Back to Journals » Drug Design, Development and Therapy » Volume 11
EUREKA study – the evaluation of real-life use of a biophotonic system in chronic wound management: an interim analysis
Authors Romanelli M , Piaggesi A, Scapagnini G, Dini V , Janowska A , Iacopi E, Scarpa C, Fauverghe S, Bassetto F
Received 25 May 2017
Accepted for publication 17 October 2017
Published 11 December 2017 Volume 2017:11 Pages 3551—3558
DOI https://doi.org/10.2147/DDDT.S142580
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Marco Romanelli,1 Alberto Piaggesi,2 Giovanni Scapagnini,3 Valentina Dini,1 Agata Janowska,1 Elisabetta Iacopi,2 Carlotta Scarpa,4 Stéphane Fauverghe,5 Franco Bassetto4
1Wound Healing Research Unit, Division of Dermatology, School of Medicine, University of Pisa, Pisa, 2Diabetic Foot Section, Department of Medicine, University of Pisa, Pisa, 3Department of Medicine and Health Sciences, School of Medicine, University of Molise, Campobasso, 4Clinic of Plastic and Reconstructive Surgery, Padova University-Hospital, Padova, Italy; 5KLOX Technologies Inc., Laval, QC, Canada
Objective: Interest has grown regarding photobiomodulation (PBM) with low-level light therapy, which has been shown to positively affect the stages of the wound healing process. In a real-life context clinical setting, the objective of the EUREKA study was to investigate efficacy, safety, and quality of life associated with the use of a BioPhotonic gel (LumiHeal™) in the treatment of chronic wounds such as venous leg ulcers (VLUs), diabetic foot ulcers (DFUs), and pressure ulcers (PUs). This BioPhotonic gel represents a new, first-in-class emission spectrum of light, including fluorescence, to induce PBM and modulate healing.
Design: The multicenter, prospective, interventional, uncontrolled, open-label study enrolled 100 patients in 12 wound centers in Italy. We performed an early interim analysis based on the first 33 subjects (13 VLU, 17 DFU, 3 PU) in seven centers who completed the study.
Main results: Seventeen patients (52%) achieved total wound closure (full re-epithelialization for 2 weeks) during the study period. Two patients (6%) were considered “almost closed” (decrease of the wound area of more than 90% at study end) and three others (9%) were considered “ready for skin grafting”. No related serious adverse events were observed, and the compliance was excellent. After the treatment, the average time to “pain-free” was 11.9 days in the VLU group. Quality of life was improved with overall increase of 26.4% of the total score (Cardiff Wound Impact Schedule, p=0.001).
Conclusion: The study revealed a positive efficacy profile of the BioPhotonic gel in promoting wound healing and reactivating the healing process in different types of chronic, hard-to-heal wounds. The treatment was shown to be safe and well tolerated by the patients, and a reduction of pain perception was also detected during the treatment period. The improvement of the quality of life was accompanied by a high level of clinician satisfaction.
Keywords: photobiomodulation, fluorescence biomodulation, biophotonics, phototherapy, light, venous leg ulcers, VLUs, pressure ulcers, PUs, diabetic foot ulcers, DFUs, hard-to-heal wounds
Introduction
Chronic wounds such as pressure ulcers (PUs), venous leg ulcers (VLUs), and diabetic foot ulcers (DFUs) remain a challenging clinical problem and efficient wound management is crucial to effectively assist the healing process.1,2 The socioeconomic burden of chronic wounds represents an enormous annual cost for health care systems. The impact of chronic wounds and their high rate of occurrence is also worsened by the aging global population.3,4 For example, it has been estimated that chronic wounds have an incidence rate of 120 per 100,000 people aged between 45 and 65 years and it rises to 800 per 100,000 people >75 years of age.4–6 In addition, it is important to highlight that underlying pathologies, particularly diabetes, may explain the failure of healing of these chronic wounds.7 The prevalence of DFUs ranges from 4% to 10% among patients with diabetes, and the lifetime incidence is reported to be as high as 25%.8,9 Moreover, in the case of VLUs, it has been reported that they recur in 70% of cases, and recent data demonstrate high VLU recurrence rates, ranging from 0% at 6 months to 56% at 54 months.10,11 Therefore, new treatment options for chronic skin ulcers are needed. In this context, the use of low energy level light therapy that relies on the effect of photobiomodulation (PBM) is an attractive alternative therapy to enhance wound healing and a promising strategy for the management of complex chronic wounds.12,13 There has been an increasing amount of biomedical research to substantiate physiological responses to visible light. The first consideration involves the assumption that for low power visible light to have an effect on a living biological system such as the skin, the photons must be absorbed by electronic absorption bands belonging to some molecular chromophore or photoacceptor.14 The second important consideration involves the use of the definition of PBM as the most suitable term to describe the molecular process and resulting beneficial photobiological responses involved in non-thermal low-dose light therapies.15
