Methods
Design and Participants
This prospective, correlational study was performed to investigate the correlation between mood and physical abilities. One hundred and nineteen participants were recruited from organizational contacts of the principal and co-investigators, local health fairs, fitness centers, and independent living facilities in the Midwest by word of mouth presentations (active recruitment), and flyers posted in senior living facilities (passive recruitment). Data collection was conducted by researchers from the University of Indianapolis, at local fitness centers and senior living facilities from January 10th 2015 through April 10th 2016. The sample size was determined by the number of participants that could be recruited over the course of three university semesters. Participants were tested privately unless consent was given for other investigators and participants to be within the room at the same time.
Participants were included in the study if they were: 60 years of age or older, able to walk independently with or without an assistive device, able to read newsprint with or without glasses, and able to hear well enough to carry on a conversation either with or without a hearing aid.
Participants were excluded if they: were currently receiving physical therapy; had an orthopedic surgery within the last 6 months; had been diagnosed with a progressive neurological condition such as Parkinson’s disease, multiple sclerosis, Guillain-Barre syndrome, polio, stroke, or Alzheimer disease; or had a history of vertigo, dizziness, or Meniere’s disease. As described in more detail in the next section, for the current study, participants (n = 8) who scored at or below 22 out of 30 on the Montreal Cognitive Assessment (MoCA) were tested but excluded from analysis. The purpose of the study was verbally explained to the participants, and informed consent was individually obtained.
The University of Indianapolis’ IRB approved this research study (#1134). The consent was written, informed consent and the study was conducted in accordance with the Declaration of Helsinki.
Procedures
Participants completed a general health intake form that collected socio-demographic information such as age, race/ethnicity, years of education completed, fall history, perceived health status, and relevant medical history (ie, medication use, level of activity, and exercise participation). All measurement tools were administered by a physical therapist who is a geriatric clinical specialist, a research psychologist, and physical therapy and psychology doctoral students.
The MoCA is a rapid screening tool with high specificity that was administered to assess general cognitive functioning.21 It was determined from previous studies that participants who scored less than 20 out of 30 may demonstrate significant cognitive deficits that would impair their ability to complete the self-report surveys used to assess mood.22 For the purpose of this study, the MoCA was used as a screening tool and was not considered to be a dependent variable. Based on the original research,21 it was determined that participants scoring 22 or lower would be excluded from involvement in the study due to mild cognitive impairment (MCI). The presence of MCI may interfere with memory of a fall and/or appropriate subjective answers to the questionnaires utilized in this study. Eight people were excluded from the study due to this concern. To prevent any concerns of the individual that they were not qualified for any reason, the eight people completed all the tests and were excluded only for data analysis. Figure 1 summarizes the recruitment, eligibility determination, data collection, and data analysis process.
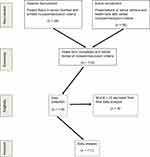 |
Figure 1 Participant recruitment, eligibility and exclusion for analysis. |
Physical and psychological measures were then completed in a randomized order. Upon conclusion of the study, all participants were given the opportunity to enter into a drawing for one of five $20 gift cards to a local retail establishment.
Physical Measures
Functional mobility measures of balance and gait speed were assessed using the Timed Up and Go (TUG) and 10-Meter Walk Test (10MWT). The TUG has demonstrated excellent validity and reliability in predicting risk of falls in older adults, and a meta-analysis has shown the TUG to be an excellent predictor of falls.23 Individuals are given verbal instructions to stand up from a chair, walk 3 m as quickly and safely as possible to a cone on the floor, turn around the cone, walk back, and sit down.23 The average score from two trials were calculated for data analysis.
The 10MWT has also been shown to be both valid and reliable in older adults with excellent correlation with other measures of mobility.24 Per standard instruction, each individual was instructed to walk a set distance of 14 m as quickly and as safely as possible. Time was recorded once the participant reached the 2-m marker and ended at the 12-m marker in order to allow for acceleration and deceleration. The distance covered was divided by the time it took the individual to walk 10 m to produce the gait speed. Three trials were collected and averaged for data analysis.
Grip strength was measured using a JAMAR hand dynamometer (Model #BK-7498; Fred Sammons Inc, Burr Ridge, Illinois).25 The individual was seated with their humerus at 0 degrees abduction and neutral rotation, 90 degrees of elbow flexion, and wrist in neutral.26,27 The average score of the three trials was collected for each hand, and data for the dominant hand was used for analysis.25
Psychological Measures
Patient levels of anxiety and depression were assessed with the Geriatric Anxiety Scale 1.0 (GAS) and the Geriatric Depression Scale Short Form (GDS-SF). For community-dwelling older adults, the GAS is valid and reliable in measuring symptoms of anxiety.28,29 The GAS is a 30-item, self-report measure where participants rate their symptoms from 0 (not at all) to 3 (all the time) on a Likert-type scale. Higher scores indicate an increased severity of anxiety.
