Back to Journals » Journal of Blood Medicine » Volume 5
Erythropoiesis-stimulating agents for the treatment of chemotherapy-induced anemia: comparisons from real-world clinical experience
Authors Rodriguez Garzotto A, Heine O, Turner M, Rebollo Laserna F, Lorenz A
Received 20 November 2013
Accepted for publication 13 February 2014
Published 28 April 2014 Volume 2014:5 Pages 43—48
DOI https://doi.org/10.2147/JBM.S57887
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Analia Rodriguez Garzotto,1 Oliver Heine,2 Matthew Turner,3 Francisco Rebollo Laserna,4 Andreas Lorenz5
1Hospital Universitario 12 de Octubre, Ctra Andalucía, Madrid, Spain; 2Zentralklinikum Suhl, Suhl, 3Sandoz International GmbH, Holzkirchen, Germany; 4Sandoz Farmaceutica SA, Madrid, Spain; 5Frauenarztpraxis, Hildburghausen, Germany
Background: The purpose of this paper is to report real-world data on the relative effectiveness of a biosimilar erythropoiesis-stimulating agent (ESA; Binocrit®), and other available ESAs for the treatment of chemotherapy-induced anemia.
Methods: Data were collected retrospectively from single centers in Spain (n=284) and Germany (n=145). Hemoglobin outcomes, transfusion requirements, and serious drug-related adverse events were assessed for each ESA.
Results: Hemoglobin outcomes and transfusion requirements were generally similar in the different ESA treatment groups assessed. No serious drug-related adverse events were recorded in any of the treatment groups.
Conclusion: These data confirm the real-world effectiveness and safety of a biosimilar ESA (Binocrit®) for the treatment of cancer patients with chemotherapy-induced anemia.
Keywords: erythropoiesis-stimulating agents, chemotherapy-induced anemia, biosimilar
Corrigendum for this paper has been published
Introduction
Patients receiving chemotherapy for cancer frequently experience anemia due to the cytotoxic effect on erythroid precursors in bone marrow.1 Other chemotherapy agents, particularly platinum-based compounds, can directly affect the cells in the kidney responsible for producing erythropoietin.2,3 Patients suffering from chemotherapy-induced anemia (CIA) experience profound effects on their quality of life due to extreme fatigue, reduced physical capacity, and in some cases, impaired cognitive function.4–6
Erythropoiesis-stimulating agents (ESAs) have been shown to steadily increase hemoglobin levels, reduce the need for blood transfusions, and improve overall quality of life.7–9 Binocrit® (Sandoz GmbH, Kundl, Austria) is a biosimilar epoetin alfa approved by the European Medicines Agency in 2007 for several indications, including the treatment of CIA. Biosimilar is a regulatory term used to describe medicines with similar properties to that of an approved biological medicine for which the patent has expired.10 For a biosimilar to be approved by the European Medicines Agency, the manufacturer must demonstrate comparability with the reference product in terms of quality, safety, and efficacy.10
Several biosimilar epoetins have been approved by the European Medicines Agency. The uptake of these medicines will depend, in part, on physicians’ confidence in the efficacy and safety of these products. There is currently a lack of data on the relative effectiveness of biosimilar ESAs and other available ESAs (short-acting and long-acting) for the treatment of CIA. Thus, it is important to report real-world clinical experience data for biosimilars in order for physicians to make informed treatment decisions for their patients.11 Here we report such data with a biosimilar epoetin alfa (Binocrit®) from two separate centers, one from Spain and one from Germany.
Patients and methods
The findings we report were obtained as part of the normal clinical management of the patients, which was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice. All ESAs were used in accordance with current European labels (ie, patients were receiving concurrent chemotherapy and ESA treatment was initiated at a hemoglobin level ≤10 g/dL).
Spanish center
Data are presented from a retrospective clinical experience audit of CIA treatment with ESAs in a large oncology department, including patients treated by multiple (n=20) physicians. The department is within the 1,300-bed Hospital 12 de Octubre in Madrid, and treated more than 21,000 patients (with more than 56,000 consultations) in 2011. Over the last 3 years, the department has treated more than 500 patients with ESAs.
