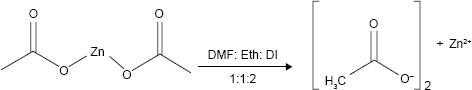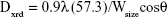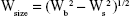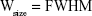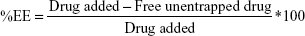Back to Journals » Drug Design, Development and Therapy » Volume 12
Efficient drug delivery of β-estradiol encapsulated in Zn-metal–organic framework nanostructures by microwave-assisted coprecipitation method
Authors Ranjbar M , Pardakhty A , Amanatfard A, Asadipour A
Received 7 May 2018
Accepted for publication 4 July 2018
Published 28 August 2018 Volume 2018:12 Pages 2635—2643
DOI https://doi.org/10.2147/DDDT.S173324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Mehdi Ranjbar,1,2 Abbas Pardakhty,1,3 Arezou Amanatfard,1 Ali Asadipour1,3
1Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran; 2Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran; 3Faculty of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran
Abstract: Metal–organic frameworks (MOFs) are structures made up of inorganic nodes, which can be either single ions or clusters of ions and organic linkers. This study reports on a novel processing route for producing β-estradiol encapsulated in Zn-MOF nanocomposites by microwave-assisted coprecipitation as a facile and fast method. Zn-MOF nanocomposites were synthesized with the aid of Zn(OAc)2·2H2O and 2,6-pyridine dicarboxylic acid ammonium as an organic ligand. Furthermore, we studied encapsulated β-estradiol which is one of the most important classes of estrogenic compounds that are used in the treatment of prostate cancer and breast cancer. The effects of β-estradiol concentration and microwave irradiation on the morphology, particle size, distribution, and in vitro photoluminescence spectroscopy experiments of β-estradiol entrapped in Zn-MOF nanocomposites were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, ultraviolet–visible spectroscopy, Fourier transform infrared spectroscopy, and Brunauer–Emmett–Teller spectroscopy. These nanostructures can be a good option for thawing hydrophilic and hydrophobic drugs over time. Zn-MOF nanocomposites with high porosity, total pore volume (0.04665 cm3g-1), and nanostructures have provided the platform to load β-estradiol such as low soluble drugs. Maximum of drug release was about 82% at pH 8.9 after 8 h.
Keywords: nano structures, drug delivery, encapsulation, drug design, β-estradiol
Introduction
Nano controlled release drug systems and solid-phase sorbents as target material are attracting a lot of attention currently and have created a new perspective through their ability to target delivery to a large surface area; this strategy could provide an important contribution for human health.1,2 Solid-phase sorbents as molecularly imprinted polymers have individual physical/chemical adsorption and high specific surface area for the specific applications.3
In the past two decades, metal–organic frameworks (MOFs), as nanoporous particulate materials, have shown well-known properties such as desirable pore size, pore shape, large surface areas, and the ability to encapsulate compound and drugs.4 Magnetic MOFs, a special type of MOFs, have a wide range of applications such as adsorbents and separation.5 Many metals such as zirconium-based magnetic MOFs6 and Fe3O4@SiO2@UiO-66 core–shell magnetic microspheres can be used as sorbents in solid-phase extraction.7 Another study shows that magnetic MOFs have been used as magnetic solid-phase extraction adsorbents in preconcentration of pyrazole/pyrrole pesticides in environmental water samples8 and also in chemical sensing9 and drug delivery.10 MOFs are a new class of hybrid crystalline material networks that have regular cavities in solid phase with many applications.11 Furthermore, MOFs are constructed via covalent linkages between metal ions/metal clusters and organic ligands generally called a linker with high porosity and thermal stability materials.12–14 MOF structures that are the novel formulations of drug delivery systems can provide important properties for low soluble drugs.15 MOF composites can be prepared by using various synthetic methods such as microwave,16 hydrothermal (solvothermal),17 electrochemical,18 mechanochemical,19 assisted heating, and ultrasound.20 Microwave technique has increased the reaction rates and functions as a rapid volumetric heating, providing an improved engineering control over the separation of nucleation and growth stages of nanomaterials.21,22 Steroid hormones are a fat-loving group with a low molecular weight and are biological compounds that act as hormones as well.23–25 The steroids are altered by the functional groups attached to the ring structure, which alter the oxidation state of the rings.26 One of the identified problems is that the hormones are released in the target organs.27 This research attempted to study whether the MOF structures can be used more efficiently in medications and dietary supplements at low doses when β-estradiol hormone is encapsulated in the MOF structures. In this study, we report the synthesis and preparation of ZnO-MOF from organic ligand (2,6-pyridine dicarboxylic acid ammonium) and encapsulated β-estradiol by using the coprecipitation method in aqueous solution. MOFs are one of the most important medications that are used to deliver lipophilic drugs by improving drug absorption. In the last decade, different strategies have been used in improving the encapsulation efficiency and release time of drugs. In this study, MOFs were considered because of their ability to encapsulate and absorb. On the other hand, MOFs are able to control the release of low soluble drugs.