The GDS-SF is a self-rating scale of 15 yes/no items that has been shown to be as valid and reliable as the long version in screening for symptoms of depression in the geriatric population.30,31 Yesavage et al suggest scores of 0 to 4 to be in the normal range, 5 to 9 to indicate mild depression, and 10 to 15 to indicate moderate to severe depression.32
Statistical Analysis
IBM SPSS Statistics 21 was utilized for the analysis of data. A multivariate, nonparametric (Spearman’s rho) correlation was performed to examine the relationships between variables of age, fall history, and physical and psychological measures. A two-tailed correlational significance was determined at the 0.01 and 0.05 levels (p < 0.01 and p < 0.05).
Results
Of the 111 participants, 87 were female with a mean age of 77.1 ±3.8, and 34 were male with a mean age of 74.9 ± 7.2. All analyzed participants identified as Caucasian (white) except eight African-American women, and three African-American men. Participants had a vast array of co-morbidities, but all were independently ambulatory. Significant correlations were found between the following: GAS and grip strength, GDS and TUG, GDS and 10MWT, TUG and 10MWT, GAS and GDS, grip strength and 10MWT, and grip strength and the TUG. Based on information collected from the general health intake form, participants’ 6-month fall history was found to be significantly correlated with their average TUG and 10MWT scores. However, there was no correlation between 6-month fall history and grip strength, GAS, or GDS (Table 1). Predictive values were also found for the TUG, 10MWT, GAS, and GDS (Table 2). Strength of correlation coefficients were based on ranges reported by Portney and Watkins.33
 |
Table 1 Correlations Between Age, Physical Measures, and Psychological Measures |
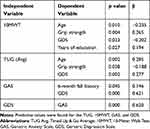 |
Table 2 Significant Predictive Variables |
As expected, 10MWT and TUG were highly correlated (r = −0.869), which is similar to a study by Lin et al that found a moderate to good correlation (r = 0.66).34 A strong correlation was anticipated as both 10MWT and TUG are measures of ambulation and gait speed, however there are slight differences. The 10MWT only assesses gait in one direction from a standing start, and the TUG incorporates sit to/from stand and changes in gait direction.15,35,36 In this study, the correlation is inverted because the data for the 10MWT was documented in speed (meters per second), and the TUG data were documented as total time to complete the task (seconds). Therefore, an increased time to complete the TUG would mean a slower or decreased gait speed.
Fair correlations were found between grip strength and 10MWT (r = 0.358) and between grip strength and the TUG (r = −0.339). Grip strength was found to be the strongest independent predictor of 10MWT (β = 0.265) but the weakest predictor of the TUG (β = −0.188). Although not as obvious, it makes sense that grip would be correlated and predictive of these physical measures of mobility. Like ambulation, grip has been demonstrated to have a direct relationship with mobility limitations, frailty, and physical disability.12–14 Just as TUG and 10MWT had an inverse relationship, so did TUG and grip strength. Decreased or low grip strength would infer physical disability or weakness, and an increased TUG time means slower walking speed and therefore infers increased disability or difficulty with ambulation.
The GAS and GDS were moderately and significantly correlated (r = 0.43), which is consistent with values reported by Brenes et al; r = 0.49, though symptoms of anxiety and depression were measured differently in their study.37 A correlation between GAS and GDS was expected as these disorders often co-exist.38–41 Our data also show that symptoms of depression and anxiety were predictive of each other (β = 0.620 and 0.621, respectively), with 6-month fall history being an additional predictor of anxiety (β = 0.146).
Discussion
The current body of literature offers limited research regarding the association between measures of physical activity, depression, and anxiety. This study found significant correlations between and within these physical and psychological measures.
