Back to Journals » Drug Design, Development and Therapy » Volume 14
Efficacy of Sub-Tenon Micro-Perfusion of Cyclophosphamide in Rabbits with Severe Ocular Inflammation
Authors Zhao L, Peng M, Lin W , Tan Q , Khan MA, Lin D
Received 19 February 2020
Accepted for publication 5 August 2020
Published 20 August 2020 Volume 2020:14 Pages 3407—3416
DOI https://doi.org/10.2147/DDDT.S250541
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Libei Zhao, Manqiang Peng, Wenxiang Lin, Qian Tan, Muhammad Ahmad Khan, Ding Lin
Department of Ophthalmology, Changsha Aier Eye Hospital, Aier School of Ophthalmology, Central South University, Changsha, People’s Republic of China
Correspondence: Ding Lin
Department of Ophthalmology, Changsha Aier Eye Hospital, Aier School of Ophthalmology, Central South University, No. 388, Section 2, Furong Middle Road, Changsha City, Hunan Province 410005, People’s Republic of China
Tel +86 13787255158
Email [email protected]
Purpose: To explore the feasibility of cyclophosphamide (CP) via a sub-Tenon micro-perfusion system (SMS) in rabbits, and assess its therapeutic efficacy in severe ocular inflammation.
Materials and Methods: Distribution and pharmacokinetics of CP were evaluated in vivo, and the concentrations of CP in plasma, vitreous humor, and retina/choroid were quantitated by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) at different time points. After induction of severe experimental uveitis, rabbits were divided into three groups (n=8 in each): the SMS group, subconjunctival injection (SI) group, and control group. Clinical inflammatory score was assessed in rabbits. Electroretinography and histopathology were performed on post-treatment day 8. Statistical analyses were performed using Mann–Whitney and Kruskal–Wallis tests. P-value less than 0.05 was considered significant.
Results: The concentrations of CP in vitreous humor and retina/choroid in the SMS group were significantly higher than that of the SI group at 3, 6, 10, and 24 hours (P< 0.01), while plasmatic CP concentrations were comparable at all time points in the SMS group and SI group (P> 0.05). The SMS group showed significantly less inflammation compared to the control group and SI group. Furthermore, the restoration of retinal structure and function were more obvious in the SMS group compared with conventional SI application.
Conclusion: Sub-Tenon micro-perfusion of CP exhibited satisfied therapeutic efficacy in rabbits with severe ocular inflammation and may provide a promising alternative for controlling ocular inflammatory disease and immune-mediated ocular diseases.
Keywords: cyclophosphamide, sub-Tenon drug delivery, ocular inflammation, treatment, rabbit
Introduction
Uveitis, a group of diseases characterized by intraocular inflammation, is one of the leading causes of preventable blindness all over the world.1 Particularly, severe and refractory inflammation is still a major challenge due to poor targeted drug delivery of effective anti-inflammatory agent. Since uveitis is an immune-mediated inflammatory ocular disease, a variety of immunosuppressants have been widely used to control inflammation in severe and refractory cases.2–5 Among them, cyclophosphamide (CP) displays better steroid-sparing and disease remission-inducing effect.6 It was first introduced in 1952, initially for managing uveitis of unknown etiology.7 Subsequently, it was used to treat severe inflammatory ocular disorders.8–10 Nevertheless, systemic administration of CP can cause undesirable side-effects that limit its clinical application.11,12
CP must be metabolized by cytochrome P450 enzymes in order to become biologically active. Cytochrome P450 enzymes were detected in the ocular tissues (including corneal epithelium, ciliary body, and retinal pigment epithelium), where CP is supposed to be activated in the eye.13,14 To minimize the potential systemic side-effects, ocular drug delivery (via intravitreal route or transscleral route) has been investigated to specifically deliver drug to the target tissue. Intravitreal delivery of immunosuppressants has gained popularity for treating uveitis.15–17 However, this route is invasive and has a multitude of risks, such as endophthalmitis, subconjunctival and vitreous hemorrhage, intraocular pressure elevation, retinal detachment, and aggravating vitreous opacity.18,19 In comparison, the transscleral route is safer to deliver to the posterior ocular segment.20 Sub-Tenon delivery involves introducing the drug to the sub-Tenon space located between the Tenon’s capsule and sclera, which enables the drug to diffuse into the vitreous directly from sclera. It is also believed that the transscleral route is suitable for delivering formulation into vitreous with optimal therapeutic level and minimal systemic exposure.21,22 However, it is difficult to maintain the sustained drug concentrations at target tissue by single sub-Tenon administration due to the rapid clearance of drug by blood and lymphatic flow.23,24 Thus, development of a sustained release platform using sub-Tenon administration may provide an approach to achieve stable drug concentrations and prolonged drug duration.