Methods
Materials and characterization
All the chemical reagents and raw materials used in our experiments were of analytical grade and were used as received without further purification. Zn(OAc)2·2H2O, glucose (C6H12O6), dimethylformamide (DMF), ammonia, and ethanol were purchased from EMD Millipore (Billerica, MA, USA). β-Estradiol was obtained from Alfa Aesar chemical company (Haverhill, MA, USA). We used many technical instruments for the characterization of products such as X-ray diffraction (XRD) patterns that were recorded by a Philips-X’Pert Pro (PANalytical, Westborough, MA, USA), X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10<2θ<80 in Tehran University, Iran. Scanning electron microscopy (SEM) images were obtained using LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. Transmission electron microscope (TEM) images were obtained using Philips EM208S with an accelerating voltage of 100 kV. The Brunauer–Emmett–Teller (BET) analysis for the surface area and pore size analysis were carried out using a BET Sorptometer (Porous Materials, Inc., Ithaca, NY, USA). Fourier transform infrared (FTIR) spectra were recorded on Shimadzu Varian 4300 spectrophotometer (KK company, Kyoto, Japan) in KBr pellets. UV–visible (UV–Vis) diffuse reflectance spectroscopy analysis was carried out using Shimadzu UV-2600 UV–Vis spectrophotometer (KK Company) with an integrating sphere attachment and BaSO4 was used as a reference.
Synthesis of Zn nanostructures
In this work, the stoichiometric amount of Zn(OAc)2·2H2O powder which is the starting reagent was dissolved in 15 mL of mixed solvent (DMF: ethanol: demineralization water =1:1:2), and the solution was stirred vigorously and heated up to 50°C for 120 min. The synthesized precipitate was obtained, centrifuged, and washed several times with DMF and finally dried at room temperature for 3 days.
