Back to Journals » Drug Design, Development and Therapy » Volume 9
Enzalutamide for the treatment of metastatic castration-resistant prostate cancer
Authors Rodriguez-Vida A, Galazi M, Rudman S, Chowdhury S, Sternberg C
Received 18 March 2015
Accepted for publication 12 May 2015
Published 29 June 2015 Volume 2015:9 Pages 3325—3339
DOI https://doi.org/10.2147/DDDT.S69433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shu-Feng Zhou
Alejo Rodriguez-Vida,1 Myria Galazi,1 Sarah Rudman,1 Simon Chowdhury,1 Cora N Sternberg2
1Medical Oncology Department, Guy’s and St Thomas’ NHS Foundation Trust, London, UK; 2Medical Oncology Department, San Camillo and Forlanini Hospitals, Rome, Italy
Abstract: In recent years, several nonhormonal and hormonal agents, including enzalutamide, have been approved for the treatment of metastatic castration-resistant prostate cancer (CRPC) on the basis of improved overall survival in prospective clinical trials. The incorporation of these agents has revolutionized the treatment of CRPC but has also raised the question of what is the ideal sequence of administering them. Enzalutamide is a nonsteroidal second-generation antiandrogen that has been approved for the treatment of metastatic CRPC both in the post-docetaxel and chemotherapy-naïve settings. This article reviews the pharmacological characteristics of enzalutamide, the efficacy studies which led to its approval, its safety profile, and quality of life-related parameters as well as its place in the sequential treatment and management of metastatic prostate cancer.
Keywords: enzalutamide, antiandrogen, ADT, androgen receptor, castration resistant prostate cancer, overall survival
Introduction
Prostate cancer is the most common cancer in men and the third most frequent cause of death from cancer in men.1 Androgen deprivation therapy (ADT) with luteinizing hormone-releasing hormone analogs is the standard of care for first-line treatment in patients with metastatic prostate cancer. However, despite an initial response, most patients will ultimately experience disease progression after a median time of 18–24 months, developing castration-resistant prostate cancer (CRPC).2 Disease at this stage was previously named androgen-independent or hormone-refractory prostate cancer. However, these terms are misleading and no longer in use for recent studies have shown that even at this stage, disease progression is still mainly driven by androgen receptor (AR) signaling, partly due to overexpression of the AR itself. Consequently, in recent years, several second-generation AR signaling inhibitors have been successfully tested in patients with metastatic CRPC confirming that prostate cancer growth remains dependent on androgen stimulation. The cytochrome P450 (CYP) 17 inhibitor abiraterone acetate and the novel antiandrogen enzalutamide have shown improved overall survival (OS) and quality of life in metastatic CRPC patients both in the pre- and post-docetaxel setting.3–6 In addition, several other nonhormonal treatments such as docetaxel and cabazitaxel chemotherapies, radio-isotope radium-223, and autologous cellular immunotherapy agent sipuleucel-T have recently been approved for the treatment of metastatic CRPC on the basis of improved OS in prospective clinical trials.7–11 The incorporation of these agents has revolutionized the treatment of CRPC and significantly improved the OS of patients but has also raised the question of what is the ideal sequence of administering them. This article reviews the pharmacological characteristics of enzalutamide, the efficacy studies which led to its approval, its safety profile, and quality of life-related parameters as well as its place in the sequential treatment and management of metastatic prostate cancer.
Clinical pharmacology
Mode of action
Enzalutamide (formerly MDV3100) is a nonsteroidal second-generation antiandrogen that acts on different steps in the AR signaling pathway. It competitively inhibits androgen binding to AR, but unlike first-generation antiandrogen, it also inhibits nuclear translocation of the AR, DNA binding, and coactivator recruitment (Figure 1). Enzalutamide was first identified in a drug development study that was looking for nonsteroidal antiandrogens that retain activity in the setting of increased AR expression as a model of castration resistance.12 A library of 200 thiohydantoin derivatives was evaluated, and enzalutamide was selected in view of its favorable drug properties,12 its inhibitory effect on CRPC cell models, its high AR-binding affinity, and its lack of agonistic activity.13 A competition assay was used to compare the relative AR-binding affinity of bicalutamide and enzalutamide in a model of hormone-sensitive cells overexpressing AR (LNCaP/AR human prostate cancer cells), where bicalutamide has an antagonist effect. Importantly, enzalutamide bound to AR with five- to eightfold greater affinity than bicalutamide.13 In order to assess the potential agonistic activity, the authors compared the effects of enzalutamide vs bicalutamide on androgen-dependent gene expression in LNCaP/AR cells. Expression of the AR target genes prostate-specific antigen (PSA) and transmembrane protease serine 2 (TMPRSS2) was induced by bicalutamide but not by enzalutamide indicating that the latter does not have agonistic activity in a castration-resistant setting.13
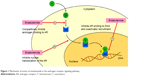  | Figure 1 Mechanism of action of enzalutamide in the androgen receptor signaling pathway. |
In the absence of androgens, AR is predominantly located in the cytoplasm. Following stimulation by androgens, AR translocates to the nucleus where it binds to DNA and promotes the expression of androgen-dependent genes. Using confocal microscopy in live LNCaP cells, investigators showed that the ratio of nuclear vs cytoplasmic AR in enzalutamide-treated cells was about fivefold reduced relative to bicalutamide demonstrating that enzalutamide impairs nuclear translocation of the AR.13 VCaP cell lines that have endogenous AR gene amplification and constitute a CRPC model were then used to assess the effect of enzalutamide in vitro. Enzalutamide suppressed growth and induced apoptosis, whereas bicalutamide did not, suggesting an inhibitory effect of enzalutamide in the CRPC setting.13
In a subsequent study by Guerrero et al the in vivo activity of enzalutamide was tested in a mouse xenograft model of CRPC using castrated male animals implanted with human LNCaP/AR cells overexpressing wild-type AR.14 Mice were administered enzalutamide or bicalutamide, and tumor volume was measured at 2- to 3-day intervals for 28 days. Enzalutamide inhibited tumor growth and significantly induced the regression of tumor volume compared with bicalutamide-treated mice.14 Interestingly, tumors in the bicalutamide-treated mice initially responded, but after day 16, they grew continuously. In contrast, enzalutamide-treated mice maintained tumor regression throughout the 30 days of the study. Immunohistochemical analysis of staining for Ki67 revealed a decrease in cell proliferation in enzalutamide-treated mice compared with control mice. Moreover, enzalutamide treatment induced cell apoptosis, as determined by an increase in activated caspase-3 levels.14 The study also confirmed the capacity of enzalutamide to inhibit AR nuclear translocation and its lack of agonistic activity using LNCaP and LNCaP-derived androgen-independent C4-2 cell lines.