Our previous work showed that sub-Tenon sustained delivery of dexamethasone effectively inhibited the severity of uveitis in rabbits.25 However, management of severe and refractory ocular inflammation recalcitrant to corticosteroids therapies often requires combined application of CP. Therefore, our current study aimed to investigate the delivery efficiency of CP via a sub-Tenon micro-perfusion system (SMS) to the vitreous and retina, and assess its therapeutic efficacy in rabbits with severe experimental uveitis.
Materials and Methods
Animals
Animal experiments were handled in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research, and the protocol (No. 2019sydw0045) was approved by the Animal Ethical Committee of Central South University. Belgium pigmented rabbits with body weight of 2.5–3.0 kg (either sex) were used and provided by the Department of Laboratory Animals of Central South University (Changsha, China). The animals were anesthetized with an intramuscular injection of 0.1 mL/kg Xylazine Hydrochloride (2 mL:0.2 g, Huamu Animal Health Products Co., Jilin, China). The eyes were topically anesthetized with 0.5% proparacaine hydrochloride (Alcaine, Alcon-Couvreur, Puurs, Belgium). Animals were euthanized by intravenous injection of 3 mL lidocaine hydrochloride and 3 mL air.
In vivo Study
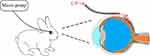
Sixty rabbits were randomly assigned into two groups: the SMS group (n=30) and subconjunctival injection (SI) group (n=30), with each group including six rabbits at each time point. In the SMS group, after anesthesia, rabbits were administered SMS in the left eyes (Figure 1), which was prepared according to our previous study.25 Briefly, a catheter with a micro-needle (BD Intima Ⅱ Closed IV Catheter System, Becton, Dickinson Co., USA) was inserted into sub-Tenon space, the micro-needle was then removed and a catheter was placed between the Tenon’s capsule and sclera, approximately 5 mm past the limbus in the superior temporal quadrant of the left eye. After that, 0.3 mL of 20 mg/mL CP (0.2 g, Jiangsu Shengdi Pharmaceutical Co., Ltd., Jiangsu, China) was released into the sub-Tenon as the initial dosage. Another end of the catheter was attached to a micro-pump releasing of CP (20 mg/mL) at the rate of 0.1 mL/h for 10 hours. In the SI group, 0.3 mL of CP (20 mg/mL) was given under topical anesthesia in the left eye using a 30-gauge needle. After administration, plasma and ocular samples were collected at 1, 3, 6, 10, and 24 hours in both groups. Blood samples obtained from auricular vein of rabbits were immediately centrifuged at 3000 g for 10 minutes to extract plasma. The rabbits were then euthanized at different time points and their eyeballs were enucleated and washed with 0.9% normal saline to remove blood, residual drug, and the conjunctiva. Ocular tissues including vitreous humor and retina/choroid compound were dissected and weighted respectively. All samples were stored in Eppendorf tubes at −20°C until analysis.