The fluorescence technology of the BioPhotonic gel is based on the unique ability of special light-capturing molecules (chromophores) to convert light emissions from a multi-light emitting diode (LED) lamp into a different photons emission spectrum with broader and longer range of wavelengths with lower energy (fluorescence). As a result of this technology, when skin is exposed to fluorescence it induces modulation of biological processes (PBM).16
The beneficial effects of PBM on wound healing might be attributed to anti-inflammatory signaling, cell proliferation, protein synthesis, and decreased bacterial infection.17 In this exploratory clinical study named EUREKA, we have used an interim analysis to investigate efficacy and safety of a BioPhotonic gel, known as LumiHeal™ (KLOX Technologies Inc., Laval, QC, Canada). The BioPhotonic gel consists of a gel containing light-absorbing molecules (chromophores) that are illuminated by a multi-LED lamp. We evaluated the effects of BioPhotonic gel in the treatment of chronic, hard-to-heal wounds such as PUs, DFUs, and VLUs.
Materials and methods
The Clinical trial identifier at Clinicaltrials.gov is NCT03021811.
One hundred subjects were enrolled in this study. There were very few inclusion and exclusion criteria and it was designed as a real-life study. If the investigator believed, based on clinical data, that the BioPhotonic treatment would be an appropriate option, the patient might be included.
This interim analysis presents the data of the first 33 subjects who completed the study, with the data being obtained from seven clinical sites. Patients were treated for a maximum period of 16 weeks (PU, VLU) to 24 weeks (DFU) or until wound closure. Once their wound closed, the patients were seen again three times over an 8-week period to confirm persistence of wound closure. Sixteen patients completed the last follow-up visit. The trial was conducted in compliance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Protocol and informed consent procedures were approved by the local ethics committees of the 12 sites involved in the study (complete list of local ethics committees in Table 1), and all patients signed an informed consent form. The sites were all centers specializing in the management of chronic wounds (VLUs, DFUs, PUs). All of them were monitored at regular intervals throughout the study period. This interim analysis includes only subjects who completed the study and for whom complete data were available for review.
  | Table 1 List of ethics committees |
Patients
A total of 33 patients (mean age 67.60 years) with DFUs, PUs, and VLUs were enrolled and treated, at least once. PUs were categorized according to grades numbered from one to four of the European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel.18 The grading system used for DFUs was the University of Texas Wound Classification System.19 For VLUs, the criterion was the presence of a diagnosed VLU (open leg ulcer, with the presence of a venous disease).
Treatment
BioPhotonic gel is a photo-converter wound gel containing specific light-absorbing molecules (chromophores) which are not absorbed by the skin. The chromophores are illuminated by a multi-LED lamp, which is a device delivering blue light with wavelengths between 440 and 460 nm and a power density of between 55 and 129 mW/cm2 at a distance of 5 cm from the light source.
Upon illumination by the blue light, the chromophores release an ultra-fast micropulsed emission of photons in the form of fluorescence, with different wavelengths in the spectra of visible light, ranging from 500 to 610 nm. The use of the BioPhotonic gel illuminated by a multi-LED lamp was proven to be non-irritating for the skin and safe to use on wounds according to in vitro studies, in vivo studies performed in rabbits, rats and pigs, and previous clinical trials performed with the same gel on VLUs, DFUs, and PUs. The photo-converter wound gel is presented in two jars, which have to be mixed together just prior to application.