Pharmacokinetics
The pharmacokinetics of enzalutamide and its major active metabolite (N-desmethyl-enzalutamide) was evaluated in patients with metastatic CRPC and healthy male volunteers.15,16 Enzalutamide plasma pharmacokinetics showed a linear two-compartment model with first-order absorption at the studied dosages ranging between 30 mg and 600 mg given orally. After administration of one dose of enzalutamide, the drug was absorbed rapidly, and time to maximum concentration was between 30 minutes and 4 hours. The half-life was about 1 week (range 3–13 days in individual patients), and was not affected by dose.16 Enzalutamide plasma concentrations reached steady state after 1 month of daily treatment with low daily fluctuations.15,16 Regarding protein binding, enzalutamide is 97%–98% bound to plasma proteins, primarily albumin, whereas N-desmethyl-enzalutamide is 95% bound to plasma proteins.15
Following a single oral administration of 14C-enzalutamide, plasma samples were analyzed for enzalutamide and its metabolites up to 77 days post-dose. Metabolism of enzalutamide was found to be carried out by the CYP mixed-function oxidase system, especially by in vitro human CYP2C8 and CYP3A4. CYP2C8 is also responsible for the formation of the active metabolite, N-desmethyl-enzalutamide. Enzalutamide is primarily eliminated by hepatic metabolism and is excreted in urine (71%) and feces (14%) mainly as inactive metabolites.15
Plasma pharmacokinetics of enzalutamide was examined in 59 healthy male volunteers and 926 patients with metastatic CRPC, with normal renal function (creatinine clearance [CrCL] ≥90 mL/min, n=512) and mild (CrCL 60–90 mL/min, n=332) and moderate (CrCL 30 mL/min to <60 mL/min, n=88) renal impairment. The apparent clearance of enzalutamide was similar in patients with preexisting mild and moderate renal impairment (CrCL 30–90 mL/min) compared to patients and volunteers with normal renal function. Similarly, plasma pharmacokinetics was analyzed in volunteers with normal hepatic function (n=16) and with preexisting mild (n=8, Child–Pugh Class A) or moderate (n=8, Child–Pugh Class B) hepatic impairment. The apparent composite area under the curve of enzalutamide was similar in patients with preexisting mild-to-moderate baseline hepatic impairment, compared to volunteers with normal hepatic function.15 Consequently, no initial enzalutamide dose adjustments or modifications are needed for patients with mild-to-moderate renal or hepatic impairment. Population pharmacokinetic analyses also showed that weight (range 46–163 kg) and age (range 41–92 years) do not have a clinically meaningful influence on the exposure to enzalutamide.15
Safety and efficacy studies
Based on the promising results seen in the preclinical studies mentioned before, the drug development of enzalutamide was pursued in several subsequent clinical trials in humans, which are summarized in Table 1.
Phase I/II clinical trial
Scher et al conducted the first Phase I/II trial to assess pharmacokinetics, safety, and tolerability of enzalutamide, and to define a maximum tolerated dose.16 Eligible patients had histologically proven prostate cancer, and progressive castration-resistant disease, which was defined by rising PSA despite castrate levels of testosterone (<1.7 nmol/L), with or without detectable metastases. One hundred and forty patients were recruited at multiple American centers and treated with escalating doses of enzalutamide in a traditional 3+3 design Phase I trial. The doses studied were 30 mg/day, 60 mg/day, 150 mg/day, 240 mg/day, 360 mg/day, 480 mg/day, and 600 mg/day. Most patients had metastatic disease (95%) among whom 78% had bone metastases, 54% had lymph node disease, and 9% had visceral metastases. Around 44% of patients had previously not received radical treatment to the primary tumor, whereas 30% had previously been treated with prostatectomy, and 26% had previously undergone radical radiotherapy to the prostate. Around 77% of patients had received at least two lines of previous hormone therapy, and 54% have previously received chemotherapy.
Antitumor activity was seen across all tested doses of enzalutamide in both patients with and without previous chemotherapy. Median time to radiological progression was 47 weeks in all patients combined, not reached for patients without previous chemotherapy, and 29 weeks for patients who had received chemotherapy (without vs with previous chemotherapy, P=0.01).16 Among patients with visceral metastases (n=59), 22% experienced a partial response, and 49% had stable disease. Of the patients with bony disease, 56% had stable disease on bone scan lasting 12 weeks or more. Circulating tumor cell counts were available for 128 out of 140 patients. Ninety-one percent of patients (70/77) with favorable pretreatment counts (<5 cells/7.5 mL of blood) remained favorable posttreatment, while 49% of patients (25/51) with unfavorable pretreatment counts (≥5 cells/7.5 mL of blood) converted to favorable posttreatment, suggesting a positive effect in this poor prognostic group.
PSA decreases were observed at all studied doses of enzalutamide. PSA response rate ≥50% was 55.7% in the whole group. The proportion of patients with a maximum PSA response ≥50% was similar between patients previously treated and not treated with chemotherapy (51% vs 62%, P=0.23). Rates of PSA decline were also similar regardless of the number of prior hormone therapies. Median time to PSA progression was 32 weeks for all patients, and it was greater in the chemotherapy-naïve group (41 weeks) than in the chemotherapy-exposed group (21 weeks, P not shown). Since PSA expression is dependent on AR, changes in PSA concentrations in serum following enzalutamide treatment were used as a pharmacodynamic marker of AR inhibition. The extent of PSA decrease and proportion of patients showing a fall in PSA were dose dependent up to 150 mg/day, with no obvious additional benefit with increased doses.16 Given the frequency of treatment discontinuations needed at higher doses, the maximum tolerated dose was identified at 240 mg/day. Consequently, a dose of 160 mg daily of enzalutamide (four tablets of 40 mg) was chosen for subsequent clinical trials. The results of this Phase I/II trial validated in man the preclinical studies indicating maintained AR signaling as the driver in CRPC and allowed the continuation of enzalutamide drug development.
