Back to Journals » Drug Design, Development and Therapy » Volume 14
Epigallocatechin-3-Gallate Protects H2O2-Induced Nucleus Pulposus Cell Apoptosis and Inflammation by Inhibiting cGAS/Sting/NLRP3 Activation
Authors Tian Y, Bao Z, Ji Y, Mei X, Yang H
Received 22 March 2020
Accepted for publication 29 April 2020
Published 27 May 2020 Volume 2020:14 Pages 2113—2122
DOI https://doi.org/10.2147/DDDT.S251623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yixing Tian, Zhaohua Bao, Yiming Ji, Xin Mei, Huilin Yang
Department of Orthopedic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China
Correspondence: Huilin Yang Email [email protected]
Background: Intervertebral disc degeneration (IDD) is the most common diagnosis of patients with lower back pain. IDD is the underlying lesion of many spinal degenerative diseases; however, the role of cGAS/Sting/NLRP3 pathway and epigallocatechin gallate (EGCG) in the development of IDD remained unclear.
Methods: The expressions of cGAS, Sting and NLRP3 mRNA of intervertebral disc (IVD) samples from IDD patients and controls were detected by RT-PCR. The nucleus pulposus cells (NPCs) were induced by hydrogen peroxide (H2O2) and used as an in-vitro model. Both 5 μM and 25 μM EGCG treatment were used to detect the effect of EGCG on the in-vitro model. Cell viability was detected by the MTT method, and cell apoptosis and cell cycle would be detected by flow cytometry. Western blot was used in the detection of the expression of cGAS/Sting/NLRP3 as well as apoptosis-related protein level. ELISA was used in the detection of pro-inflammatory factors, including IL-1β, TNF-α, IL-6 and IL-10.
Results: The expressions of cGAS, Sting and NLRP3 mRNA were significantly increased in the IVD samples from IDD patients and NLRP3 was associated with cGAS and Sting. Advanced in-vitro study showed that H2O2 significantly increased the expression of cGAS, Sting and NLRP3 protein levels. Advanced experiments showed that EGCG treatment demonstrated significant protective effects in cell viability, apoptosis, cell cycle arrest and inflammatory status through down-regulation of cGAS/Sting/NLRP3 pathway.
Conclusion: It was shown that the cGAS, Sting and NLRP3 up-regulation was associated with the incidence of IDD. Our findings also suggest that EGCG treatment would provide anti-apoptosis, anti-inflammation and promote cell viability in H2O2 treatment-incubated NPCs through inhibiting cGAS/Sting/NLRP3 pathway.
Keywords: intervertebral disc degeneration, nucleus pulposus cells, epigallocatechin gallate, cGAS, sting, NLRP3
Introduction
Intervertebral disc degeneration (IDD) is the most common diagnosis of patients with lower back pain. IDD is the underlying lesion of many spinal degenerative diseases, including cervical spondylosis, lumbar disc herniation, and lumbar spinal stenosis.1 With the aging status of population and changes in lifestyles, the prevalence of spinal degenerative diseases kept increasing year by year; thus, it demonstrated the importance of IDD management. As spinal degenerative diseases would cause both serious influences of the life quality of patients and extreme healthy burden to society, it usually would need extensive medical management.2 Patients with severe spinal degenerative diseases usually need surgery; however, the normal physiological structure would be changed in both fusion and non-fusion methods. Besides, spinal surgery might bring failure of internal fixation, prosthesis wear, and adjacent segment regression.3 These situations highlighted the importance in the medical management of IDD; however, the treatment of IDD achieves no satisfactory outcomes.
IDD is a multi-etiology and age-related degenerative disorder while its detailed pathological mechanisms remained unclear by now. Generally speaking, the occurrence and development of IDD were related to the process of heredity, aging, trauma, excessive weight-bearing and smoking.4 The molecular mechanisms of IDD included cell senescence, oxidative stress, inflammation and extracellular matrix degradation.4 The apoptosis and oxidative stress of nucleus pulposus cells (NPCs) in intervertebral disc (IVD) would induce inflammation and thus played a key role in promoting IDD. The mechanism of apoptosis was related to oxidative stress and H2O2-induced oxygen-free radical damage was an important effector in the progression of IDD.5 The local accumulation of H2O2 would lead to chronic inflammation and might be used as a target of IDD.