The bi-weekly BioPhotonic gel treatment regimen was used in combination with standard of care specific to each type of chronic wound (PUs, DFUs, VLUs). Typically, upon evaluation, any excess fibrin or necrotic tissue was debrided. The wound was cleansed with normal saline and a 2 mm thick layer of the gel was applied on the ulcers. The wound was then illuminated with the multi-LED light for 5 minutes at a distance of 5 cm. Once the treatment was completed, the gel was removed from the wound with gentle saline irrigation. A non-adherent dressing was then applied to prevent any contact between the wound and the external environment. Standard of care adapted to each ulcer was then followed, including: compression bandage systems, offloading, pressure reduction, management of a moist wound environment, use of barrier creams, management of wound infection, and nutritional assessment.
Efficacy analysis
Efficacy was assessed through the following endpoints: rate of complete wound closure; time to complete wound closure; wound area reduction over time; incidence of wound breakdown, following closure; impact of treatment quality of life (QOL); and ease of use by health care professionals. Wound area evaluation (mean change in wound area over time) was performed via the Silhouette™ Imaging System (ARANZ Medical, Christchurch, New Zealand), a device linked to a computerized system, allowing wound pictures and assessments of key characteristics (area, volume, depth, etc), as previously reported by Romanelli et al.20 Moreover, the following criteria were developed by the sponsor and used to define the response to the treatment: 1) full responder: decrease of the wound size area of more than 90% at the end of the study period and/or decrease of more than 50% of the size in 15 days or less; 2) partial responder: decrease of the size of the wound during the study period, but without meeting the criteria of full responders; 3) non-responder: increase of the size of the wound during the study period. The same criteria were used for all the types of wounds (PUs, DFUs, VLUs).
Safety analysis
Safety was documented via the collection of the following parameters: adverse events; serious adverse events; device incidents; clinical laboratory parameters; vital signs; physical examinations; pain; proportion of subjects with wound clinical infection requiring systemic antimicrobial therapy.
QOL measurements
The Cardiff Wound Impact Schedule (CWIS) is a wound-specific tool designed for subjects affected by chronic ulcers. This questionnaire was chosen for its consistency, its ability to discriminate between health states, and its good reproducibility.21 It was used to assess the impact of treatment on the QOL of patients involved in the study. The CWIS includes a total score and three main domains (“sub-scores”): “social life”, “well-being”, and “physical symptoms and daily living”. The questionnaire is designed to be self-administered and it was administered at baseline as well as at the first follow-up visit.
Pain assessment
Specific information on pain was collected by investigators. They were asked to assess the presence or absence of wound pain at each treatment visit. Intensity of this pain was not assessed.
Ease of use/satisfaction
The ease of use of the lamp and the gel was assessed by the investigators at the first and last treatment visits, through specific questionnaires developed by the sponsor.
As 33 subjects completed the study and were included in this interim analysis, a total of 66 questionnaires (33 at visit 1 or study start, and 33 at “end of study”) were collected.
For each question, investigators had to assess their satisfaction by selecting one box on a seven-box scale ranging from “very satisfied” to “very unsatisfied”.
Statistical analysis
As it was a real-life reproducibility study, analyses were primarily descriptive in nature in this interim analysis. There was no formal sample size calculation. Sample size was based on clinical considerations and no statistical power calculations. A maximum number of 100 patients were to be enrolled in this study. There was no blinding in this study. Safety analysis was carried out on the intent-to-treat population, which was the same as the efficacy population; it consisted of all patients having received at least one treatment. CWIS scores were analyzed using paired Student’s t-tests, and a p-value <0.05 was considered as statistically significant.
Results
Among the 33 subjects, 17 had DFUs (five stage 1A and 12 stage 2A), three had PUs (two stage II and 1 stage III), and 13 had VLUs. The average duration of the chronic ulcers under study was 72.3 weeks at screening (52.0 weeks for PUs, 54.7 weeks for DFUs, and 98.6 weeks for VLUs). As it was a real-life study, the dimensions of the chronic ulcers treated in the study were different depending on the type of wound. The average area of PUs was 2.9 cm2 at screening (SD: 3.1 cm2), while the average area of DFUs and VLUs was 1.8 cm2 (SD: 2.02 cm2) and 13.2 cm2 (SD: 10.9 cm2), respectively.