Phase III clinical trials
Phase III trial: the AFFIRM study
Following the promising results of enzalutamide in the Phase I/II trial, a Phase III trial was designed to study the role of enzalutamide in metastatic CRPC patients progressing after docetaxel. The AFFIRM study was an international, Phase III, randomized, double-blind, placebo-controlled study of enzalutamide in patients with prostate cancer who had previously been treated with one or two chemotherapy regimens, at least one of which contained docetaxel.5 Patients were eligible for enrollment if they had a histologically confirmed diagnosis of prostate cancer, castrate levels of testosterone, previous treatment with docetaxel, and adequate organ function, and were of Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2. Patients with visceral metastases, excluding central nervous system involvement, were allowed. Patients were randomly assigned in a 2:1 ratio to receive enzalutamide (160 mg orally once daily) or placebo. They were stratified according to the baseline ECOG PS and pain score at baseline. Use of glucocorticoids was permitted but not required. The primary endpoint of the study was OS. Secondary endpoints included PSA response, soft-tissue response, quality of life, time to PSA progression, radiographic progression-free survival (rPFS), and time to the first skeletal-related event (SRE).
The study enrolled 1,199 patients who were randomly assigned 2:1 to receive either enzalutamide (800 patients) or placebo (399 patients). Patient characteristics were well balanced between the two arms. One-third of the patients had undergone prior radical prostatectomy, and 39% had received prior radical radiation therapy. Most patients had bone metastases (91.6%). Around 70% of the patients had soft-tissue metastases among whom 23% had visceral metastases in the lung or liver. Most patients were of ECOG PS 0–1 (91.5%) and no pain or mild pain on baseline pain score (71.5%). Around 27% of the patients had received at least two prior lines of chemotherapy, and 50% of the patients had received at least three previous lines of hormone therapy.5
At the time of the prespecified interim analysis, the use of enzalutamide significantly improved median OS compared to placebo (18.4 months, 95% confidence interval [CI]: 17.3–not reached vs 13.6 months) resulting in a 37% reduction in the risk of death (hazard ratio [HR] 0.63, P<0.001).5 In view of these results, an independent data- and safety-monitoring committee recommended the study to be halted and unblinded, and patients on placebo were allowed to cross over to receive enzalutamide. At the interim analysis, median time on treatment was 8.3 months in the enzalutamide group and 3.0 months in the placebo group. The OS benefit with enzalutamide was seen in all subgroups, included in poor-risk categories such as an ECOG PS 2, the presence of moderate or severe pain, visceral metastases, and the presence of >20 bone lesions. On multivariate analysis, enzalutamide treatment, ECOG PS 0–1, PSA progression only at study entry, no pain or mild pain, no visceral metastases, normal hemoglobin, and alkaline phosphatase were independently associated with significant prolonged OS (P<0.05).5
Enzalutamide was also superior to placebo for all secondary endpoints, including PSA response rate ≥50% (54% vs 2%, P<0.001), soft-tissue objective response rate (29% vs 4%, P<0.001), and rPFS (8.3 months vs 2.9 months, HR 0.40, P<0.001). Enzalutamide also significantly prolonged the time to first SRE as compared to placebo (16.7 months vs 13.3 months, HR 0.69, P<0.001) where SRE was defined as need for radiotherapy or surgery to bone, pathologic bone fracture, spinal cord compression, or change in anticancer therapy to treat bone pain.5 The beneficial effect of enzalutamide in terms of SRE was independent of the use of bisphosphonates at baseline.17
An unplanned post hoc analysis was conducted to study the impact of baseline use of corticosteroids (around 30% in both arms) on the efficacy of enzalutamide.18 Interestingly, median OS was significantly shorter in patients taking corticosteroids at baseline compared to those who were not (11 months vs not reached, HR 0.54, P<0.05). Moreover, use of steroids at baseline was also significantly associated with reduced OS in the multivariate model after adjusting for stratification and known prognostic factors (coefficient: −0.62±0.091, HR 0.54, P<0.001).18 In view of these results, the impact of concomitant steroids combining baseline use of steroids and steroids added while on study was also evaluated.19 Around 46% of the patients received concomitant steroids in both arms. Regardless of treatment arm, use of concomitant steroids was associated with inferior median OS compared to the non-corticosteroid group (11.5 months vs not reached, HR 0.40, P<0.001). Similarly, patients receiving corticosteroids also had a significantly worse rPFS and time to PSA progression (P<0.001). Nevertheless, enzalutamide consistently improved OS and rPFS independently of use of corticosteroids.19 One potential explanation of this deleterious effect is that patients who received steroids were generally sicker (33.6% and 24% of patients with moderate or severe bone pain, respectively) and had more advanced disease (higher median baseline PSA; 14.5% and 7.1% of visceral metastases and 46.8% and 29.8% of >20 bone lesions, respectively) than patients who did not receive steroids. The authors also suggest that steroids may also stimulate prostate cancer growth by activation of promiscuous ARs among other potential molecular mechanisms.19
Another post hoc analysis was carried out to assess the efficacy of enzalutamide in elderly patients (≥75 years).20 Median OS was significantly prolonged with enzalutamide compared to placebo both in patients <75 years (not reached vs 13.6 months, HR 0.63, P<0.001) and in patients ≥75 years (18.2 months vs 13.3 months, HR 0.61, P=0.004). rPFS, time to PSA progression, and PSA response rate were also significantly improved with enzalutamide over placebo in both subgroups, confirming the efficacy of enzalutamide also in the elderly population.20 Based on the outstanding results seen with enzalutamide in terms of efficacy, toxicity profile (“Safety and Tolerability”), and quality of life (“Patient-reported quality of life”) in the AFFIRM study, both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved the use of enzalutamide for men with metastatic CRPC progressing to docetaxel on August 31, 2012 and June 21, 2013, respectively.