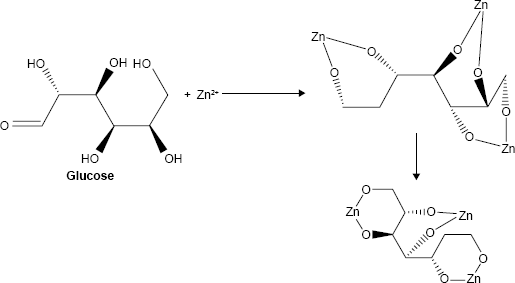
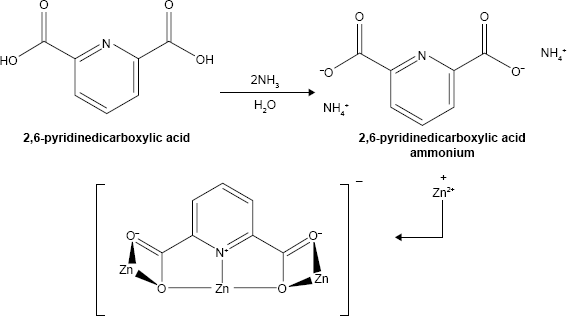
Preparation of mixed-linker frameworks
For the synthesis of mixed-linker MOFs (MIXMOFs), at first 2,6-pyridinedicarboxylic acid and C6H12O6 as linkage precursors in the molar ratio 1:2 were dissolved in the molar ratio 2:1:1 (DMF: ethanol: demineralization water) under vigorous stirring at room temperature. 2,6-Pyridinedicarboxylic acid was synthesized according to a previous study.2 In the next step, in another beaker, the two solutions were mixed together in a reflux system at 50°C for 2 h under weak stirring, while 5 mL of 1 M NaOH solution was added dropwise to the reflux system. The proposed structure of the Zn-MOF nanocomposites by mixed-linker frameworks is shown in Equations 1–3:
Preparation of β-estradiol encapsulated in MOF nanostructures
After performing the reaction in the reflux system, 0.01 g (0.036 mmol) β-estradiol was added to the mixture. The final solution was loaded into a microwave teflon and exposed to microwave irradiation in a microwave box for 15 minutes at 600 W. In order to prevent the deposits of nanostructures at periodic and regular intervals, the microwave irradiation was ON for 30 s and OFF for 60 s. In the final step, after the heating process, the system was allowed to cool to room temperature naturally, and the obtained precipitate was collected and dried in vacuum at 50°C for 48 h. The as-synthesized powders were characterized by XRD, SEM, TEM, BET, and FTIR. Scheme 1 shows the summary of the synthesis of Zn-MOFs nanocomposites containing β-estradiol.
  | Scheme 1 Summary of the synthesis of Zn-MOF nanocomposites containing β-estradiol. |
|
|
|
|
|
|
Results and discussion
Drug handling and delivery are the most important uses of nanotechnology in pharmaceutical science.28 β-Estradiol, a main naturally occurring estrogenic compound, can be used in the treatment of cancer and primary ovarian failure. Molecular formula of β-estradiol is C18H24O2, which is shown in Figure 1, is an estrane steroid,29 and has a molecular weight of 272.38 g/mol. In this study, Zn(OAc)2·2H2O was applied as a metal component in the formation of Zn-MOF structures. X-ray powder diffraction (XRD), an accurate analytical technique, was primarily used to investigate the phase of the crystalline materials and structures produced. The XRD pattern of Zn-MOF nanocomposites and Zn-MOF nanocomposites containing β-estradiol is shown in Figure 2A and B, respectively. Based on the XRD data, the crystallite diameter (Dc) of Zn-MOF nanocomposites was calculated to be 90–120 nm from the full width of the half maximum (FWHM) by using the Debay–Scherer equation (Equations 4–6).30
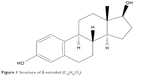  | Figure 1 Structure of β-estradiol (C18H24O2). |
  | Figure 2 XRD patterns of Zn-MOF nanocomposites (A) and Zn-MOF nanocomposites containing β-estradiol (B). |
|
|
where
|
|
|
|
where Wsize is the breadth of the observed diffraction line at its half-maximum intensity and K is the shape factor. The XRD patterns show that the structures have lost their crystalline state when β-estradiol was trapped in the MOF, which is probably due to the filling of the pores of the network with β-estradiol.