A total of 274 patients (those who had records/data in relation to their ESA treatment readily available) were included in the present analysis. Patients were treated with biosimilar epoetin alfa 40,000 IU once a week (n=116) or 30,000 IU once a week (n=14), darbepoetin alfa 500 μg every 3 weeks (n=99), or darbepoetin alfa 150 μg once a week (n=45). Use of parenteral iron supplementation was considered for patients with serum ferritin <300 ng/mL and transferrin saturation <20%. The primary aim of ESA treatment was transfusion avoidance, with the requirement for red blood cell transfusion assessed during the period of ESA therapy and for 2 months after the end of therapy. Treatments were also assessed for hemoglobin outcomes at the start and end of therapy, and comparisons performed of the different hemoglobin outcomes according to the different ESA treatments using Tukey’s honestly significant difference test.
German center
This was a retrospective matched-cohort analysis of 145 patients from a community-based single center with solid tumors and CIA. The center includes three physicians and treats around 1,000 patients with cancer per year; most also routinely receive ESA therapy. Patients were treated with biosimilar epoetin alfa 40,000 IU once weekly (n=95) or darbepoetin alfa 500 μg once every 3 weeks (n=50). Iron supplementation (sodium ferric gluconate complex in sucrose injection; 62.5 mg) was given if serum ferritin levels fell below 200 ng/mL. The aim of treatment was to achieve a hemoglobin level of 12 g/dL, at which point ESA treatment was stopped permanently. Both treatments were assessed against hemoglobin outcomes and red blood cell transfusion requirements. Comparisons were performed of the different hemoglobin outcomes for the different ESA treatments using t-tests.
Comparison of pooled data on hemoglobin outcomes and transfusion requirements
Data on hemoglobin outcomes and transfusion requirements from the two centers were pooled for comparison. Data were combined based on ESA product, rather than product and dose, given the relatively low number of patients who received biosimilar epoetin alfa 30,000 IU once weekly and darbepoetin alfa 150 μg once a week. For hemoglobin, the mean (standard deviation) increase during the study was calculated for each product, and a comparison was performed using a t-test. For transfusion requirements, pooled data for each product (proportion of patients who required a transfusion) was compared using a chi-squared (Pearson) test.
Results
Spanish center
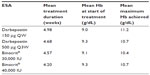
The most common tumor types were non-small-cell lung cancer (30%), breast cancer (12%), head and neck cancer (7%), and ovarian cancer (6%), as shown in Table 1. The most commonly used chemotherapy agents overall were carboplatin (108/284 patients) and paclitaxel (68/284 patients); the most common chemotherapy combination was carboplatin/paclitaxel (49/284 patients). Among patients treated with darbepoetin (all doses combined, n=146), the most common regimens were carboplatin/paclitaxel (22 patients), carboplatin/gemcitabine (eight patients), and paclitaxel alone (eight patients). The same three regimens were also the most commonly used in patients who received biosimilar epoetin alfa (all doses combined, n=116): carboplatin/paclitaxel (20 patients), carboplatin/gemcitabine (seven patients), and paclitaxel alone (six patients). Twenty-seven percent of patients received ESA treatment for 4 weeks, and 42% were treated for more than 4 weeks. Mean duration of ESA by treatment group was as follows: biosimilar epoetin alfa 40,000 IU once a week, 4.20 weeks; biosimilar epoetin alfa 30,000 IU once a week, 4.57 weeks; darbepoetin alfa 500 μg every 3 weeks, 4.68 weeks; and darbepoetin alfa 150 μg once a week, 4.98 weeks. The mean overall hemoglobin level prior to treatment was 9.3 g/dL; 19% of patients had a hemoglobin level <8.5 g/dL and 42% had a hemoglobin level <9 g/dL at the start of the study.
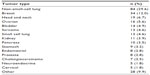  | Table 1 Characteristics of patients at the Spanish center |
The mean overall hemoglobin at the end of the study was 10.8 g/dL. There were no significant differences (P>0.05) between the different treatment groups in terms of hemoglobin level at the start of ESA treatment, hemoglobin levels achieved at the end of the treatment period, or the highest hemoglobin level achieved on ESA treatment (Table 2). The number of patients overall who required a transfusion was low (38 patients, 13.4%), and generally similar across the different treatments. For example, 13 patients (11.1%) in the biosimilar epoetin alfa 40,000 IU once a week group and 11 patients (11.2%) in the darbepoetin alfa 500 μg every 3 weeks group received a transfusion. No serious drug-related adverse events were reported in any ESA group.