Although the disorders of depression and anxiety have similar symptoms, recognizing the differences between each is important. Anxiety is characterized by an irrationalized fear response, despite the absence of any real danger, and can have physical symptoms that include fatigue, irritability, and difficulty sleeping.42 Anxiety often interferes with an individual’s confidence and ability to perform normal tasks. Depression is a condition characterized by decreased motivation to perform daily activities, decreased energy levels, feelings of pessimism, and restlessness.42 Brenes et al also found that symptoms of anxiety and depression alone are associated with increased disability in older adults and that levels of disability are even greater when the two are combined.37
Our analysis shows that grip strength, which is also a predictor of frailty,10 was minimally correlated with anxiety as measured by the GAS (r = −0.200). Other studies have shown that anxiety may also be a contributing factor in frailty.43,44 Also, it has been found that anxiety is significantly correlated with disability and is a significant predictor of ability to complete activities of daily living and light housework in a sample of older women,37,45 which supports the findings in the current study’s primarily female-based population. Interestingly, our study found that measures of mobility and gait speed, such as the 10MWT and TUG that were used to measure frailty,10 were not correlated to anxiety in this study. A recent longitudinal study that investigated age-related functional decline and muscle loss in a large sample of community-dwelling older adults found that grip strength declined at a considerably faster rate than gait speed over a 2-year period.46 As grip strength declines more quickly than gait speed, it can be assumed that decreased grip strength would be found prior to other criteria for frailty and anxiety, which may explain why grip strength was associated with anxiety in this study but measures of gait speed were not. Perhaps, individuals with decreased grip strength have noticed this decline in strength, which could increase their anxiety about getting older or losing independence during functional activities. Grip strength has also been found to have a direct effect on the quality of life in older adults.47
In this study, it is plausible that grip strength was correlated with symptoms of anxiety because many participants had a preconceived notion that they would score poorly on this task. In addition, grip strength may not have been correlated with symptoms of depression because the physical and psychological effort involved in the measurement of grip strength was much less than the effort required to perform multiple trials of the 10MWT or TUG.
Symptoms of depression, as measured by GDS, were found to have a direct, though minimal, association with the physical measures of the TUG (r = 0.255) and the 10MWT (r = −0.255). Our data also show that the GDS was a small predictor of both the TUG (β = 0.277) and 10MWT (β = −0.202). The relationship between depression and physical activity has been examined frequently in the literature, and many studies have found that physical activity can be utilized as a treatment to decrease symptoms of depression.7,48-50 Brenes et al also found that depression is associated with increased disability (r = 0.10).37 Correlations in our study were actually higher than in Brenes et al’s study,37 which could be due to the fact that our study used objective measures of physical ability rather than self-report surveys, which obtain perceptions of ability rather than actual ability.45,51 Therefore, individuals with symptoms of depression may have greater risks for decreased mobility performance, and this functional limitation may also lead to an increase in those symptoms.
As expected, 6-month fall history was correlated with the TUG and 10MWT as cut-off scores that predict fall risk in community-dwelling older adults have been well established,52 as well as across a wide array of other populations. The use of the 10MWT has proven to be an invaluable clinical tool in measuring gait speed to determine functional mobility, fall risk, and natural physical decline.46,53,54
The correlations found in this study have several clinical implications for physical therapists (PTs). First, PTs tend to spend more time with their patients than most other medical professionals. This allows PTs to develop a greater therapeutic alliance and may aid in catching mood patterns that seem “out of the ordinary”. Secondly, PTs play a crucial role in helping patients develop an awareness of their deficits so that they can work together to improve functional mobility and return patients to the activities they enjoy doing. Grip strength, gait speed and mobility should be routinely measured in this population. Additionally, successful rehabilitation instills confidence in patients’ ability to perform at home and in the community, consequently preventing potential health risks associated with physical inactivity. Lastly, PTs are in the best position to promote health and wellness through home exercise programs, education, and social engagement via community activities. Integrating a holistic, biopsychosocial approach to patient care helps us to determine what is meaningful to the individual and can motivate them to remain active throughout the lifespan.
There are limitations to this study regarding lack of generalizability. The nature of the study, along with the inclusion criteria, limited the amount of variability of the participants. Also, a convenience sample was used, which may have positively skewed the results and also decreased the generalizability of the study. The measures of mood and self-reported fall history may be influenced by participant attitude toward the questions. A number of variables were not included in data collection that may have better clarified the results (eg, a direct, objective measure of fear of falling, a medication profile, body mass index, sleep patterns).
Future studies are needed to further examine these results with a more diverse population of community-dwelling older adults. Further investigation should explore and determine the relationship to fear of falling in this population.