Drug Assay
CP standard (>98% purity) and ifosfamide (>98% purity) were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). CP was used to construct the standard curve and ifosfamide was used as an internal standard (IS). All other chemical agents used were of analytical grade. The concentration of CP in the samples was determined by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method. Liquid chromatographic separation was performed on a C18 Hypersil ODS column (150 mm×2.1 mm, i.d., 5 μm particle size; Thermo Scientific, USA) at 40°C using an Ultimate 3000 system with a Chromeleon Xpress ver2.4 workstation. The mobile phase consisted of ammonium acetate (pH=3.2) and acetonitrile (20:80, v/v) at a flow rate of 0.2 mL/min. The total running time was 7.0 minutes, and the injection volume was 5 μL. Mass spectrometric detection was performed on a Thermo ORBITRAP VELOS PRO in the positive ionization and MS/MS (selective ion monitoring) mode. The protonate molecular ions [M+H]+ with m/z 261 and m/z 233 were selected as precursor ions for CP and ifosfamide, respectively. XcaliburQual Browser software was used for data acquisition and analysis.
Weighted retina/choroid compound was homogenized with 0.9% normal saline (1:5, w/v) using a Bio-Gen PRO200 Homogenizer (Pro Scientific, USA). After homogenization, a volume of 200 μL of each sample (plasma, vitreous humor, and retina/choroid homogenate) was transferred to a centrifuge tube of 1.5 mL and added with a volume of 20 μL aliquots of IS (50 ng/mL). Then the mixture was vortexed for 30 seconds and extracted with n-hexane (0.5 mL) and acetate ester (0.5 mL) for 1 hour, and centrifuged at 14,000 rpm for 5 minutes. The organic layer was separated and the extract was evaporated under a gentle stream of nitrogen gas at 45°C until it was completely dry. The dried residue was dissolved with 50 μL of mobile phase and centrifuged at 14,000 r/min for 5 minutes, and 5 μL of the supernatant was injected into the LC-MS/MS system for analysis.
Pharmacokinetic Parameter Estimation
The pharmacokinetic parameters were estimated using noncompartmental analysis (Kinetica version 5.1, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The pharmacokinetic parameters for each sample, including the area under the concentration–time curve from zero to 24 hours (AUC0–24), elimination half-life (T1/2), the peak concentration (Cmax), and the time to reach peak concentration (Tmax) were analyzed.
Experimental Uveitis Rabbit Model and Treatment
The anti-inflammatory efficacy of CP-SMS was assessed in a severe experimental uveitis rabbit model. Rabbits were intravitreally injected with Mycobacterium tuberculosis H37Ra antigen (Becton, Dickinson Co., USA) suspended in phosphate-buffered saline (PBS; 80 µg; 1 µg/µL) after pre-immunization to establish the severe experimental uveitis.25–27 Rabbits were then randomly assigned to three groups: (1) SMS group (n=8): rabbits were treated with SMS of 20 mg/mL CP for 7 days (10 h/day); (2) SI group (n=8): rabbits received SI of 20 mg/mL CP for 7 days; (3) control group (n=8): rabbits did not receive any treatment.
Clinical Observation
The clinical signs of inflammatory response in the anterior chamber and vitreous humor were observed with slit-lamp biomicroscopy and indirect ophthalmoscopy (with a 20-diopter aspheric lens) on days 3 and 7 and images of ocular appearance were taken as well. The signs of anterior chamber fibrin and vitreous opacity were graded 30 minutes after treatment using a 0–4 grading scale (0=none, 0.5=trace, 1=mild, 2=moderate, 3=severe, and 4=totally opacified).28,29 Clinical scores were assessed by a single observer (MQP) who was blinded to the allocation.
Electroretinography (ERG)
Retinal function was evaluated by recording scotopic ERG using a Ganzfeld Q450 SC universal electrophysiological diagnostic system (Roland Consult, Germany) on post-treatment day 8. Following overnight dark adaptation, the rabbits were anesthetized, and the pupils were dilated with 1% tropicamide and 2.5% phenylephrine eye drops (Santen, Osaka, Japan). The gold-ring electrode was placed on the cornea. The reference platinum needle electrode was inserted subcutaneously on the forehead, and the tail root of the rabbit was connected to ground electrode. All procedures were performed under dim red light. Scotopic ERG responses were stimulated using a white light of 3.0 cd s/m2 intensity. The amplitude of the b-wave was calculated and recorded on a commercial RETImap system (Roland Consult, Germany).