German center
The two cohorts were well matched (Table 3). Most patients in each group had breast cancer (85% of patients in the biosimilar epoetin alfa group and 82% in the darbepoetin alfa group). More than 50% of patients in each group were treated with one of the following chemotherapy regimens: docetaxel/adriamycin/cyclophosphamide or 5-fluorouracil/epirubicin/cyclophosphamide with or without docetaxel. All patients were treated with concomitant intravenous iron along with their ESA therapy.
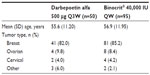  | Table 3 Patient characteristics by treatment group at the German center |
The mean overall hemoglobin level prior to treatment was 9.85 g/dL and 9.92 g/dL for the biosimilar epoetin alfa and darbepoetin alfa groups, respectively. Hemoglobin outcomes and red blood cell transfusion requirements are shown in Table 4. The mean duration of ESA treatment was 4.66 weeks in the Binocrit® group and 4.28 weeks in the darbepoetin alfa group. The mean hemoglobin level at the end of ESA treatment was 11.91 g/dL in the biosimilar epoetin alfa group and 11.93 g/dL in the darbepoetin alfa group. There were no significant differences (P>0.05) between the two groups in hemoglobin level at the start of ESA treatment or at the end of the treatment. In both treatment groups, the median time to achieve a hemoglobin increase >1 g/dL and 2 g/dL was 2 and 4 weeks, respectively. Four patients (4.2%) in the biosimilar epoetin alfa group and four (8%) in the darbepoetin group required a red blood cell transfusion during the period of ESA treatment. No deaths, thromboembolic events, or other serious adverse drug reactions were observed under ESA treatment.
Pooled analysis of hemoglobin outcomes and transfusion requirements
Based on pooled data for each product, the mean increase in hemoglobin was 1.98±1.53 g/dL with biosimilar epoetin alfa, and 1.82±1.01 g/dL with darbepoetin alfa (P=0.5). Blood transfusion was required by 18 patients (8%) who received biosimilar epoetin alfa and 28 patients (14.3%) who received darbepoetin alfa (P=0.039).
Discussion
Binocrit® is a biosimilar ESA that has been approved for the treatment of CIA since 2007. Previous studies have demonstrated the efficacy and safety of this agent for the treatment of CIA, both in a randomized, controlled trial and also in real-life clinical practice.12,13 However, to date, there has been a lack of data on the relative effectiveness of biosimilar ESAs and other available ESAs in this setting. The availability of such real-world data is important if physicians are to make informed treatment decisions when considering options for ESA treatment. The data presented here confirm the real-life clinical effectiveness and safety of managing CIA with biosimilar epoetin alfa. They also indicate that treatment outcomes are similar when patients with cancer and CIA are treated with biosimilar epoetin alfa or darbepoetin alfa. The proportion of patients in both product groups who required a blood transfusion was low compared with data from previously published randomized studies.12,14,15 Pooled data from the two centers showed a statistically significant difference in transfusion requirement favoring biosimilar epoetin alfa. However, caution is warranted when considering the clinical significance of this finding, given the constraints of the retrospective nature of the study. It is interesting to note that, based on the data from the German center, once weekly treatment with biosimilar epoetin alfa appears to be as effective as darbepoetin once every 3 weeks for raising hemoglobin levels and avoiding the need for red blood cell transfusions.
The European labels for the use of ESAs in patients with cancer and CIA have been revised in recent years to recommend more conservative use. Current labels advise that patients must be receiving chemotherapy and that treatment is initiated at a hemoglobin level ≤10 g/dL, with the aim of treatment to achieve a hemoglobin level in the range 10–12 g/dL. It is noteworthy that ESA use differed slightly between the two centers analyzed in this study, while still falling in line with these recommendations. Hemoglobin levels at initiation of ESA were higher in the German center than in the Spanish center, as were the hemoglobin levels achieved at the end of the ESA treatment period. Despite these differences in ESA use and practice patterns in the two centers, no serious drug-related adverse events (including thromboembolic events) were reported in either center. Previously, meta-analyses have reported a thromboembolic event rate of approximately 4% in ESA-treated patients.16,17 However, these analyses included studies of what would today be considered off-label use of ESAs (eg, patients not receiving chemotherapy, initiation of ESA treatment at hemoglobin levels >12 g/dL).