Efficacy of study endpoints
Despite the heterogeneity of the different chronic wounds, the use of this novel technology demonstrated a significant efficacy profile. Table 2 shows the response of the 33 subjects included in this interim analysis. Twenty-one patients were considered as full responders (64%), six as partial responders (18%), and six as non-responders (18%). The wound size of full responders decreased on average by 78% at day 30 (20 subjects), and by 99% at day 60 (17 subjects). By type of wound, these percentages were respectively: 78% and 100% for PUs; 76% and 99% for DFUs; 81% and 97% for VLUs. As shown in Table 3, the percentage of full responders was higher for DFUs (12 out 17 subjects – 70%) and VLUs (eight out of 13 subjects – 62%). As only three patients were included in the PU group, results were not interpretable for this category.
  | Table 2 Incidence of wound closure |
Among the full responders, 17 subjects achieved complete ulcer closure (complete wound closure rate of 100.0%) with a mean time to closure of 39.8 days. In particular, nine were from the DFU group (mean time: 28.3 days), seven from the VLU group (mean time: 53.6 days), and one was from the PU group (47.0 days). Moreover, no case of wound breakdown was observed during the 2-week follow-up period after wound closure. Figures 1 and 2 show the mean wound area variation in the full responders of DFU and VLU groups, respectively. As shown in these figures, the mean time to reach a 50% decrease of the wound size area in full responders was 19 days for DFUs and 22 days for VLUs. Among the six partial responders, three were from the DFU group and three from the VLU group. Two of them became ready for skin grafting according to investigators. The size of these partially responding wounds decreased from 24% to 43% for DFUs, and from 38% to 60% for VLUs during the study period. There were six non-responders, two were from the PU group, two from the DFU group, and two from the VLU group.
  | Figure 1 Mean wound area reduction (in % vs the size at screening) in DFU full responders. |
  | Figure 2 Mean wound area reduction (in % vs the size at screening) in VLU full responders. |
Safety and tolerability
There was only one related adverse event of the study leading to a discontinuation of a subject. The patient reported intermittent erythema following the use of the BioPhotonic gel. However, this adverse event was considered of mild intensity by the investigator. No related serious adverse events were reported in any subject receiving the treatment. The safety profile reported in this interim analysis is similar to what has been observed in previous clinical trials using the BioPhotonic gel in subjects with chronic wounds.22,23 Furthermore, the treatment did not cause any clinically significant abnormal values in laboratory analyses, either biochemical, hematological or in urine. There was no clinically significant impact on vital signs, and no negative impact observed during the physical examinations. Compliance was also excellent with only two subjects missing more than two expected visits.
Tolerability was also assessed through the assessment of pain. Presence of pain was assessed by investigators at every treatment visit through a questionnaire. After the treatment, wound pain disappeared in 100% of the patients who declared having pain at baseline. The VLU group is the population of subjects most concerned by pain among chronic ulcer patients. In this group, the pain was present at baseline in 54% of the subjects. Pain systematically disappeared once the treatment with the BioPhotonic gel was initiated, with an average time to “no pain” of 11.9 days (meaning a maximum of four treatments with the BioPhotonic gel); the maximum time being 14 days (Table 4). Once disappeared, the wound pain never reappeared during the treatment period. Additionally, although unrelated to the treatment, only one wound on a VLU patient developed an infection (erysipelas) during the study period. Except for this case, no other wound infection (related or unrelated to the study treatment) was observed. No wound presenting clinical signs of wound colonization was observed in the DFU and PU groups during the treatment period. These data, already observed in previous clinical studies with the same medical device,22,23 may support the hypothesis of control of wound colonization. Taken together, these results clearly demonstrate a favorable tolerability and safety profile.
  | Table 4 Impact on pain (VLU only) |
QOL outcomes
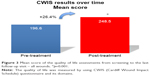
The standardized questionnaire CWIS was used to evaluate the impact of the treatment on the QOL of the patients. Despite a high score at screening, the overall QOL index was significantly improved during the study period, with an overall increase of 26.4% of the mean total score, from 196.6 (SD: 56.4) at screening to 248.5 (SD: 54.2) at follow-up (p=0.001) (Figure 3). This positive increase was observed in both full and partial responders. It is reasonable to speculate that this improvement of QOL may be linked to the positive impact observed on pain reduction. There was an overall improvement in all domains of the CWIS. The greatest improvement was seen in the well-being component, for each type of wound, with a mean increase of 44.7% (p=0.002) (Figure 3). A significant improvement was also observed in the physical symptoms (+18.4%, p=0.019) and social life domains (+22.3%, p=0.001) (Figure 3). Therefore, these data indicate a high confidence of the subjects in the efficacy of the treatment throughout the study period.