Nowadays, cyclic GMP-AMP synthase (cGAS) and stimulator of IFN gene (Sting) have been reported to play a key role in virus detection, infection reaction as well as a variety of degenerative disorders, including cardiovascular disease, neurodegenerative diseases, diabetes as well as cancers.6–8 Besides, inhibiting the cGAS/Sting pathway provided potential therapeutic methods for kinds of degenerative disorders, including progeria, neurodegeneration and age-related macular degeneration.9–12 In recent reviews, cGAS, which is a sensor of cytoplasmic DNA, played a key role in the aging progression.13,14 As both degenerative progress and chronic inflammation were regarded as key biological progresses of IDD, cGAS/Sting pathway, which was a connection of these two pathways, might play key regulation roles in the IDD incidence. Inflammasomes were multiprotein signaling platforms that controlled the inflammatory responses15 and coordinated antimicrobial host defenses. Previous studies demonstrated that NLRP3 inflammasome plays a vital role in IDD;16 however, no previous study focused the effect of cGAS/Sting pathway on NLRP3 inflammasome activation in the development of IDD.
Epigallocatechin gallate (EGCG), which is a catechins monomer isolated from tea, is the main component of green tea polyphenols. It was reported that EGCG plays important antibacterial, antiviral, antioxidant, anti-arteriosclerosis, antithrombotic, antiangiogenic, anti-inflammatory and anti-tumor effects.17 In a previous case control, it was found that tea drinking habit is a protective factor for IDD.18 The effect of EGCG on the oxidative stress and inflammatory response in intervertebral disc has been studied in animal model and in-vitro cell culture.19,20 In this study, we conducted an experiment based on clinical samples and in-vitro studies to investigate the expression of cGAS, Sting and NLRP3 in the IDD cases and detect the effects of EGCG in the H2O2-treated NPCs.
Materials and Methods
Ethics Statement
The use of human material followed the Declaration of Helsinki in the use of human subjects. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, Suzhou, RPC. Written informed consents were obtained from all the participants in this study.
Study Subjects
All the participants included in this study are patients from January 2019 to January 2020. A total of 37 cases of lumbar degenerative diseases were collected as IDD group and 15 cases of spinal cord injury patients were included as control group. Among the IDD group, there were 17 males and 20 females and the average age is 54.2±15.6 years. For the IDD cases, 18 patients are with lumbar disc herniation, 5 patients with lumbar disc derived lumbago, 7 patients with lumbar stenosis, and 7 patients with lumbar spondylolisthesis pathological segment. In the control group, the patients with acute spinal cord injury after trauma admitted to the control group. The mean age of the control group is 46.8±18.3 years and 6 male and 9 female patients are included in this study. No patients in the control group were diagnosed as IDD before injury. Any patients with tumor, infection, immune and endocrine diseases were excluded from any group. Once the intervertebral disc samples were removed during surgeries, they will be kept in TRIzol reagent (Invitrogen, CA, USA) at −80°C until the following experiment.
RNA Extraction and Quantitative Reserve Transcription PCR (RT-PCR)
For the RNA extraction in the clinical samples from both IDD and control groups, total RNA extraction was conducted with TRIzol reagent according to the manufacturer’s instruction. The extracted RNA was treated with the DNA-free™ kit (Ambion, TX, USA) to remove the genomic DNA. After RNA extraction, RNA concentration and quality were detected using the NanoDrop spectrophotometer (Thermo Scientific, CA, USA). The relative abundance of mRNA expression of cGAS, Sting and NLRP3 in all the sample genes was determined with quantitative PCR using Reverse transcription was performed with 1.25 μg of total RNA in a final reaction volume of 50 μL. The quantitative real-time PCR was conducted with Novostart SYBR qPCR Super Mix Plus (Novoprotein, CA, USA) following the manufacturers’ instructions. We used β-actin as the endogenous control gene in this study. The reaction program was set as 1 cycle at 95°C for 15 min, 40 cycles at 95°C for 15 s, and then 65°C for 1 min. The primer sequences used in this study were as the following: cGAS: forward: 5ʹ‑AAGGATAGCCGCCATGTTTCT‑3ʹ, reverse: 5ʹ‑TGGCTTTCAGCAAAAGTTAGG‑3ʹ; Sting: forward: 5ʹ‑AGCATTACAACAACCTGCTACG‑3ʹ, reverse: 5ʹ‑GTTGGGGTCAGCCATACTCAG‑3ʹ; NLRP3: forward: 5ʹ‑GATCTTCGCTGCGATCAACA‑3ʹ, reverse: 5ʹ‑GGGATTCGAAACACGTGCATTA‑3ʹ; β-actin: forward: 5ʹ‑TCCCTGGAGAAGAGCTACG‑3ʹ, reverse: 5ʹ‑GTAGTTTCGTGGATGCCACA‑3ʹ. The relative gene expression was calculated using the comparative cycle threshold method (CT, 2−ΔΔCt).