Phase III trial: the PREVAIL study
In order to assess the role of enzalutamide in the prechemotherapy setting, another Phase III trial was designed. The PREVAIL study was a multinational, double-blind, randomized, placebo-controlled, Phase III trial of enzalutamide in men with metastatic CRPC who have progressed after ADT but have not undergone chemotherapy.6 Eligible patients had histologically confirmed adenocarcinoma of the prostate with documented metastases, had experienced PSA and/or radiographic progression despite castrate levels of testosterone, were of ECOG PS 0–1, and asymptomatic or mildly symptomatic on baseline pain score. Previous treatment with cytotoxic chemotherapy, ketoconazole, or abiraterone was not allowed. Prior antiandrogen therapy and concurrent corticosteroids were permitted but not required. Patients with visceral metastases, excluding central nervous system involvement, were allowed. Patients were randomly assigned in a 1:1 fashion to receive enzalutamide (160 mg orally once daily) or placebo and were stratified according to the study site. Median OS and rPFS were coprimary endpoints. Secondary endpoints included time to cytotoxic chemotherapy initiation, time to first SRE, soft-tissue response, time to PSA progression, PSA response rate, and quality of life.6
A total of 1,717 patients were enrolled in the study, with 872 being randomized to the enzalutamide arm and 845 to the placebo arm. Baseline demographics were well balanced between treatment arms. Most patients were of ECOG PS 0 (68%) and asymptomatic on baseline pain score (66%). The majority of patients had bone metastases (83.3%), half of the patients had lymph node involvement (50.7%), and 11.8% had visceral metastases in the lung or liver. Most patients had received prior antiandrogen therapy (86.7%), and 20.7% had received two or more antiandrogen treatment lines. Only 4.1% of the patients were taking corticosteroids at baseline.6
Median time on treatment was 16.6 months in the enzalutamide group and 4.6 months in the placebo group. At the preplanned interim analysis, treatment with enzalutamide resulted in a 29% decrease in the risk of death compared to placebo (HR 0.71, P<0.001) with a median OS estimated at 32.4 months in the enzalutamide group and 30.2 months in the placebo group. The benefit seen with enzalutamide on OS was consistent across all prespecified subgroups including in elderly people (≥75 years), patients with visceral metastases, and patients with low hemoglobin levels at study entry. An updated analysis of OS with 116 additional events revealed a median OS not yet reached in the enzalutamide group and of 31.0 months in the placebo group (HR 0.73, P<0.001). The efficacy of enzalutamide over placebo was also shown for the other coprimary endpoint of the study. Enzalutamide resulted in an 81% reduction in the risk of radiographic progression or death as compared with placebo (HR 0.19, P<0.001) with a median rPFS not reached in the enzalutamide arm and of 3.9 months in the placebo arm. In view of these results, the data- and safety-monitoring committee recommended halting the study and allowing patients receiving placebo to cross over to enzalutamide.
The superiority of enzalutamide over placebo was also seen in all secondary endpoints including PSA response rate ≥50% (78% and 3%, respectively, P<0.001), objective response rate in measurable disease (59% and 5%, respectively, P<0.001), and time to first SRE (31.1 months vs 31.3 months, HR 0.72, P<0.001). Enzalutamide significantly prolonged the median time to initiation of cytotoxic chemotherapy (28.0 months vs 10.8 months, HR 0.35, P<0.001).6
A post hoc analysis was conducted to assess the outcomes of patients with baseline visceral metastases.21 For this analysis, the visceral subgroup was further divided into liver and lung subsets. Patients with both the liver and lung metastases were included in the liver subset. Patients with visceral disease had higher baseline median PSA than those without visceral disease (72.5 ng/mL and 46.8 ng/mL, respectively), worse ECOG PS (61.8% and 68.9% had ECOG PS 0, respectively), and higher rates of lymph node disease (57.8% and 49.8%, respectively), but similar rates of bone disease (80.4% and 83.7%, respectively). Treatment with enzalutamide prolonged rPFS in both visceral subsets (HR 0.44, 95% CI: 0.22–0.90 for the liver subset and HR 0.14, 95% CI: 0.06–0.36 for the lung subset) but improved OS only in the lung subset (HR 1.03, 95% CI: 0.57–1.9 for the liver subset and HR 0.59, 95% CI: 0.33–1.1 for the lung subset). Treatment with enzalutamide also resulted in benefits in all secondary endpoints in both visceral subsets including an objective response rate of 29% in the liver subset and 73% in the lung subset. Interestingly, radiological complete responses in measurable disease with enzalutamide were seen in 6% of the patients with liver metastases (n=34) and 11% of the patients with lung metastases (n=37). In both treatment arms, patients with the liver metastases had worse outcomes than those with the lung metastases, confirming the negative prognosis associated with the liver metastases.21
Another prespecified analysis was conducted to assess the efficacy of enzalutamide in elderly patients (≥75 years). Median OS was significantly prolonged with enzalutamide compared to placebo both in patients <75 years (31.5 months vs not reached, HR 0.77, P=0.022) and in patients ≥75 years (32.4 months vs 25.1 months, HR 0.61, P<0.001). rPFS and time to PSA progression were also significantly improved with enzalutamide over placebo in both subgroups, confirming the efficacy of enzalutamide also in the elderly population.22 Given the excellent results with enzalutamide in the PREVAIL study, both the FDA and the EMA expanded the indication for enzalutamide to include the prechemotherapy setting.
Sequential retrospectives studies
Several new drugs have recently emerged as standard therapies in metastatic CRPC, and some of them such as abiraterone and enzalutamide target AR pathway signaling. While cross-resistance between these agents when used in sequence has not been proven prospectively, several retrospective case series have suggested that the antitumor activity of enzalutamide is limited when given after abiraterone or chemotherapy as compared to when given upfront. A selected list of these case series is shown in Table 2.