Histopathological Examination
Histopathological analyses were performed on post-treatment day 8. All rabbits were sacrificed and the eyeballs were enucleated instantly, and immersed in a fixative solution containing 4% paraformaldehyde for 2 hours, and embedded in paraffin. The paraffin sections of 4-µm were cut through the papillary optic nerve plane, and stained with hematoxylin and eosin (H&E). Images were taken under light microscopy (Olympus, Tokyo, Japan). Histopathology of the retina was examined by a masked pathologist for the severity of retinal damage and graded using a 0–5 grading scale as previously reported.30
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The results of the differences between the two groups at each time point were analyzed by Mann–Whitney test. Statistical comparisons for the clinical evaluation and b-wave amplitude among different groups were performed using Kruskal–Wallis test with Dunn post-hoc tests. P-value<0.05 was considered statistically significant.
Results
CP Concentrations and Distribution Characteristics
CP and distribution characteristics in plasma, vitreous humor, and retina/choroid via SMS and SI are shown in Figure 2. Compared with plasmatic CP concentrations, the retina/choroid and vitreous humor showed relatively higher levels of CP in both SMS and SI groups. As shown in Table 1, the concentrations of CP in retina/choroid and vitreous humor at 3, 6, 10, and 24 hours were notably higher in the SMS group (P<0.01) and without a significant difference at 1 hour (P>0.05), as compared with the SI group. For plasma, no statistically significant differences at all time points were seen between the SMS group and the SI group (P>0.05). In addition, the CP concentration of plasma was negligible at 24 hours following either SMS or SI.
 |
Table 1 Cyclophosphamide Concentrations in the Plasma, Vitreous Humor, and Retina/Choroid in SMS and SI Groups |
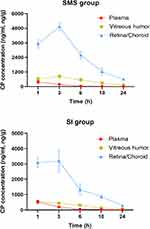 |
Figure 2 CP distribution characteristics in plasma, vitreous humor, and retina/choroid via SMS and SI. |
Pharmacokinetics
The pharmacokinetic parameters of CP in plasma, vitreous humor, and retina/choroid are presented in Table 2. Cmax of CP in plasma, vitreous humor, and retina/choroid in the SMS group were 381.23±108.06 ng/mL, 832.31±137.46 ng/mL, and 4929.68±323.02 ng/g, respectively, while those in the SI group were 522.33±110.25 ng/mL, 560.88±87.59 ng/g, and 3200.88±699.74 ng/g, respectively. The peak CP levels of vitreous humor and retina/choroid were greater in the SMS group than the SI group. AUC0–24 of CP in plasma was lower in the SMS group than the SI group. However, AUC0–24 of CP in ocular tissues were significantly higher (1.81-times in vitreous humor and 1.57-times in retina/choroid) for the SMS group than the SI group. Tmax of CP in plasma and retina/choroid were similar between the two groups, which were 1 hour in plasma, and 3 hours in retina/choroid. Vitreous Tmax was shorter in the SI group (1 hour) than that in the SMS group (3 hours). In the SMS group, the T1/2 in plasma, vitreous humor, and retina/choroid were 6.63±3.75 hours, 8.07±4.43 hours, and 7.59±1.46 hours, respectively. In the SI group, the T1/2 were 5.82±2.76 hours, 7.56±3.02 hours, and 8.30±2.06 hours, respectively.
 |
Table 2 Pharmacokinetics of Cyclophosphamide in Plasma, Vitreous Humor, and Retina/Choroid via SMS and SI |
Efficacy Outcomes
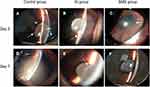
Images of the ocular appearance on post-treatment days 3 and 7 for all groups are displayed in Figure 3. The rabbits developed severe anterior chamber fibrin, pupil synechiae, and vitreous opacity in the control group (Figure 3A and D). However, less clinical signs of inflammation were observed in the SMS group and SI group (Figure 3B, C, E, and F). Furthermore, there were no signs of inflammation in the SMS group on post-treatment day 7 (Figure 3F).