Ease of use/satisfaction
High levels of satisfaction have been reported by the health care providers throughout the study period, with 95% of the assessments reporting that investigators would recommend the BioPhotonic gel to their colleagues.
Discussion
This study was designed to address the safety and efficacy of the BioPhotonic gel for the treatment of chronic wounds such as DFUs, PUs, and VLUs. The clinical evaluation of the use of PBM through the BioPhotonic gel was positive. In this interim analysis, the use of the BioPhotonic gel was associated with an excellent safety profile. There were no treatment-related serious adverse events and only one patient reported a mild intermittent erythema following the treatment. Tolerability was also evaluated through the assessment of pain. Despite the fact that procedures for treatment of chronic ulcers, particularly VLUs, may be generally considered as painful and/or uncomfortable,24,25 the results showed that, when the pain was initially present before the first treatment it disappeared once this first treatment was initiated. We observed an average pain-free time of 9.8 days (all wounds), meaning that on average a maximum of three treatments was needed to remove that pain. Compared to the existing treatment solutions for chronic ulcers, this is an additional advantage of the BioPhotonic gel.
Even if compliance to the study may be an issue with patients affected by chronic wounds, in this interim analysis the treatment regimen led to a high rate of compliance. As reported by Lavery et al for DFUs,26 wound infections are frequent and responsible for delayed wound healing and wound breakdowns. We previously observed that the BioPhotonic gel treatment on chronic wounds decreased the susceptibility to wound infection.22 The overall incidence of infection in our study was lower than we estimated, reporting only one case of erysipelas unrelated to the treatment on a VLU patient. Although these results need to be further investigated, the absence of wound infections may be associated with the effects of the treatment to control bacteria involved in colonization in wound surfaces.
Efficacy profile may also be considered as very satisfying, with 17 wounds (52% of total wounds) totally closed and 21 wounds (67%) considered as full responders. These efficacy results will however need to be confirmed by a randomized controlled trial. In addition, no case of wound breakdown was observed 2 weeks after wound closure in the PU, VLU, and DFU groups and we were also able to assess the perseverance of wound closure in the majority of the subjects during the 8-week follow-up period. The study has shown that the BioPhotonic gel enhances wound healing through promotion of the healing process of chronic, hard-to-heal wounds, which were reactivated with an appropriate and efficient wound bed preparation. This efficacy was particularly remarkable on VLUs and DFUs, especially considering the age of these wounds at screening and their hard-to-heal status.27–29 Even if excellent and superior to the majority of the results observed in the literature,30–34 the results in DFUs will however need to be confirmed as the majority of these DFU wounds were of small size and of stages 1a and 2a.
Hard-to-heal wounds have a negative impact on patient well-being. For this reason, we investigated the impact of the treatment on the QOL of the subjects. The total score of CWIS increased during the study period for all wounds (+26.4%), confirming the positive impact of the treatment on overall aspects of QOL for subjects with chronic wounds. This positive tendency was also observed with the three sub-scores: social life (+22.3%), well-being (+44.7%), and physical symptoms/daily living (+18.4%) (Figure 3).
A critical success factor of the efficacy of this treatment was the clinical usability of the BioPhotonic gel. The high levels of satisfaction reported in the study support the positive experience of using the BioPhotonic gel in the treatment of complex chronic wounds.
There are some limitations in this study that should be noted. Since the purpose of this study was to assess the efficacy of the BioPhotonic gel in a real-life setting in different investigating centers, there was no formal sample size calculation for each group, and no control group. There was also a limited number of inclusion and exclusion criteria in this multi-center study. This resulted in a diversity of wound characteristics, especially in terms of wound size and previous standard of care. Another limitation is the fact that the multi-LED lamp needs to be used in a clinical setting.
Conclusion
The device used in this study offers an important and promising new therapeutic option in the treatment of hard-to-heal chronic wounds. Effective features of the system in this non-controlled trial include the efficacy profile associated with promotion of wound healing in different types of chronic wounds, safety and tolerability, improvement of the QOL, and positive impact on pain reduction. These preliminary data suggest that the studied BioPhotonic gel has the potential to become an alternative for unmet needs in the management of chronic ulcers.