Conclusion
This study was performed to let health professionals know that there is a risk in older adults for mood disorders. Health providers must understand the risk for psychological issues is present with physical problems in order to guide their treatment. We found that, in a population of older adults, there is a significant relationship between some physical and psychological measures. Grip strength could be an indicator of fall risk in older adults and falls can have serious consequences. Grip strength may prove useful in objectively screening for symptoms of anxiety along with its current use of identifying progressive decline in physical function. Given the results of this study, PTs should screen for depression when an older adult performs poorly on the TUG or 10MWT. The screening for depression or anxiety can then be used to refer these patients to the appropriate mental health professional. This study demonstrated that it is imperative for clinicians to consider the relationship between depression or anxiety and physical performance within a biopsychosocial model in older adult populations which should lead to better health outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. U.S. Census Bureau. The population 65 years and older in the United States: 2016 American Community Survey Reports; October 2018. Available from: https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf.
2. Maurer DM, Raymond TJ, Davis BN. Depression: screening and diagnosis. Am Fam Physician. 2018;15:508–515.
3. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371:1228–1236. doi:10.1056/NEJMcp1402180
4. National Institute of Mental Health. Major depression. Available from: https://www.nimh.nih.gov/health/statistics/major-depression.shtml.
5. Geriatric Mental Health Foundation. Anxiety and older adults: overcoming worry and fear. Available from: https://www.aagponline.org/index.php?src=gendocs&ref=anxiety.
6. Centers for Disease Control and Prevention. Physical activity. Available from: https://www.cdc.gov/physicalactivity/inactivity-among-adults-50plus/index.html.
7. Azevedo Da Silva M, Singh-Manoux A, Brunner EJ, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol. 2012;27(7):537–546. doi:10.1007/s10654-012-9692-8
8. Fay P, Edmond SL, Baron JK, et al. Depression screening by physical therapists: practices, beliefs, barriers. J Back Musculoskelet Rehabil. 2017;30:1221–1229. doi:10.3233/BMR-169551
9. Ferenchick EK, Ramanuj P, Pincus HA. Depression in primary care: part 1-screening and diagnosis. BMJ. 2019;365:1794.
10. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146
11. Bohannon RW, Magasi SR, Bubela DJ, et al. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46:555–558.
12. Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and ageing in men project. J Am Geriatr Soc. 2010;58:2055–2062. doi:10.1111/j.1532-5415.2010.03145.x
13. Sallinen J, Stenholm S, Rantanen T, et al. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726. doi:10.1111/j.1532-5415.2010.03035.x
14. Xue QL, Walston JD, Fried LP, et al. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: the Women’s Health and aging study. Arch Intern Med. 2011;171:1119–1121. doi:10.1001/archinternmed.2011.252
15. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi:10.1111/j.1532-5415.1991.tb01616.x
16. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi:10.1046/j.1532-5415.2003.51104.x
17. LeBrassuer NK. Gait as an integrative measure and predictor of health across species. J Gerontol a Biol Sci Med Sci. 2019;74:39–52.
18. van Milligen BA, Lamers F, de Hoop GT, et al. Objective physical functioning in patients with depressive and/or anxiety disorders. J Affect Disord. 2011;131:193–199. doi:10.1016/j.jad.2010.12.005
19. van Milligen BA, Vogelzangs N, Smit JH, et al. Physical function as predictor for the persistence of depressive and anxiety disorders. J Affect Disord. 2012;136:828–832. doi:10.1016/j.jad.2011.09.030
20. Warraich HJ, Kitzman DW, Whellan DJ, et al. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail. 2018;11:1–11. doi:10.1161/CIRCHEARTFAILURE.118.005254
21. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;2005(53):695–699. doi:10.1111/j.1532-5415.2005.53221.x
22. Waldron-Perrine B, Axelrod BN. Determining an appropriate cutting score for indication of impairment on the Montreal Cognitive Assessment. Int J Geriatr Psychiatry. 2012;27:1189–1194. doi:10.1002/gps.v27.11
23. Barry E, Galvin R, Keogh C, et al. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta-analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
24. Middleton A, Fritz SL. Assessment of gait, balance, and mobility in older adults: considerations for clinicians. Curr Transl Geriatr Exp Gerontol Rep. 2013;2:205–214. doi:10.1007/s13670-013-0057-2
25. Fess EE. Grip Strength.
26. Bhuanantanondh P, Nanta P, Mekhora K. Determining sincerity of effort based on grip strength test in three wrist positions. Saf Health Work. 2018;9:59–62. doi:10.1016/j.shaw.2017.06.001
27. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi:10.1093/ageing/afr051
28. Segal DL, June A, Payne M, et al. Development and initial validation of a self-report assessment tool for anxiety among older adults: the Geriatric Anxiety Scale. J Anxiety Disord. 2010;24:709–714. doi:10.1016/j.janxdis.2010.05.002
29. Yochim BP, Mueller AE, June A, et al. Psychometric properties of the geriatric anxiety scale: comparison to the beck anxiety inventory and geriatric anxiety inventory. Clin Gerontol. 2011;34:21–33. doi:10.1080/07317115.2011.524600
30. Aikman GG, Oehlert ME. Geriatric depression scale: long form versus short form. Clin Gerontol. 2001;22(3/4):63–70. doi:10.1300/J018v22n03_07
31. Wall JR, Lichtenberg PA, MacNeill SE, et al. Depression detection in geriatric rehabilitation: geriatric depression scale short form vs. long form. Clin Gerontol. 1999;20(3):13–21. doi:10.1300/J018v20n03_03
32. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi:10.1016/0022-3956(82)90033-4
33. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice.