The high-resolution shape and size of the nanosized materials or nanostructures were investigated by using SEM and TEM images (Figure 3A and B, respectively). Observations show that MOFs have been prepared with a lot of porosity and that there are relatively strong bonds between β-estradiol with double ligands. The particle size obtained with the electron microscopy is in accordance with the data of the X-ray diffraction pattern. The dynamic light scattering measurements of the Zn-MOF nanocomposites and the Zn-MOF nanocomposites containing β-estradiol composite are shown in Figure 4A and B, respectively. As the results show particle size distribution of Zn-MOF nanocomposites containing β-estradiol compared with Zn-MOFs nanocomposites is greater which could be because of loading β-estradiol in structures and an increase in the size of cavities in Zn-MOF nanocomposites.
  | Figure 4 The DLS measurements for the Zn-MOFs nanocomposites (A) and Zn-MOFs nanocomposites containing β-estradiol (B). |
To evaluate the thermal stability of nanostructures, we used thermogravimetric analysis (TGA). In Figure 5, the TGA graph of Zn-MOF nanocomposites containing β-estradiol between 100°C and 400°C show that the thermal behavior of the nanocomposites can be classified into two separated regions. By increasing the temperature, thermal decomposition occurs in temperature range between 110–130°C, which is related to the loss of water, the most significant weight loss happens in this step (mass change: 11.89%). In the second region due to the final thermal degradation of the nanocomposites (mass change: 2.58%), the temperature ranges from 310°C to 350°C. N2 adsorption–desorption behavior of Zn-MOF nanocomposites shows the surface area, pore size, and pore volume of structures. The results indicate that the organize regular structures of mixed-linker frameworks (2,6-pyridinedicarboxylic acid and C6H12O6) in products causes, the volume, and the diameter of the cavities are regular and uniform. Therefore, β-estradiol was encapsulated uniformly in MOFs. The adsorption/desorption isotherm diagram of Zn-MOF nanocomposites is shown in Figure 6. Average pore diameter and total pore volume (p/p0 =0.990) were 37.1 nm and 0.046656 cm3 g−1, respectively. In the study of stability of the particle size, the specified product was kept in a refrigerator at an average temperature of 4°C for a week. The results show that the stability of nanostructures had been maintained over the previous 1 week, which indicates that the nanostructures are well distributed and dispersed in the final products. The graph remains as a normal bell shape and the desired formulation is stable. Figure 7 shows the stability graph of β-estradiol encapsulated in Zn-MOF nanostructures exactly after 1 day and 1 week.
  | Figure 7 Stability graph of the Zn-MOF nanocomposites containing β-estradiol after 1 day and 1 week. |
FTIR spectroscopy is an analytical technique used to characterize and form different links in the composition of organic (and in some cases inorganic) materials. The FTIR spectrum of Zn-MOF nanocomposites and Zn-MOF nanocomposites containing β-estradiol and reference peak related to β-estradiol31,32 are shown in Figure 8. As shown in Figure 8A, the vibration peaks corresponding to the spinel structure are identified at about 593 and 875 cm−1, which confirms the presence of links Zn-O and Zn-N33 and stretching bands in 1,042 and 1,645 cm−1 range in Figure 8B, associated with C−C, C−N, and C=O bands in the β-estradiol encapsulated Zn-MOF nanocomposites. Weak and broad peaks centered at about 2,931 and 3,423 cm−1 wavenumbers are related to C−H and O−H bands, respectively. UV–Vis spectroscopy is used to determine the concentration of the analyte by measuring light absorption across the UV and visible light wavelengths. The MOF structures are highly sensitive to pH environment and show different behaviors in various pH. The increased pH of the reaction environment in MOF structures leads to packing and shrinkage, and hence, β-estradiol gets released faster.