Eight retrospective studies have analyzed the antitumor activity of enzalutamide following progression on docetaxel and abiraterone accounting for a total number of 380 patients with metastatic CRPC.23–30 All efficacy endpoints were significantly poorer compared to the results of the pivotal Phase III trial of enzalutamide in the post-chemotherapy setting. Median treatment duration with enzalutamide ranged from 2.8 months to 4.9 months as compared to 8.3 months in the AFFIRM trial. PSA response rates ≥50% and ≥90% were seen in 10%–28.6% and 2.5%–4.3% of patients, respectively, compared to 54% and 25%, respectively, in the AFFIRM trial. Moreover, in two studies, around 36% and 55% of patients experienced continuous PSA rising as best PSA response to enzalutamide.25,29 Objective partial responses in measurable disease were described in 2.9%–11.8% of patients (n=123) in contrast to 29% in the AFFIRM trial. Time to event endpoints was also significantly lower in the retrospective studies. Median time to PSA progression, median PFS, and median OS were 2.7–4.0 months, 2.7–4.9 months, and 4.8–8.3 months, respectively, as compared to 8.3 months, 8.3 months, and 18.4 months, respectively, in the AFFIRM trial.5 Interestingly, PSA responses ≥50% with enzalutamide among patients who had been abiraterone-refractory were only seen in 0%–23.1% of patients suggesting primary resistance and cross-resistance between the two agents in the post-chemotherapy setting.23,24,26,28 Conversely, among abiraterone-sensitive patients, around 13.3%–60% also had a PSA response rate ≥50%, suggesting that there is a subset of patients who respond to both agents when given sequentially.23,24,26,28 Similar poor results with enzalutamide were described in a pooled analysis of ten retrospective case series of 536 patients treated with enzalutamide following progression on docetaxel and abiraterone.31 Overall, the pooled PSA response rate ≥50% was 22.9% (95% CI: 19.3–27.1) in the whole group, 26%–34% among abiraterone responders, and 13%–20% among abiraterone-refractory patients. Median PFS was 3.1 months (range 1.4–4.9), and median OS was 8.3 months (range 2.85–10.6).
One retrospective study compared the activity of enzalutamide after abiraterone in 115 docetaxel-pretreated and docetaxel-naïve metastatic CRPC patients.32 Median enzalutamide treatment duration was 4.1 months and did not significantly differ between docetaxel-pretreated and docetaxel-naïve patients (HR 1.09, P=0.7). Confirmed PSA response rates ≥50% did not differ between docetaxel-pretreated and docetaxel-naïve patients (22% vs 25.5%, P=0.8) and were significantly lower than those seen in the AFFIRM (54%) and PREVAIL (78%) trials.5,6,32 Median time to progression and OS on enzalutamide were not significantly different between docetaxel-pretreated and docetaxel-naïve patients (4.6 months vs 6.6 months, HR 0.87, P=0.6 and 10.6 months vs 8.6 months, HR 1.58, P=0.2, respectively). A univariate analysis conducted to identify any predictive factors of response to enzalutamide failed to show any factors significantly correlated with a confirmed PSA response to enzalutamide including prior docetaxel (P=0.7), response to prior abiraterone (P=0.2), and duration of prior abiraterone (P=0.3).32 The authors conclude that prior docetaxel does not have a large impact on the activity of enzalutamide. However, the antitumor activity shown by enzalutamide in this study is also significantly limited compared to the AFFIRM and PREVAIL trials.
One retrospective study analyzed 61 patients who progressed on abiraterone therapy and subsequently received either enzalutamide or docetaxel.33 There were no significant differences between the two groups in terms of PSA response, time to PSA progression, or PFS (P=0.4, P=0.32, and P=0.25, respectively). Importantly, PSA response rate ≥50% in the enzalutamide arm was seen in 41% of patients, as compared to 78% in the PREVAIL study. Similarly, time to PSA progression and PFS were 4.1 months and 4.7 months with enzalutamide as compared to 11.2 months and not reached, respectively, in PREVAIL, suggesting also some cross-resistance between the two agents in the prechemotherapy setting.6,33
One retrospective study examined the potential cross-resistance between docetaxel and enzalutamide.34 One hundred and seven patients treated with enzalutamide were included: 60 were docetaxel pretreated, and 47 were docetaxel naïve. All the outcome measures were significantly poorer in the docetaxel-pretreated group including treatment duration (3.2 months vs 4.9 months, P=0.01), PSA response rate (25.4% vs 43.2%, P=0.06), time to PSA progression (2.6 months vs 7.2 months, P<0.001), PFS (3.3 months vs not reached, P<0.001), and OS (11.6 months vs 16.2 months, P=0.003).34 These results suggest some cross-resistance between docetaxel and enzalutamide and are in keeping with the relatively worse results seen in the AFFIRM trial as compared to the PREVAIL trial.5,6
One recent study evaluated the effect of prior treatment with abiraterone and/or docetaxel in 310 enzalutamide-treated CRPC patients. Among these patients, 12% received neither prior abiraterone nor docetaxel, 25% received prior abiraterone, 10% received prior docetaxel, and 53% received both prior abiraterone and docetaxel. Within these groups, respectively, PSA decline ≥30% was achieved among 67%, 28%, 43%, and 24% of patients, PSA PFS were 5.5 months, 4.0 months, 4.1 months, and 2.8 months, and 12-month OS was 78%, 64%, 77%, and 51%.35 Taken together, these results suggest that enzalutamide has reduced antitumor activity when given after docetaxel and/or abiraterone in both the pre- and post-chemotherapy settings. However, these results could also be partially explained by the fact that patients on third-line therapy have usually a more advanced and aggressive disease than those on second line of treatment and need to be taken as hypothesis generating. Randomized trials are therefore needed to prospectively validate these results and determine the best sequence of treatment.
Ongoing clinical trials
Several clinical trials evaluating the use of enzalutamide alone or in combination in CRPC are currently ongoing. These are summarized in Table 3.
Comparison with bicalutamide
Two randomized Phase II studies are analyzing the efficacy of enzalutamide vs bicalutamide in different settings: metastatic CRPC progressing on ADT and nonmetastatic and metastatic CRPC progressing on ADT (NCT01288911 and NCT01664923, respectively).