As mentioned previously, ESA use in our centers reflects the current, more conservative recommendations and this may explain why no cases of thromboembolic events were noted. In the future, adequately designed and appropriately powered studies would be of interest to determine the impact of different ESA practice patterns on safety outcomes such as thromboembolic events.
The potential for ESAs to affect disease progression and/or mortality in cancer patients has been the subject of much debate in the literature. Preclinical studies have suggested that erythropoietin receptor (Epo-R) mRNA and/or protein may be present in a range of human tumors and cancer cell lines, and that ESAs have growth-modulating effects on tumor cells.18–20 However, several methodological issues have been raised that may limit the validity of some of these findings.18,21 These include use of bulk tumor tissue (which may contain stromal cells and other cell types that infiltrate from blood) in studies examining Epo-R mRNA levels, use of commercially available antibodies for Western blot and immunohistochemistry analyses that were subsequently shown to lack specificity for Epo-R, and use of supraphysiological ESA doses. More recently, new antibodies have been developed that appear to have improved specificity for Epo-R;22 such antibodies may become valuable new tools in this area of research. A number of clinical studies have also contributed to concerns that ESAs may impact disease progression and overall survival in patients with cancer, although it is important to highlight that these involved use of ESAs that would now be considered off-label (eg, target hemoglobin levels in excess of 14 g/dL, patients who were not receiving chemotherapy).23–26 These and other studies have prompted several meta-analyses to assess the use of ESAs and safety outcomes in patients with cancer.17,27–30 Importantly, none of these has identified an adverse effect of ESA use on survival when considering studies in patients with CIA. Similarly, based on the balance of evidence to date from clinical trials, ESAs do not have an adverse effect on disease progression.21,30
In many countries, expenditure on health care is increasing to unsustainable levels, with cancer medicines a leading driver of these increases.31 Use of biosimilar medicines has been identified as one way of controlling these escalating costs. A recent study evaluated the comparative cost efficiency of different ESAs under different scenarios of fixed and weight-based dosing in the management of CIA.32 Managing CIA with biosimilar epoetin alfa was shown to be consistently cost-efficient over treatment with originator epoetin alfa, epoetin beta, and darbepoetin alfa (both once weekly and once every 3 weeks) under both fixed and weight-based dosing scenarios. Patient exposure to biosimilars continues to increase as adoption of these agents becomes more widespread. For example, the current (as of February 2014) estimated exposure to Binocrit® is over 300,000 patient-years, with more than 5,000 patients studied in clinical trials.33 A recent review identified no difference in safety profiles between biosimilar and reference products.34 Similarly, a prospective randomized clinical study, conducted since licensing, demonstrated equivalent pharmacokinetic and pharmacodynamic profiles, safety, and clinical efficacy between originator and biosimilar epoetins.35
As indicated previously, a clear limitation of our data is that they were generated from retrospective chart reviews rather than in a randomized, controlled setting; an adequately designed, prospective study is necessary to confirm our findings. Nevertheless, reporting of such real-world data is of use to physicians when considering the choice of ESA therapy for patients with cancer and CIA.
In summary, data from these single-center audits confirm the real-world effectiveness and safety of biosimilar epoetin alfa (Binocrit®) for the treatment of cancer patients with CIA. The findings should inform and reassure physicians when making treatment decisions on use of ESAs in this setting.
Disclosure
FRL and MT are employees of Sandoz. The other authors have no conflicts to declare. Medical writing assistance in the preparation of this paper was provided by Tony Reardon of Spirit Medical Communications Ltd and funded by Sandoz International GmbH.