In vitro drug release of β-estradiol encapsulated in Zn-MOF nanostructures was performed by dialysis approach at pH 3.2, 5.8, 7.4, 8.1, and 8.9 after 8 h and the results are shown in Figure 9. The results indicate that the drug release was ~64%, 68%, 75%, 78%, and 82% at pH 3.2, 5.8, 7.4, 8.1, and 8.9 after 8 h, respectively. The results obtained from the interpretation of the patterns in lower levels of acidity show that the release was slower due to the sustainability of β-estradiol at acidic pH. In other words, under acidic conditions, drug release rates are lower due to the erosion of nanostructures. By increasing the pH to 7.4 gradually, the structural destruction occurs in MOF nanostructures at pH 8.9 after 8 h. The nanostructures destroyed completely after 8 h, and drug release was about 82% compared to other pH. The encapsulation efficiency (EE) of β-estradiol encapsulated in Zn-MOF nanostructures is shown in Figure 10. The %EE of β-estradiol was determined by measuring the residual in the aqueous bulk phase after encapsulation of β-estradiol in Zn-MOF nanostructures. The calculation was based on the theoretical drug loading (Equation 7):
|
|
  | Figure 9 In vitro drug release of β-estradiol encapsulated in the Zn-metal–organic framework nanostructures at pH 3.2, 5.8, 7.4, 8.1, and 8.9 after 8 h. |
  | Figure 10 Encapsulation efficiency (%EE) of β-estradiol encapsulated in Zn-metal–organic framework nanostructures. |
The most promising formulation with high encapsulation efficiency (92%) of the β-estradiol encapsulated in Zn-MOF nanostructures introduces these structures as very suitable drug carriers.
Conclusion
MOFs with significant porosity and regular network structures have high potential for drug delivery of low soluble drugs such as β-estradiol. In this study, for first time, MOFs were synthesized by the microwave-assisted coprecipitation with MIXMOFs (2,6-pyridinedicarboxylic acid and C6H12O6) as linkage precursors, and we determined that the encapsulated β-estradiol is one of the most important classes of estrogenic compounds that are used in the treatment of prostate cancer and breast cancer. The Zn-MOF nanocomposites containing β-estradiol were characterized by XRD, SEM, TEM, UV-Vis, IR, and BET. Total pore volume (p/p0 =0.990) of Zn-MOF nanocomposites was estimated 0.046656 [cm3 g−1] that these structures can be used to trap β-estradiol.
Acknowledgment
The authors are grateful to the Council of Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
Disclosure
The authors report no conflicts of interest in this work.
References
Wen Y, Chen L, Li J, Liu D, Chen L. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. Trends Analyt Chem. 2014;59:26–41. | ||
Ranjbar M, Taher MA, Sam A. Mg-MOF-74 nanostructures: facile synthesis and characterization with aid of 2,6-pyridinedicarboxylic acid ammonium. J Mater Sci. 2016;27(2):1449–1456. | ||
Płotka-Wasylka J, Szczepańska N, de La Guardia M, Namieśnik J. Modern trends in solid phase extraction: new sorbent media. Trends Analyt Chem. 2016;77:23–43. | ||
Zheng H, Zhang Y, Liu L, et al. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138(3):962–968. | ||
Ma J, Wu G, Li S, et al. Magnetic solid-phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J Chromatogr A. 2018;1553:57–66. | ||
Wang X, Deng C. Preparation of magnetic graphene @polydopamine @Zr-MOF material for the extraction and analysis of bisphenols in water samples. Talanta. 2015;144:1329–1335. | ||
Zhang W, Yan Z, Gao J, Tong P, Liu W, Zhang L. Metal-organic framework UiO-66 modified magnetite@silica core-shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish samples. J Chromatogr A. 2015;1400:10–18. | ||
Ma J, Yao Z, Hou L, et al. Metal organic frameworks (MOFs) for magnetic solid-phase extraction of pyrazole/pyrrole pesticides in environmental water samples followed by HPLC-DAD determination. Talanta. 2016;161:686–692. | ||