Combination studies
The role of combining enzalutamide with abiraterone is currently under evaluation in different studies. The safety of this combination is being studied in a single-arm Phase II study in metastatic CRPC patients with bone metastases (NCT01650194). A randomized Phase III trial is comparing enzalutamide alone or in combination with abiraterone in metastatic chemotherapy-naïve CRPC patients (NCT01949337).
One randomized Phase II study and one Phase III study are evaluating the combination of enzalutamide and radium-223 in men with metastatic CRPC to the bone. The Phase II study is assessing safety and efficacy of radium-223 alone or in combination with abiraterone or enzalutamide in patients of ECOG PS 0–2 (NCT02034552), whereas the Phase III study is comparing enzalutamide alone or in combination with radium-223 in asymptomatic or mildly symptomatic patients (ECOG PS 0–1, NCT02194842). An ongoing single-arm Phase II study is assessing the combination of enzalutamide and vascular endothelial growth factor receptor inhibitor tivozanib in metastatic CRPC patients previously treated with docetaxel (NCT01885949).
Several studies are evaluating the role of combining enzalutamide with immunotherapy. One randomized Phase II trial is comparing the combination of enzalutamide and the PROSTVAC vaccine to enzalutamide alone in the castration-resistant chemotherapy-naïve metastatic setting (NCT01867333). In addition, preliminary results of a Phase II trial of sipuleucel-T with concurrent vs sequential enzalutamide in metastatic CRPC were recently presented at the European Society for Medical Oncology annual meeting in 2014. A prime-boost effect on antigen-presenting cells (APCs) was evident in both arms, as indicated by greater APC activation at infusions 2 and 3 than at infusion 1, suggesting an immune-related effect of the combination treatment. Toxicity profile was generally mild (only G1 and G2 adverse events [AEs] seen), similar between the arms, and consistent with those previously reported with sipuleucel-T and enzalutamide (“Safety and Tolerability”).36 Study results on peripheral immune responses are eagerly awaited.
In terms of Phase I trials, several studies are currently evaluating the safety of enzalutamide combined with different drugs in metastatic CRPC: docetaxel (NCT01565928), everolimus (NCT02125084), glucocorticoid-receptor antagonist mifepristone (NCT02012296), crizotinib (NCT02207504), insulin-like growth factor inhibitor-1 BI836845 (NCT02204072), and phosphoinositide 3-kinases β-selective inhibitor GSK2636771 (NCT02215096).
Maintenance therapy
To ascertain whether enzalutamide treatment can be safely continued despite disease progression, two randomized studies are studying the role of maintenance enzalutamide. A placebo-controlled Phase III trial is analyzing the role of continuing enzalutamide in chemotherapy-naïve metastatic CRPC patients who are starting treatment with docetaxel following disease progression to enzalutamide alone (NCT02288247). Eligible patients will receive open-label enzalutamide, and upon disease progression, they will be randomized to continue enzalutamide or receive placebo in combination with docetaxel. A Phase IV study with the same design is analyzing the role of maintained enzalutamide beyond progression in patients starting abiraterone–prednisolone (NCT01995513). Both studies have a primary endpoint of PFS and are restricted to asymptomatic or minimally symptomatic chemotherapy-naïve patients.
Sequential therapy
In view of the retrospective case series suggesting limited antitumor activity of enzalutamide when given after other AR inhibitors or chemotherapy (“Sequential retrospectives studies”), several clinical trials have been designed to ascertain prospectively whether there is cross-resistance between these new drugs. A single-arm Phase IV is evaluating the efficacy of enzalutamide treatment in patients with metastatic CRPC previously treated with abiraterone–prednisolone (NCT02116582). Eligible patients need to have received at least 6 months of abiraterone and have stopped it because of disease progression.
Another randomized open-label Phase II study is trying to determine the optimal sequencing of abiraterone and enzalutamide in the pre-chemotherapy setting. Eligible patients will be randomized to either abiraterone or enzalutamide, and upon PSA progression, they will be crossed over to the opposite agent (NCT02125357). A similar randomized Phase II study is assessing the best sequence of abiraterone or enzalutamide with respect to cabazitaxel in poor prognosis metastatic CRPC patients. Poor prognostic factors are defined by the presence of visceral metastases, development of castration-resistance within 12 months of ADT, high lactate dehydrogenase, ECOG PS 2, low albumin, and/or increased alkaline phosphatase. Patients will be randomized to receive cabazitaxel vs abiraterone or enzalutamide and will be allowed to cross over to the opposite arm on disease progression (NCT02254785).
Nonmetastatic setting with biochemical recurrence only
Two studies are evaluating the role of enzalutamide in nonmetastatic prostate cancer patients with PSA recurrence only following radical treatment for localized disease. A Phase III study is comparing the efficacy of enzalutamide vs placebo in patients with nonmetastatic CRPC and PSA progression only despite ADT with a PSA doubling time of ≤10 months (NCT02003924). The primary endpoint of this study is metastasis-free survival.
Safety and tolerability
Enzalutamide is generally well tolerated and has a favorable toxicity profile. The rates of serious AEs were similar between enzalutamide and placebo in both the AFFIRM and PREVAIL Phase III trials (34% vs 39% and 32% vs 27%, respectively).5,6 Table 4 summarizes the most common AEs with enzalutamide in Phase III trials. Most enzalutamide-related AEs are usually mild, and grade ≥3 AEs are uncommon (28% for all AEs and between 1% and 6% for each individual AE).5,6 The most common AE is fatigue (34%–36%), but it rarely reaches a grade ≥3 level (2%–6%). Other frequent AEs are osteoarticular pain (14%–27%), constipation (22%), diarrhea (16%–21%), hot flushes (18%–20%), and hypertension (6.6%–13%). Cardiac disorders were noted in 6%–10% of patients with disorders of grade ≥3 only in 1%–3%.5,6 No relevant hematological, biochemical, or electrolyte AEs were reported in any of the two Phase III trials. AEs leading to treatment discontinuation or death were rare (6%–8% and 3%–4%, respectively).