References
Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40(15):2293–22306. | |
Macpherson IR, Lindsay CR, Reed NS. Recombinant human epoetin beta in the treatment of chemotherapy-related anemia. Ther Clin Risk Manag. 2009;5(1):261–270. | |
Horiguchi H, Oguma E, Kayama F. Cadmium and cisplatin damage erythropoietin-producing proximal renal tubular cells. Arch Toxicol. 2006;80(10):680–686. | |
Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25(3 Suppl 7):43–46. | |
Cunningham RS. Anemia in the oncology patient: cognitive function and cancer. Cancer Nurs. 2003;26(Suppl 6):38S–42S. | |
Jacobsen PB, Garland LL, Booth-Jones M, et al. Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. J Pain Symptom Manage. 2004;28(1):7–18. | |
Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888–895. | |
Fallowfield L, Gagnon D, Zagari M, et al. Multivariate regression analyses of data from a randomised, double-blind, placebo-controlled study confirm quality of life benefit of epoetin alfa in patients receiving non-platinum chemotherapy. Br J Cancer. 2002;87(12):1341–1353. | |
Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19(11):2865–2874. | |
Weise M, Bielsky MC, De Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–5117. | |
Aapro M. What do prescribers think of biosimilars? Target Oncol. 2012;7 Suppl 1:S51–S55. | |
Weigang-Köhler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie. 2009;32(4):168–174. | |
Kerkhofs L, Boschetti G, Lugini A, Stanculeanu DL, Palomo AG. Use of biosimilar epoetin to increase haemoglobin levels in patients with chemotherapy-induced anemia: real-life clinical experience. Future Oncol. 2012;8(6):751–756. | |
Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211–1220. | |
Auerbach M, Silberstein PT, Webb RT, et al. Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol. 2010;85(9):655–663. | |
Bohlius J, Langensiepen S, Schwarzer G, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst. 2005;97(7):489–498. | |
Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–714. | |
Österborg A, Aapro M, Cornes P, Haselbeck A, Hayward CR, Jelkmann W. Preclinical studies of erythropoietin receptor expression in tumour cells: impact on clinical use of erythropoietic proteins to correct cancer-related anaemia. Eur J Cancer. 2007;43(3):510–519. | |
Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res. 2011;17(20):6373–6380. | |
Todaro M, Turdo A, Bartucci M, et al. Erythropoietin activates cell survival pathways in breast cancer stem-cell like cells to protect them from chemotherapy. Cancer Res. 2013;73(21):6393–6400. | |
Aapro M, Jelkmann W, Constantinescu SN, Leyland-Jones B. Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer. Br J Cancer. 2012;106(7):1249–1258. | |
Singh S, Verma R, Pradeep A, et al. Dynamic ligand modulation of EPO receptor pools, and dysregulation by polycythemia-associated EPOR alleles. PLoS One. 2012;7(1):e29064. | |
Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362(9392):1255–1260. | |
Smith RE Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26(7):1040–1050. | |
Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25(9):1027–1032. | |
Overgaard J, Hoff CM, Hansen HS, et al. Randomized study of darbepoetin alfa as modifier of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): final outcome of the DAHANCA 10 trial. J Clin Oncol. 2009;27:15 Suppl:Abstr 6007. | |
Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–1542. | |
Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ. 2009;180(11):E62–E71. | |
Ludwig H, Crawford J, Österborg A, et al. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol. 2009;27(17):2838–2847. | |
Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102(2):301–315. | |
Cornes P. The economic pressures for biosimilar drug use in cancer medicine. Target Oncol. 2012;7 Suppl 1:S57–S67. | |
Aapro M, Cornes P, Sun D, Abraham I. Comparative cost efficiency across the European G5 countries of originators and a biosimilar erythropoiesis-stimulating agent to manage chemotherapy-induced anemia in patients with cancer. Ther Adv Med Oncol. 2012;4(3):95–105. | |
Aapro M. Biosimilars in oncology: current and future perspectives. GaBI Journal. 2013;2(2):91–94. | |
Abraham I, MacDonald K. Clinical safety of biosimilar recombinant human erythropoietins. Expert Opin Drug Saf. 2012;11(5):819–840. | |
Lissy M, Ode M, Roth K. Comparison of the pharmacokinetic and pharmacodynamic profiles of one US-marketed and two European-marketed epoetin alfas: a randomized prospective study. Drugs R D. 2011; 11(1):61–75. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.