Clinical signs of inflammatory response were assessed by anterior chamber fibrin and vitreous opacity. As shown in Figure 4, the SMS and SI groups significantly reduced the clinical inflammatory scores on post-treatment days 3 and 7 in comparison to the control group (P<0.01). The SMS group had the lowest clinical score among the three groups. For anterior chamber fibrin, there was no significant difference between the SMS group and the SI group on post-treatment day 3 (P=0.783), but the SMS group had a lower score than the SI group on post-treatment day 7 (0.06±0.18 vs 0.63±0.23; P<0.01; Figure 4A). Moreover, the mean vitreous opacity score was notably lower in the SMS group compared with the SI group at any observation time point (Figure 4B).
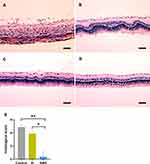
Histopathological section images and histological scores of the retina in all groups are illustrated in Figure 5. The control group manifested severe inflammation and remarkable inflammatory cell infiltration in the retina. The retinal structure was disorganized with a photoreceptor layer fully damaged (Figure 5A). Meanwhile, moderate disruption of the retinal structure and photoreceptor layer could be observed in the SI group (Figure 5B). There were no significant differences in the mean histological score of the retina between control group and SI group (4.87±0.35 vs 3.88±0.35; P=0.108; Figure 5E). In contrast, the retinal structure was rescued with normal or only slight damage to the photoreceptor outer layer found in the SMS group (Figure 5C). The mean histological score of the retina in the SMS group was significantly lower than the SI group (P=0.039; Figure 5E) and control group (P<0.01; Figure 5E).
Retinal Function Evaluation
Scotopic ERGs were used to evaluate the retinal function in uveitis rabbits, the results of b-wave amplitude in all groups are shown in Figure 6. The rabbits in the SI group and SMS group showed significantly greater b-wave amplitudes as compared to the control group (P<0.01). Furthermore, the b-wave amplitude in the SMS group was comparable to the normal rabbits (P>0.05) and notably greater than the SI group (P<0.01).
Discussion
In the present study, we demonstrated the ability of SMS to achieve satisfied concentrations of CP in the vitreous and retina with low systemic exposure. Additionally, we further proved that SMS loaded with CP effectively resolved inflammatory symptoms and preserved retinal function in experimental uveitis rabbits.
Treatment of severe uveitis requires efficient ocular drug level to rapidly control the initial inflammation and subsequent sustained maintenance drug level to achieve prolonged therapeutic action to prevent disease relapse.31 Trans-Tenon’s retrobulbar infusion of drug using a blunt cannula is a safe and effective treatment for uveitis.32 In the current study, SMS was optimized by inserting a catheter directly into the sub-Tenon through a micro-needle without the need for a surgical cut down or an incision, which is as convenient as the intravenous route to provide an easy and safe repeated injection process. The releasing amount of CP into the sub-Tenon was controlled by an auto-infusion pump which delivered 6 mg CP as an initial dose and subsequently dropped down to a maintenance dose (2 mg every hour). This drug delivery strategy is suitable for treating severe uveitis because it can provide personalized drug therapy according to the severity of disease.