Primary NPC Isolation and Culture
The primary NPC isolation and culture were conducted as described previously.21 The nucleus pulposus (NP) was extracted from IVD tissues, and its morphology was observed under a light microscope. Then, NP samples were dissected and washed with PBS and incubated with 0.25% trypsin solution and 0.2% type II collagenase (sigma, St. Louis, Missouri) at 37°C for 4~6 h. The NPCs were purified from tissue debris through a 200 μm filter and then cultured in DME/F-12 media (HyClone, UT, USA) with 10% fetal bovine serum (Gibco, CA, USA) and 1× penicillin/streptomycin (Gibco, CA, USA) at 37°C and 5% CO2. NPCs in 3~5 generations would be used for further in-vitro experiments.
H2O2 Treatment in NPCs
H2O2 treatment in NPCs was conducted following previous protocols with modifications.22,23 Briefly, 5 × 104 cells per well were placed in six-well cell culture plates, and the cultured cells were incubated with complete medium containing 800 µM H2O2 for 2 h followed by PBS wash for three times for 5 continuous days. After H2O2 treatment, the processed NPCs cells were digested with trypsin, passaged, and plated on new palates for experiments.
Cell Viability Assay
The cell viability was conducted with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (Liaohua BioTec, Nanjing, RPC). After H2O2 treatment in cultured NPCs, cells were treated with EGCG (0, 5 and 25 μM) in 96-well plates for 24 h. The cells were exposed to 430nm radiation for 30 min, and then incubated at 37°C for 16 h. After lysing the cells in 100 μL per well lyse reagent and incubated at 37°C overnight, MTT labeling reagent (10 μL per well) was added for 4 h. The absorbance was read at 570 nm using a Multiplate reader (Molecular Device Corporation, CA, USA).
Cell Migration Assay
The migration ability of NPCs in different groups was analyzed using an 8-micron well (Corning, Corning, NY, USA). A final amount of 2 × 104 cells/mL was added to the upper cavity, and then 0.5 mL of the complete medium would be added to the lower cavity. After incubation at 37°C for 24 h, 0.5% crystal violet (Sigma-Aldrich, Inc, MO, USA) was used to stain migrating NPCs and quantify them under a light microscope.
Flow Cytometry (FCM)
FCM was used to detect NPC apoptosis and cell cycle arrest induced by H2O2. After trypsin treatment, the isolated cells were fixed in 70% ethanol at 4°C for 4 h. For apoptosis analyses, the cells were stained with Annexin-V fluorescein isothiocyanate and PI solution, and the samples are kept in the dark for 20 min. For cycle arrest analyses, the cell in different groups was stained with 50 μg/mL RNase A and 50 μg/mL PI for 30 min at 37°C. Samples were analyzed with a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA), and at least 8000 cells were collected from each sample. Data were analyzed using Kaluza For Gallios 1.0 software.