34. Lin M, Hwang H, Hu M, et al. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. 2004;52:1343–1348. doi:10.1111/j.1532-5415.2004.52366.x
35. Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29:64–68. doi:10.1519/00139143-200608000-00004
36. Wolf SL, Catlin PA, Gage K, et al. Establishing the reliability and validity of measurements of walking time using the emory functional ambulation profile. Phys Ther. 1999;79:1122–1133. doi:10.1093/ptj/79.12.1122
37. Brenes GA, Penninx BWJ, Judd PH, et al. Anxiety, depression and disability across the lifespan. Aging Ment Health. 2008;12:158–163. doi:10.1080/13607860601124115
38. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–599. doi:10.1037/0021-843X.110.4.585
39. Da Silva Santos L, de Oliveira CB, Souza EC, et al. The effects of physical activity on anxiety, depression, and quality of life in elderly people living in the community. Trends Psychiatry Psychother. 2019;41:36–42. doi:10.1590/2237-6089-2017-0129
40. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorders: results from the national comorbidity survey replication (NCS-R). J Am Med Assoc. 2003;289:3095–3105. doi:10.1001/jama.289.23.3095
41. Regier DA, Rae DS, Narrow WE, et al. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry. 1998;173(Suppl 34):24–28. doi:10.1192/S0007125000293483
42. Ninan PT, Berger J. Depression and anxiety. Depress Anxiety. 2001;14:94–104. doi:10.1002/da.1049
43. Bernal-López C, Potvin O, Avila-Funes JA. Frailty is associated with anxiety in community-dwelling elderly adults. J Am Geriatr Soc. 2012;60:2373–2374. doi:10.1111/jgs.2012.60.issue-12
44. Ní Mhaoláin AM, Fan CW, Romero-Ortuno R, et al. Frailty, depression, and anxiety in later life. Int Psychogeriatr. 2012;24:1265–1274. doi:10.1017/S1041610211002110
45. Brenes GA, Guralnik JM, Williamson JD, et al. The influence of anxiety on the progression of disability. J Am Geriatr Soc. 2005;53:34–39. doi:10.1111/jgs.2005.53.issue-1
46. Auyeung TW, Lee SWJ, Leung J, et al. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr Gerontol Int. 2014;14:76–84. doi:10.1111/ggi.12213
47. Kwak Y, Kim Y. Quality of life and subjective health status according to handgrip strength in the elderly: a cross-sectional study. Aging Ment Health. 2019;23:107–112. doi:10.1080/13607863.2017.1387766
48. Jantunen H, Wasenius N, Kautiainen H, et al. Change in physical activity and health-related quality of life in old age- a10-year follow-up study. Scand J Med Sci Sports. 2019;29:1797–1804. doi:10.1111/sms.13501
49. Ku PW, Fox KR, Chen LJ, et al. Physical activity and depressive symptoms in older adults: 11-year follow-up. Am J Prev Med. 2012;42:355–362. doi:10.1016/j.amepre.2011.11.010
50. Panza GA, Taylor BA, Thompson PD, et al. Physical activity intensity and subjective well-being in healthy adults. J Health Psychol. 2019;24:1257–1267. doi:10.1177/1359105317691589
51. Mehta KM, Yaffe K, Brenes GA, et al. Anxiety symptoms and decline in physical function over 5 years in the health, aging and body composition study. J Am Geriatr Soc. 2007;55:265–270. doi:10.1111/jgs.2007.55.issue-2
52. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80:896–903. doi:10.1093/ptj/80.9.896
53. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign” [corrected]. J Geriatr Phys Ther. 2009;32(2):2–5. doi:10.1519/00139143-200932020-00002
54. Samah AZ, Nordin NAM, Shahar S, et al. Can gait speed test be used as a falls risk screening tool in community dwelling older adults? A review. Polish Ann Med. 2016;23:61–67. doi:10.1016/j.poamed.2015.04.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