Disclosure
S Fauverghe is Director of Clinical and Medical Affairs at KLOX Technologies.
M Romanelli, G Scapagnini, V Dini, C Scarpa, and F Bassetto are medical consultants for KLOX Technologies. The authors have no other conflicts of interest to report.
References
Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. Br J Dermatol. 2015;173(2):379–390. | ||
Jones KR, Fennie K, Lenihan A. Evidence-based management of chronic wounds. Adv Skin Wound Care. 2007;20(11):591–600. | ||
Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. | ||
Oliverio J, Gero E, Whitacre KL, Rankin J. Wound care algorithm: diagnosis and treatment. Adv Skin Wound Care. 2016;29(2):65–72. | ||
Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. Am J Clin Dermatol. 2000;1(5):269–275. | ||
Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38(2):72–140. | ||
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4(9):560–582. | ||
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. | ||
Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag. 2007;3(1):65–76. | ||
Finlayson K, Edwards H, Courtney M. Factors associated with recurrence of venous leg ulcers: a survey and retrospective chart review. Int J Nurs Stud. 2009;46(8):1071–1078. | ||
Reeder SW, Eggen C, Maessen-Visch BM, de Roos KP, Neumann HA. Recurrence of venous leg ulceration. Rev Vasc Med. 2013;1(4):63–65. | ||
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. | ||
Kuffler DP. Photobiomodulation in promoting wound healing: a review. Regen Med. 2016;11(1):107–122. | ||
Sutherland JC. Biological effects of polychromatic light. Photochem Photobiol. 2002;76(2):164–170. | ||
Kim HP. Lightening up light therapy: activation of retrograde signaling pathway by photobiomodulation. Biomol Ther (Seoul). 2014;22(6):491–496. | ||
Nielsen ME, Devemy E, Jaworska J, Scapagnini G. Photobiomodulation by low energy chromophore-induced fluorescent light. Abstract presented at SPIE BiOS, San Francisco, CA, USA; 28 January 2017. | ||
de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3). pii: 7000417. | ||
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. National Pressure Ulcer Advisory Panel; 2014. Available from: http://www.npuap.org/resources/educational-and-clinical-resources/prevention-and-treatment-of-pressure-ulcers-clinical-practice-guideline/. Accessed October 22, 2017. | ||
Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 2001;24(1):84–88. | ||
Romanelli M, Dini V, Rogers LC, Hammond CE, Nixon MA. Clinical evaluation of a wound measurement and documentation system. Wounds. 2008;20(9):258–264. | ||
Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J. 2004;1(1):10–17. | ||
Nikolis A, Grimard D, Pesant Y, Scapagnini G, Vézina D. A prospective case series evaluating the safety and efficacy of the KLOX BioPhotonic System in venous leg ulcers. Chronic Wound Care Management and Research. 2016;3:101–111. | ||
Nikolis A, Fauverghe S, Vezina D, Scapagnini G. Evaluation of biophotonic therapy in a non-healing diabetic foot ulcer: a case report. Diabetic Foot Canada. 2016;4(2):25–30. | ||
Woo K, Sibbald G, Fogh K, et al. Assessment and management of persistent (chronic) and total wound pain. Int Wound J. 2008;5(2):205–215. | ||
Price P, Fogh K, Glynn C, Krasner DL, Osterbrink J, Sibbald RG. Managing painful chronic wounds: the Wound Pain Management Model. Int Wound J. 2007;4 Suppl 1:4–15. | ||
Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29(6):1288–1293. | ||
Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol. 1999;135(8):920–926. | ||
Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages? Am J Med. 2000;109(1):15–19. | ||
Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12(2):163–168. | ||
Blume P, Driver VR, Tallis AJ, et al. Formulated collagen gel accelerates healing rate immediately after application in subjects with diabetic neuropathic foot ulcers. Wound Repair Regen. 2011;19(3):302–308. | ||
Zelen CM, Orgill DP, Serena T, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. 2017;14(2):307–315. | ||
Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554–560. | ||
Richard JL, Martini J, Bonello Faraill MM, et al. Management of diabetic foot ulcers with a TLC-NOSF wound dressing. J Wound Care. 2012;21(3):142–147. | ||
Edmonds M; European and Australian Apligraf Diabetic Foot Ulcer Study Group. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8(1):11–18. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.