Meng J, Bu J, Deng C, Zhang X. Preparation of polypyrrole-coated magnetic particles for micro solid-phase extraction of phthalates in water by gas chromatography-mass spectrometry analysis. J Chromatogr A. 2011;1218(12):1585–1591. | ||
Kang BK, Lee JS, Chon SK, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274(1–2):65–73. | ||
Huo SH, Yan XP. Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst. 2012;137(15):3445–3451. | ||
Doherty CM, Buso D, Hill AJ, Furukawa S, Kitagawa S, Falcaro P. Using functional nano- and microparticles for the preparation of metal-organic framework composites with novel properties. Acc Chem Res. 2014;47(2):396–405. | ||
Han Z, Shi W, Cheng P. Synthetic strategies for chiral metal-organic frameworks. Chinese Chem Lett. 2018;29(6):819–822. | ||
Liu X-L, Yin Q, Huang G, Liu T-F. Stable pyrazolate-based metal-organic frameworks for drug delivery. Inorg Chem Commun. 2018;94:21–26. | ||
Huxford RC, della Rocca J, Lin W. Metal-organic frameworks as potential drug carriers. Curr Opin Chem Biol. 2010;14(2):262–268. | ||
Chen Y, Ni D, Yang X, Liu C, Yin J, Cai K. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim Acta. 2018;278:114–123. | ||
Al-Janabi N, Martis V, Servi N, Siperstein FR, Fan X. Cyclic adsorption of water vapour on CuBTC MOF: sustaining the hydrothermal stability under non-equilibrium conditions. Chem Eng J. 2018;333:594–602. | ||
Yu H, Han J, An S, Xie G, Chen S. Ce(III, IV)-MOF electrocatalyst as signal-amplifying tag for sensitive electrochemical aptasensing. Biosens Bioelectron. 2018;109:63–69. | ||
Klimakow M, Klobes P, Rademann K, Emmerling F. Characterization of mechanochemically synthesized MOFs. Microporous Mesoporous Mater. 2012;154:113–118. | ||
Nadar SS, Rathod VK. Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzyme Microb Technol. 2018;108:11–20. | ||
Martín Á, Navarrete A. Microwave-assisted process intensification techniques. Curr Opin Green Sustain Chem. 2018;11:70–75. | ||
Guo Q, Sun D-W, Cheng J-H, Han Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci Technol. 2017;67:236–247. | ||
Hu Y, Cai K, Luo Z, et al. Layer-by-layer assembly of β-estradiol loaded mesoporous silica nanoparticles on titanium substrates and its implication for bone homeostasis. Adv Mater. 2010;22(37):4146–4150. | ||
des Azevedo S, Lakshmi D, Chianella I, et al. Molecularly imprinted polymer-hybrid electrochemical sensor for the detection of β-estradiol. Ind Eng Chem Res. 2013;52(39):13917–13923. | ||
Asselin-Labat ML, Vaillant F, Sheridan JM, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798. | ||
Hu C, He M, Chen B, Zhong C, Hu B. Sorptive extraction using polydimethylsiloxane/metal-organic framework coated stir bars coupled with high performance liquid chromatography-fluorescence detection for the determination of polycyclic aromatic hydrocarbons in environmental water samples. J Chromatogr A. 2014;1356:45–53. | ||
Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32(2):183–200. | ||
Lee SH, Heng D, Ng WK, Chan HK, Tan RB. Nano spray drying: a novel method for preparing protein nanoparticles for protein therapy. Int J Pharm. 2011;403(1–2):192–200. | ||
Hong L, Colpan A, Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006;12(10):2747–2753. | ||
Rostamizadeh M, Yaripour F, Hazrati H. High efficient mesoporous HZSM-5 nanocatalyst development through desilication with mixed alkaline solution for methanol to olefin reaction. J Porous Mater. 2018;25(5):1287–1299. | ||
Lahcen AA, Baleg AA, Baker P, Iwuoha E, Amine A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-β-estradiol determination. Sens Actuat B Chem. 2017;241:698–705. | ||
Asada T, Oikawa K, Kawata K, Ishihara S, Iyobe T, Yamada A. Study of removal effect of bisphenol A and β-estradiol by porous carbon. J Health Sci. 2004;50(6):588–593. | ||
Adarsh NN, Kumar DK, Dastidar P. Zn(II) metal–organic frameworks (MOFs) derived from a bis-pyridyl-bis-urea ligand: effects of crystallization solvents on the structures and anion binding properties. Cryst Eng Comm. 2008;10(11):1565–1573. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.