Western Blot Analysis
NPCs would be lysed with RIPA buffer containing 1×protease inhibitor cocktail and EDTA. The soluble and insoluble protein fractions were separated at 13,000 rpm for 15 min at 4°C. The protein concentrations in supernatants were determined with the Bradford protein detection kit, and aliquots of protein (40 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The protein was separated on 10% SDS-PAGE and transferred to a 0.45 μm nitrocellulose membrane (Bio-Rad) for Western blot. The membrane would be blocked with a 1% skimmed milk powder on a rotary shaker at room temperature for 1 h, and then incubated with anti-cGAS (1:1000; Cell Signaling Technology), anti-Sting (1:500; Santa Cruz), anti-NLRP3 (1:500; Santa Cruz), caspase-3 (1:500; Cell Signaling Technology), caspase-9 (1:1000; Santa Cruz), Bc1-2 (1:1000; Cell Signaling Technology), Bax (1:1000; Cell Signaling Technology) and GAPDH (1:500; Santa Cruz) overnight. After 3 times washes with PBST buffer (0.1% Tween-20, 1×-PBS) and the membranes would be incubated with 1:2000 goat horseradish peroxidase-conjugated second antibody (Novus Biologicals) at room temperature for 1 h. After 3 times washes with PBST buffer, specific antibody–antigen complex was detected using an enhanced chemiluminescence Western blot detection system (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). The images were taken using ImageJ (NIH) software was used to quantify changes in protein expression.
Enzyme Immunoassay (ELISA)
Human IL-1β, TNF-α, IL-6 and IL-10 level in the culture medium in all the groups were determined with commercial ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. Protein concentrations were measured with ELISA kits for triple times per cytokine.
Statistical analysis
The data in this study were determined from at least three independent experiments and would be presented as mean ± SD. Statistical analyses were performed using unpaired Student’s t-test when comparing two groups and one-way analysis of variance with Bonferroni’s multiple comparison test for three groups or more. The correlation analyses were conducted using Pearson correlation assay and the linear correlation was simulated with linear regression method. Statistical differences were considered significant at a P value of less than 0.05.
Results
cGAS, Sting and NLRP3 Were Up-Regulated in IVD Tissues from IDD Patients
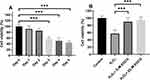
In order to compare the expression of the cGAS, Sting and NLRP3 mRNA of IDD cases and controls, we carried out real-time PCR analyses of IVD samples in both groups. Through analyzing the mRNA level of cGAS, Sting and NLRP3 in 37 IDD patients and 15 controls, it was found that cGAS, Sting and NLRP3 mRNA levels were significantly up-regulated in IDD cases (Figure 1A). In advanced correlation analyses, we detect the correction analyses between cGAS, Sting and NLRP3 in the 37 IDD cases and it was found that cGAS (P < 0.001) and Sting (P < 0.001) expressions are associated with NLRP3 levels (Figure 1B and C).
Effect of EGCG on the Viability of H2O2-Treated NPCs
To examine the effect of EGCG on the H2O2-treated NPCs, we employed an in-vitro model in which H2O2 treatment was adopted in the cultured NPCs. After treatment with 800 µM H2O2 for consequent 5 days, a significant reduced cell viability was detected in NPCs since day 3 and it would lead to almost 50% reduction of cell viability in day 5 (Figure 2A). After 5 μM and 25 μM treatment, the cell viability was significantly increased compared with the H2O2-treated group (P < 0.001, Figure 2B).
EGCG Protects Against H2O2-Treated Cell Apoptosis and Cell Cycle Arrest in NPCs
Through FCM assays, we detected the cell cycle in H2O2 with and without EGCG treatment. The apoptosis was detected with FCM by the annexin V/PI assay. As shown in Figure 3B, H2O2 significantly increased both early and late apoptosis. To further detect the protective effect of EGCG in H2O2-treated NPCs, we measured the apoptotic rates in 5 μM and 25 μM EGCG-treated group. After treatment with both 5 μM and 25 μM EGCG, a significant reduced apoptotic rate was detected (P < 0.001), and the data are presented in Figure 3A. In advanced study, it demonstrated a remarkable G1 phase arrest and preventing cells from entering S phase with H2O2 treatment. After addition with EGCG in both 5 μM and 25 μM concentration, it was found that EGCG significantly prevents cell cycle arrest in H2O2-treated NPCs (Figure 3B).