We characterized the CP distribution and pharmacokinetics in plasma, retina/choroid, and vitreous via the SMS using the conventional SI administration as a comparative modality. Our results showed that CP levels of vitreous humor and retina/choroid in the SMS group were much higher within 24 hours (except 1 hour) than inthe SI group. This indicated that CP released from SMS was more efficient to arrive at posterior segment tissues than that from SI. Several factors are attributed to this phenomenon. Firstly, higher CP concentration at the delivery site could be obtained via SMS due to slower clearance rate. It has been shown that the vascular density is low in sub-Tenon’s area,33 which can decrease drug clearance by blood flow. Furthermore, the concentration gradient can enhance drug penetration from the scleral surface to the retina/vitreous.34–36 Thus, the longer period for CP loading at the injection site, the higher possibility for CP penetrating into the sclera and getting to the ocular tissue. For pharmacokinetics study, AUC0-24 of CP in the vitreous and retina/choroid in the SMS group was greater than that in the SMS group. Although the SMS group had a later Tmax in the vitreous, higher Cmax is crucial for ocular diseases.
It has been shown that severe ocular inflammation could be observed in experimental uveitis models.25 In the current study, we started the treatment 24 hours after the model induction when severe intraocular inflammation occurred. The clinical inflammatory scores of the anterior chamber and vitreous humor in the control group were gradually increased over time, whereas those in the SI group and SMS group were decreased. Compared with the SI group, the SMS group showed a significant reduction in inflammatory scores on post-treatment day 7. These data suggested that SMS loaded with CP effectively inhibited ocular inflammation. Furthermore, retinal histopathological and ERG analysis showed that the restoration of retinal structure and function were more obvious in the SMS group, suggesting that the delivering CP via SMS was more potent in protecting retinal function compared with conventional SI application.
Although the rabbit ocular anatomy and physiology resemble that of humans, caution is necessary when extrapolating the ocular drug delivery system in humans from the experimental data obtained in the animal model.37 In contrast to humans, rabbits possess much thinner sclera, which facilitate penetration of drug from episcleral space into the choroid/retina and vitreous. Moreover, the basal metabolic rate in a rabbit is faster, which may shorten the half-life of the drug in ocular tissues due to an increase in drug clearance rate. Consequently, the dosage of CP and the release rate of the SMS should be changed and designed accordingly if it is used for patient with uveitis. The systemic and ocular complications associated with local delivery of CP via SMS remains unclear. Further study is necessary to address these concerns. Moreover, we expect the present drug delivery system can be upgraded in the future to cure other severe ocular immune-mediated diseases.
Conclusions
Releasing of CP through a sub-Tenon micro-perfusion system achieved high CP concentrations in the retina/choroid and vitreous humor, which relieved ocular inflammation and restored both retinal anatomical structure and function in the rabbit model with severe uveitis. It may become a promising candidate for treating ocular inflammation and immune-mediated ocular diseases.
Acknowledgments
This work was supported by the Key program for Hunan Provincial Science and Technology Department (No. Kc1701045). The authors would like to thank Professor Shaogang Liu (Advanced Research Center, Central South University) for guidance of the pharmaceutical analysis method. We also thank Professor Siqi Xiong (Xiangya hospital, Central South University) for his generous help and support.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. 2016;123(3):655–662. doi:10.1016/j.ophtha.2015.10.028
2. Forrester JV, Kuffova L, Dick AD. Autoimmunity, autoinflammation, and infection in uveitis. Am J Ophthalmol. 2018;189:77–85. doi:10.1016/j.ajo.2018.02.019
3. Willermain F, Rosenbaum JT, Bodaghi B, et al. Interplay between innate and adaptive immunity in the development of non-infectious uveitis. Prog Retin Eye Res. 2012;31(2):182–194. doi:10.1016/j.preteyeres.2011.11.004
4. Lee RW, Nicholson LB, Sen HN, et al. Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol. 2014;36(5):581–594. doi:10.1007/s00281-014-0433-9
5. Rosenbaum JT, Bodaghi B, Couto C, et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: a review. Semin Arthritis Rheum. 2019;49(3):438–445. doi:10.1016/j.semarthrit.2019.06.004
6. Jabs DA. Immunosuppression for the uveitides. Ophthalmology. 2018;125(2):193–202. doi:10.1016/j.ophtha.2017.08.007
7. Roda Perez E. [Nitrogen mustard therapy of uveitis of unknown etiology]. Rev Clin Esp. 1952;44(3):173–180. Spanish.
8. Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010;117(2):356–365. doi:10.1016/j.ophtha.2009.06.060
9. Suelves AM, Arcinue CA, Gonzalez-Martin JM, Kruh JN, Foster CS. Analysis of a novel protocol of pulsed intravenous cyclophosphamide for recalcitrant or severe ocular inflammatory disease. Ophthalmology. 2013;120(6):1201–1209.