  | Table 4 Most common adverse events with enzalutamide |
In the post hoc analysis of the impact of corticosteroids in the AFFIRM trial, the use of concomitant steroids was associated with higher rates of grade 3 and 4 AEs relative to the non-corticosteroid group (63.3% and 34.4%, respectively).19 Importantly, in the post hoc analysis of patients with baseline visceral metastases treated in the PREVAIL trial, AE rates in the visceral subgroup were similar to the full study population.21 In the Phase I/II trial, the most common AEs were fatigue (27.1%), nausea (8.6%), dyspnea (7.9%), anorexia (5.7%), and back pain (5.7%).16
Seizure was reported in five patients in the enzalutamide group of the AFFIRM trial (0.6%) and in one patient in each treatment arm in the PREVAIL trial (0.1% of the enzalutamide patients).5,6 In both Phase III trials, patients with a history of seizure or a condition that could confer a predisposition to seizure were excluded. However, the majority of patients who had a seizure had predisposing factors which could have lowered the threshold for seizure. Of the five patients who experienced seizures in the AFFIRM trial, two patients had brain metastases, one patient had inadvertently been administered lidocaine intravenously immediately before the seizure, and one patient with brain atrophy had an unwitnessed event classified as a seizure, in the context of a history of heavy alcohol use, after initiation of haloperidol 7 days beforehand.5 In the PREVAIL trial, both patients had a preexisting history of seizure that was unknown to the investigators at the time of enrollment. Similarly, in the Phase I/II trial of enzalutamide, two witnessed seizures occurred in patients receiving doses of 600 mg/day and 360 mg/day, and one possible seizure at 480 mg/day. Both patients who had witnessed seizures were concurrently taking drugs such as olanzapine, prochlorperazine, or methylphenidate which could contribute to a lowered seizure threshold.16 Inhibition of the γ-aminobutyric acid-gated chloride channel has been hypothesized as a potential mechanism by which enzalutamide could lower the threshold for seizures.5 Consequently, enzalutamide should not be administered to patients with central nervous system metastases, history of seizure, or who have other predisposing factors (underlying brain injury, stroke, alcoholism, concomitant medication that may lower the seizure threshold).
Patient-reported quality of life
Both the AFFIRM and the PREVAIL trials included quality of life as a secondary endpoint using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire. The FACT-P includes the score of 39 items which are ranged from 0 to 4, with higher scores indicating a better quality of life. A quality-of-life response is defined as a 10-point improvement in the global score on the FACT-P. In the AFFIRM trial, a quality-of-life response was seen more frequently with enzalutamide than placebo (43% vs 18%, P<0.001).5 In the PREVAIL trial, patients on the enzalutamide arm had a delayed time to decline in the quality of life (11.3 months vs 5.6 months, HR 0.63, P<0.001) defined as decrease of 10 points or more on the global score of FACT-P.6
Enzalutamide also showed beneficial effects on other health-related quality-of-life factors, as reported in an updated analysis of the AFFIRM trial.17 Patients in the enzalutamide group had a significantly longer median time to quality-of-life deterioration (9.0 months vs 3.7 months, HR 0.45, P<0.0001). Pain was assessed using the mean Brief Pain Inventory-Short Form (BPI-SF), a validated questionnaire for the assessment of pain severity and the degree of interference with daily activities. Enzalutamide significantly improved both pain severity and pain interference scores from baseline to week 13, as compared with placebo (P<0.001).17 Enzalutamide also resulted in a significant improvement in the median time to pain progression (not reached vs 13.8 months, HR 0.56, P=0.0004). Moreover, in post hoc analyses, the reduction in the risk of pain progression with enzalutamide was maintained regardless of the use of bisphosphonate or corticosteroid at baseline.17 In another updated analysis of the AFFIRM trial, longitudinal changes in FACT-P scores were assessed during the first 25 weeks of treatment. After 25 weeks, mean FACT-P total score decreased by 1.52 points with enzalutamide compared to 13.73 points with placebo (P<0.001).37
Similar results of other health-related quality-of-life factors were seen in an updated analysis of the PREVAIL trial.38 Patients in the enzalutamide arm experienced significantly smaller magnitudes of increase in pain interference and pain severity than patients in the placebo arm using the BPI-SF score at week 25 (P<0.001 and P=0.002, respectively). A significantly lower proportion of patients reported pain progression for both severity and interference in the enzalutamide arm than in the placebo arm at week 25 (P<0.001).38
Conclusion – place in therapy
In the last 10 years, six novel therapies have shown to improve survival in metastatic CRPC, and these include docetaxel,7,8 cabazitaxel,9 abiraterone acetate,3,4 sipuleucel-T,11 enzalutamide,5,6 and radium-223.10 Since the approval of docetaxel in 2004, three registration therapeutic spaces have appeared for drug development: pre-docetaxel, combination with docetaxel, and post-docetaxel settings. Like abiraterone acetate, enzalutamide significantly prolongs survival in men with metastatic CRPC in both the chemotherapy-naïve and chemotherapy-pretreated settings. No direct head-to-head comparison between these agents or trials comparing their sequential use has been conducted. Moreover, there are no available predictive biomarkers to select the best sequence or to guide patient selection for each individual therapy. Consequently, the selection of therapy is currently mainly based on drug availability, comorbidities, and patient/clinician preference. One advantage of enzalutamide and abiraterone over the other agents is that they are administered orally and have a more favorable toxicity profile. Because enzalutamide does not require the coadministration of steroids, enzalutamide is preferred over abiraterone for those patients where steroids are not recommended. Conversely, abiraterone might be chosen over enzalutamide for patients with history of seizures or who are receiving concomitant medications which lower the seizure threshold. However, evidence-based data to support the use of one drug over the other are currently lacking.