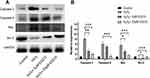
EGCG Treatment Altered Apoptosis-Related Protein Expression in H2O2-Treated NPCs
As shown in Figure 3A, EGCG could significantly improve apoptotic status in H2O2-treated NPCs. To detect the effect of EGCG on the expression of apoptosis-related proteins, we measured the expressions of cleaved caspase-3, caspase-9, Bcl-2 and Bax of different groups in this study. As showed in Figure 4A and B, it was found that caspase-3, caspase-9 and Bax were up-regulated after H2O2 treatment while Bcl-2 was down-regulated. When EGCG was added in H2O2-treated NPCs, the pro-apoptosis proteins caspase-3, caspase-9 and Bax were down-regulated, while anti-apoptosis protein Bcl2 was up-regulated. The data in this experiment confirmed the anti-apoptotic effect of EGCG in NPCs.
EGCG Treatment Promoted Cell Migration of H2O2-Treated NPCs
As shown in Figure 5, the effects of EGCG on the cell migration were detected through migration assay. Through adding 5 μM and 25 μM EGCG in H2O2-treated NPCs, cell migration ability was significantly improved compared with only the H2O2-treated NPC group (Figure 5A and B).
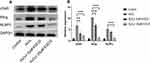
EGCG Decreases the Expression of cGAS/Sting/NLRP3 in H2O2-Treated NPCs
Considering that the mRNA content of cGAS, Sting and NLRP3 in IVD tissues of IDD patients, we conducted advanced study in the protein expressions in H2O2-treated NPCs. As shown in Figure 6A and, H2O2 treatment resulted in a remarkable increase in the protein levels of cGAS, Sting and NLRP3. Besides, it was detected that there is a significant reduction of cGAS, Sting and NLRP3 expressions in the EGCG treatment group.
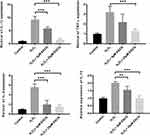
EGCG Decreases the Release of Pro-Inflammatory Factors in H2O2-Treated NPCs
The effect of EGCG on inflammation was evaluated through analyzing the levels of four pro-inflammatory mediators, IL-1β, TNF-α, IL-6 and IL-10, after treatments with or without H2O2 and EGCG. It was found that exposure of H2O2 significantly increased expression of IL-1β, TNF-α, IL-6 and IL-10 (P < 0.001). After EGCG addition was conducted, the release of L-1β, IL-6 and IL-10 was significantly reduced in 5 μM and 25 μM EGCG treatment group (P < 0.01). The level of TNF-α was significantly reduced in 25 μM but not 5 μM EGCG treatment group (P < 0.001). All the detailed data are showed in Figure 7.
Discussion
In this current study, our data showed that cGAS, Sting and NLRP3 mRNA expressions were significantly increased in the IVD samples from IDD patents. In-vitro study based on H2O2-treated NPCs, it was found that H2O2 could significantly increase the expression of cGAS, Sting and NLRP3 protein levels. Advanced experiments showed that EGCG treatment would demonstrate significantly protective effects in cell viability, apoptosis, cell cycle arrest and pro-inflammatory factors release through down-regulating cGAS/Sting/NLRP3 pathway.
Considering that cGAS/Sting/NLRP3 pathway plays a key role in various disorders, however, the expression of cGAS, Sting and NLRP3 had not been studied in IVD samples from IDD cases. It has been long recognized that cGAS played a key role in the recognition of cytoplasmic DNA thus demonstrated key effect in the virus infection,24,25 its potential roles in the autoimmune and degenerative diseases have been reported nowadays as well.26 A previous study about the effect of the cGAS/Sting pathway on cancer treatment demonstrated that the cGAS/Sting pathway was involved in immune checkpoint blockade therapy response in pancreatic cancer.27 As cGAS has been reported to be a therapeutic target of aging,28 we conducted advanced studies on the expression of cGAS and Sting pathway in the IDD cases. As far as we know, this is the first study on the expression of cGAS and Sting in IVD samples of IDD patients. As significantly increased expressions of cGAS and Sting were detected in IDD cases, the cGAS/Sting pathway might demonstrate important effects in IDD incidence. However, only mRNA expressions of cGAS, Sting and NRLP3 in IDD cases were detected in this study, their protein levels would provide us more knowledge in the potential regulation effects. We would conduct an advanced study with better study design and larger sample size focusing on the relationship of cGAS/Sting/NRLP3 expression and IDD incidence. Besides, more advanced researches are required in the circulating cGAS/Sting on the incidence of IDD as well as the local cGAS/Sting expression on the diagnosis and prognosis of IDD.