Concerns remain regarding the appearance of de novo and acquired resistance to enzalutamide. Several mechanisms of resistance to enzalutamide have been reported, and these include AR gene amplification, AR point mutation and truncation, intracrine synthesis of androgens by the tumor cells, overexpression of glucocorticoid receptor, and changes in AR cofactor balance.39 Moreover, several of these mechanisms might be shared with other AR-targeted agents such as abiraterone and ARN-509 and could potentially explain the cross-resistance seen between them. One important mechanism would be the appearance of an AR splice variant which lacks the ligand-binding domain targeted by enzalutamide and abiraterone, and remains constitutively active as a transcription factor.40 Another potential mechanism recently described is the emergence of a novel missense mutation F876L in the ligand-binding domain of the AR conferring resistance to enzalutamide and ARN-509 by converting it from an AR antagonist to an AR agonist.41 This mechanism could potentially lead to the enzalutamide withdrawal syndrome described both in the preclinical and clinical setting.42,43
The approval of enzalutamide in both the pre- and post-docetaxel setting represents a major advance in the treatment of prostate cancer. However, the increasing numbers of available treatment options for the same setting of disease hasten the need for predictive biomarkers to inform treatment selection and sequence. Gaining robust evidence to guide the sequencing of these agents is of great importance as it will maximize patient benefit.
Disclosure
The authors report no conflicts of interest in this work.
References
Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46(17):3040–3052. | ||
Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339(15):1036–1042. | ||
de Bono JS, Logothetis CJ, Molina A, et al; OU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. | ||
Ryan CJ, Smith MR, de Bono JS, et al; COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. | ||
Scher HI, Fizazi K, Saad F, et al; AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. | ||
Beer TM, Armstrong AJ, Rathkopf DE, et al; PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. | ||
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. | ||
Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. | ||
de Bono JS, Oudard S, Ozguroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. | ||
Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. | ||
Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. | ||
Jung ME, Ouk S, Yoo D, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J Med Chem. 2010;53(7):2779–2796. | ||
Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. | ||
Guerrero J, Alfaro IE, Gómez F, Protter AA, Bernales S. Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate. 2013;73(12):1291–1305. | ||
Xtandi. Highlights of Prescribing Information. Northbrook, IL: Astellas Pharma US, Inc; 2015. Available from: http://www.astellas.us/docs/12A005-ENZ-WPI.PDF. Accessed Jan 30, 2015. | ||
Scher HI, Beer TM, Higano CS, et al; Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375(9724):1437–1446. | ||
Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15(10):1147–1156. | ||
Scher H, Fizazi K, Saad F, et al. Association of baseline corticosteroid with outcomes in a multivariate analysis of the phase 3 AFFIRM study of enzalutamide (ENZA), an androgen receptor signaling inhibitor (ARSI). Ann Oncol. 2012;23(suppl 9):Abstr 899D. | ||
Scher HI, Fizazi K, Saad F, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide (ENZA), an androgen receptor inhibitor. J Clin Oncol. 2013;31(suppl 6):Abstr 6. | ||
Sternberg CN, de Bono JS, Chi KN, et al. Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol. 2014;25(2):429–434. | ||
Higano C, Alumkal J, Chowdhury S, et al. Response rates and outcomes with enzalutamide for patients with metastatic castration-resistant prostate cancer and visceral disease in the PREVAIL trial. Ann Oncol. 2014;25(suppl 4):Abstr 767. | ||
Graff JN, Baciarello G, Armstrong AJ, et al. Clinical outcomes and safety in men ≥75 and <75 years with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide in the phase 3 PREVAIL trial. J Clin Oncol. 2015;33(suppl 7):Abstr 200. | ||
Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65(1):30–36. | ||
Bianchini D, Lorente D, Rodriguez-Vida A, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50(1):78–84. | ||
Schmid SC, Geith A, Böker A, et al. Enzalutamide after docetaxel and abiraterone therapy in metastatic castration-resistant prostate cancer. Adv Ther. 2014;31(2):234–241. | ||
Thomson D, Charnley N, Parikh O. Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate-resistant prostate cancer. Eur J Cancer. 2014;50(5):1040–1041. | ||
Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120(7):968–975. | ||
Thomsen FB, Røder MA, Rathenborg P, Brasso K, Borre M, Iversen P. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand J Urol. 2014;48(3):268–275. | ||
Brasso K, Thomsen FB, Schrader AJ, et al. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur Urol. Epub 2014 Aug 6. | ||
Vera-Badillo FE, Leibowitz-Amit R, Templeton A, et al. Clinical activity of enzalutamide against metastatic castration-resistant prostate cancer (mCRPC) in patients who have progressed on abiraterone acetate: the Princess Margaret experience. J Clin Oncol. 2014;32(suppl 4):Abstr 159. | ||
Petrelli F, Coinu A, Borgonovo K, et al. Enzalutamide after docetaxel and abiraterone acetate treatment in prostate cancer: a pooled analysis of 10 case series. Clin Genitourin Cancer. 2015;13(3):193–198. | ||
Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67(1):23–29. | ||
Suzman DL, Luber B, Schweizer MT, Nadal R, Antonarakis ES. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate. 2014;74(13):1278–1285. | ||
Nadal R, Zhang Z, Rahman H, et al. Clinical activity of enzalutamide in docetaxel-naïve and docetaxel-pretreated patients with metastatic castration-resistant prostate cancer. Prostate. 2014;74(15):1560–1568. | ||
Cheng HH, Gulati R, Azad A, et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015;18(2):122–127. | ||
Small EJ, Raymond L, Gardner TA, et al. STRIDE, a randomized, phase 2, open-label study of sipuleucel-T with concurrent vs sequential enzalutamide in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2014;25(suppl 4):Abstr 774. | ||
Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol. 2015;26(1):179–185. | ||
Loriot Y, Miller K, Sternberg CN, et al. Impact of enzalutamide on skeletal-related events (SREs), pain and quality of life (QoL) in the PREVAIL trial. Ann Oncol. 2014;25(suppl 4):Abstr 762D. | ||
Claessens F, Helsen C, Prekovic S, et al. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat Rev Urol. 2014;11(12):712–716. | ||
Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. | ||
Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–1029. | ||
Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013;3(9):1030–1043. | ||
Rodriguez-Vida A, Bianchini D, Van Hemelrijck M, et al. Is there an antiandrogen withdrawal syndrome with enzalutamide? BJU Int. 2015;115(3):373–380. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