Previous studies have demonstrated a significant association between inflammasome and IDD progression.29,30 In consistence with previous researches, the results in this study demonstrated that NLRP3, which is a key factor in the inflammasome pathway, was up-regulated in IVD samples from IDD cases. Advanced analyses demonstrated the correlation between cGAS and Sting expression and NLRP3 levels in IDD cases and that provide us evidence of the potential regulation effect of cGAS/Sting on NLRP3. Even no previous studies focused on the effect of the cGAS/Sting pathway on the inflammasome in IDD, the cGAS/Sting pathway was reported to play a key role in the inflammasome activation.31 The inflammatory corpuscles of human bone marrow cells can be activated by the cGAS/Sting recognition mechanism, which can also activate the innate immune system’s response to viral DNA. The researchers found that the activation of the cGAS/Sting pathway would lead to the programmed cell death unrelated to the antiviral response. When the activation of the cGAS/Sting pathway exceeds a certain threshold, the sting protein will induce the cleavage of a kind of cell inner membrane vesicles called lysosomes and induce inflammasome.32 Considering that both cGAS/Sting and inflammasome are commonly reported biological progresses in various diseases, we propose that the cGAS/Sting pathway could trigger NLRP3 activation and contribute in the development of IDD. More in-vitro and in-vivo studies are required to confirm this hypothesis.
EGCG, which is an extract from green tea, has been reported to play an important role in different diseases, including cardiovascular disorders, neurodegenerative diseases, cancers and diabetes.33–35 A previous study demonstrated that EGCG could contribute in the extracellular matrix formation and then enhance bone-forming capacity.36 Osteoporosis is the second most common epidemic disease among the aging population in the world and previous studies have found that people who drink tea regularly have higher bone mineral density and fewer hip fractures. It was found that EGCG reduced the RANKL/OPG ratio in mRNA expression and secreted protein levels through the RANK/RANKL/OPG pathway, and ultimately reduced osteoclast formation and TRAP activity by TRAP (+) staining of osteoclasts.37 EGCG has been shown to inhibit osteoclast differentiation and EGCG could reverse bone resorption in the LPS-induced cranial bone erosion model through improving bone density and preventing bone loss. Therefore, EGCG is a viable anti-osteoclast effect both in-vitro and in-vivo, indicating its therapeutic strategy against potential bone resorption.38 However, limited knowledge about the effect of EGCG in the development of IDD has been obtained even drinking tea was related with reduced risk of IDD. In this study, we confirmed that 5 μM and 25 μM EGCG could promote cell viability and inhibit apoptosis and cell cycle arrest through anti-inflammatory factor secretion in H2O2-treated NPCs. Considering that there are a lot of apoptotic chondrocytes in human degenerative lumbar disc tissue and apoptosis is the main factor of the decrease of active cells in degenerative lumbar disc tissue, EGCG could be used as a potential treatment for IDD.39 Inflammation can also be a ubiquitous participant in the degenerative disc disease, and the relationship between inflammation and back pain is well recognized.40 EGCG has been regarded as a powerful anti-oxidative and anti-inflammation natural extract and might be applied as a potential drug for the treatment and prevention of IDD. More experiments on the effect of EGCG on the development and progression of IDD based on animal models would be conducted in the following researches.
In summary, our data in this study show that the up-regulated cGAS, Sting and NLRP3 were related to the incidence of IDD. EGCG would provide anti-apoptosis, anti-inflammation and promote cell viability in H2O2 treatment-induced NPCs. Our findings also suggest that cGAS/Sting/NLRP3 pathway might be involved in the progression of IDD and EGCG would be used as a potential protector for IDD.
Acknowledgments
We acknowledged Dr. Wei Zhu from Tongji University in the in-vitro model development in this study.
Author Contributions
YXT and ZHB performed all experiments and analyzed the data. YXT and HLY prepared the original manuscript. YXT, YMJ, XM, HLY revised the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing financial interests.
References
1. Qiu S, Shi C, Anbazhagan AN, et al. Absence of VEGFR-1/Flt-1 signaling pathway in mice results in insensitivity to discogenic low back pain in an established disc injury mouse model. J Cell Physiol. 2019.
2. Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. doi:10.1016/j.arr.2019.100978
3. Frapin L, Clouet J, Delplace V, Fusellier M, Guicheux J, Le Visage C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv Drug Deliv Rev. 2019;149–150:49–71. doi:10.1016/j.addr.2019.08.007
4. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi:10.1038/nrrheum.2013.160
5. Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019;10(7):510. doi:10.1038/s41419-019-1701-3
6. Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10(1):26–39. doi:10.1158/2159-8290.CD-19-0761
7. Ablasser A, Hur S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat Immunol. 2020;21(1):17–29. doi:10.1038/s41590-019-0556-1
8. Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. STING: a master regulator in the cancer-immunity cycle. Mol Cancer. 2019;18(1):152. doi:10.1186/s12943-019-1087-y
9. Wu Y, Wei Q, Yu J. The cGAS/STING pathway: a sensor of senescence-associated DNA damage and trigger of inflammation in early age-related macular degeneration. Clin Interv Aging. 2019;14:1277–1283. doi:10.2147/CIA.S200637
10. Mathur V, Burai R, Vest RT, et al. Activation of the STING-dependent Type I interferon response reduces microglial reactivity and neuroinflammation. Neuron. 2017;96(6):1290–1302 e1296. doi:10.1016/j.neuron.2017.11.032
11. Gonzalo S, Coll-Bonfill N. Genomic instability and innate immune responses to self-DNA in progeria. Geroscience. 2019;41(3):255–266. doi:10.1007/s11357-019-00082-2
12. Hamann L, Ruiz-Moreno JS, Szwed M, et al. STING SNP R293Q is associated with a decreased risk of aging-related diseases. Gerontology. 2019;65(2):145–154. doi:10.1159/000492972
13. Gluck S, Ablasser A. Innate immunosensing of DNA in cellular senescence. Curr Opin Immunol. 2019;56:31–36. doi:10.1016/j.coi.2018.09.013
14. Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med. 2018;215(5):1287–1299. doi:10.1084/jem.20180139
15. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. doi:10.1038/s41581-019-0234-4
16. Huang Y, Peng Y, Sun J, et al. Nicotinamide phosphoribosyl transferase controls NLRP3 inflammasome activity through MAPK and NF-kappaB signaling in nucleus pulposus cells, as suppressed by melatonin. Inflammation. 2020. doi:10.1007/s10753-019-01166-z
17. Bimonte S, Cascella M, Barbieri A, Arra C, Cuomo A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: an update of the current state of knowledge. Infect Agent Cancer. 2020;15:2. doi:10.1186/s13027-020-0270-5
18. Deng Y, Tan XT, Wu Q, Wang X. Correlations between COL2A and aggrecan genetic polymorphisms and the risk and clinicopathological features of intervertebral disc degeneration in a Chinese Han population: a case-control study. Genet Test Mol Biomarkers. 2017;21(2):108–115. doi:10.1089/gtmb.2016.0256
19. Krupkova O, Sekiguchi M, Klasen J, et al. Epigallocatechin 3-gallate suppresses interleukin-1beta-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372–386. doi:10.22203/eCM.v028a26
20. Krupkova O, Handa J, Hlavna M, et al. The natural polyphenol epigallocatechin gallate protects intervertebral disc cells from oxidative stress. Oxid Med Cell Longev. 2016;2016:7031397. doi:10.1155/2016/7031397
21. Jiao Y, Yuan Y, Lin Y, et al. Propionibacterium acnes induces discogenic low back pain via stimulating nucleus pulposus cells to secrete pro-algesic factor of IL-8/CINC-1 through TLR2-NF-kappaB p65 pathway. J Mol Med (Berl). 2019;97(1):25–35. doi:10.1007/s00109-018-1712-z
22. Li P, Gan Y, Xu Y, et al. The inflammatory cytokine TNF-alpha promotes the premature senescence of rat nucleus pulposus cells via the PI3K/Akt signaling pathway. Sci Rep. 2017;7:42938. doi:10.1038/srep42938
23. Zhu W, Wu Y, Meng YF, et al. Effect of curcumin on aging retinal pigment epithelial cells. Drug Des Devel Ther. 2015;9:5337–5344. doi:10.2147/DDDT.S84979
24. Ning X, Wang Y, Jing M, et al. Apoptotic caspases suppress Type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol Cell. 2019;74(1):19–31 e17. doi:10.1016/j.molcel.2019.02.013
25. Qin Y, Liu Q, Tian S, Xie W, Cui J, Wang RF. TRIM9 short isoform preferentially promotes DNA and RNA virus-induced production of type I interferon by recruiting GSK3beta to TBK1. Cell Res. 2016;26(5):613–628. doi:10.1038/cr.2016.27
26. Gentili M, Lahaye X, Nadalin F, et al. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 2019;26(9):2377–2393 e2313. doi:10.1016/j.celrep.2019.01.105
27. Zhang Q, Green MD, Lang X, et al. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Res. 2019;79(15):3940–3951. doi:10.1158/0008-5472.CAN-19-0761
28. Hari P, Millar FR, Tarrats N, et al. The innate immune sensor Toll-like receptor 2 controls the senescence-associated secretory phenotype. Sci Adv. 2019;5(6):eaaw0254. doi:10.1126/sciadv.aaw0254
29. Song Y, Wang Y, Zhang Y, et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J Cell Mol Med. 2017;21(7):1373–1387. doi:10.1111/jcmm.13067
30. Tang P, Gu JM, Xie ZA, et al. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radic Biol Med. 2018;120:368–379. doi:10.1016/j.freeradbiomed.2018.04.008
31. Costa Franco MM, Marim F, Guimaraes ES, et al. Brucella abortus triggers a cGAS-independent STING pathway to induce host protection that involves guanylate-binding proteins and inflammasome activation. J Immunol. 2018;200(2):607–622. doi:10.4049/jimmunol.1700725
32. Gaidt MM, Ebert TS, Chauhan D, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171(5):1110–1124 e1118. doi:10.1016/j.cell.2017.09.039
33. Ahmed F, Ijaz B, Ahmad Z, Farooq N, Sarwar MB, Husnain T. Modification of miRNA Expression through plant extracts and compounds against breast cancer: mechanism and translational significance. Phytomedicine. 2020;68:153168. doi:10.1016/j.phymed.2020.153168
34. Cao SY, Zhao CN, Gan RY, et al. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: an updated review. Antioxidants (Basel). 2019;8:6.
35. Bordoni A, Boesch C, Malpuech-Brugere C, Orfila C, Tomas-Cobos L. The role of bioactives in energy metabolism and metabolic syndrome. Proc Nutr Soc. 2019;78(3):340–350. doi:10.1017/S0029665119000545
36. Huang A, Honda Y, Li P, Tanaka T, Baba S. Integration of epigallocatechin gallate in gelatin sponges attenuates matrix metalloproteinase-dependent degradation and increases bone formation. Int J Mol Sci. 2019;20:23. doi:10.3390/ijms20236042
37. Chen ST, Kang L, Wang CZ, et al. (-)-Epigallocatechin-3-gallate decreases osteoclastogenesis via modulation of RANKL and osteoprotegrin. Molecules. 2019;24:1.
38. Zhu S, Zhu L, Yu J, Wang Y, Peng B. Anti-osteoclastogenic effect of epigallocatechin gallate-functionalized gold nanoparticles in vitro and in vivo. Int J Nanomedicine. 2019;14:5017–5032. doi:10.2147/IJN.S204628
39. Zhang Z, Lin J, Nisar M, et al. The Sirt1/P53 axis in diabetic intervertebral disc degeneration pathogenesis and therapeutics. Oxid Med Cell Longev. 2019;2019:7959573.
40. Liao Z, Wu X, Song Y, et al. Angiopoietin-like protein 8 expression and association with extracellular matrix metabolism and inflammation during intervertebral disc degeneration. J Cell Mol Med. 2019;23(8):5737–5750. doi:10.1111/jcmm.14488
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.