10. Wakefield D. Does cyclophosphamide still have a role in the treatment of severe inflammatory eye disease? Ocul Immunol Inflamm. 2014;22(4):306–310. doi:10.3109/09273948.2013.854395
11. Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: fundamentals of care for uveitis (FOCUS) initiative. Ophthalmology. 2018;125(5):757–773. doi:10.1016/j.ophtha.2017.11.017
12. Tavakolpour S. Current and future treatment options for pemphigus: is it time to move towards more effective treatments? Int Immunopharmacol. 2017;53:133–142. doi:10.1016/j.intimp.2017.10.027
13. Nakano M, Lockhart CM, Kelly EJ, Rettie AE. Ocular cytochrome P450s and transporters: roles in disease and endobiotic and xenobiotic disposition. Drug Metab Rev. 2014;46(3):247–260. doi:10.3109/03602532.2014.921190
14. Salgueiro A, Egea MA, Espina M, Valls O, Garcia ML. Stability and ocular tolerance of cyclophosphamide-loaded nanospheres. J Microencapsul. 2004;21(2):213–223. doi:10.1080/02652040310001637866
15. Sadaka A, Sisk RA, Osher JM, Toygar O, Duncan MK, Riemann CD. Intravitreal methotrexate infusion for proliferative vitreoretinopathy. Clin Ophthalmol. 2016;10:1811–1817. doi:10.2147/OPTH.S111893
16. Taylor SR, Banker A, Schlaen A, et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina. 2013;33(10):2149–2154. doi:10.1097/IAE.0b013e31828ac07d
17. Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116(4):797–801. doi:10.1016/j.ophtha.2008.10.033
18. Gupta A, Sun JK, Silva PS. Complications of intravitreous injections in patients with diabetes. Semin Ophthalmol. 2018;33(1):42–50. doi:10.1080/08820538.2017.1353811
19. Nguyen QD, Merrill PT, Clark WL, et al. Intravitreal sirolimus for noninfectious uveitis: a phase III sirolimus study assessing double-masked uveitis treatment (SAKURA). Ophthalmology. 2016;123(11):2413–2423. doi:10.1016/j.ophtha.2016.07.029
20. Agban Y, Thakur SS, Mugisho OO, Rupenthal ID. Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov Today. 2019;24(8):1458–1469. doi:10.1016/j.drudis.2019.03.023
21. Ghate D, Brooks W, McCarey BE, Edelhauser HF. Pharmacokinetics of intraocular drug delivery by periocular injections using ocular fluorophotometry. Invest Ophthalmol Vis Sci. 2007;48(5):2230–2237. doi:10.1167/iovs.06-0954
22. Shen L, You Y, Sun S, Chen Y, Qu J, Cheng L. Intraocular and systemic pharmacokinetics of triamcinolone acetonide after a single 40-mg posterior subtenon application. Ophthalmology. 2010;117(12):2365–2371. doi:10.1016/j.ophtha.2010.03.033
23. Robinson MR, Lee SS, Kim H, et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res. 2006;82(3):479–487. doi:10.1016/j.exer.2005.08.007
24. Chan JE, Pridgen TA, Csaky KG. Episcleral clearance of sodium fluorescein from a bioerodible sub-tenon’s implant in the rat. Exp Eye Res. 2010;90(4):501–506. doi:10.1016/j.exer.2010.01.001
25. Zhao L, Huang X, Peng M. et al. Sub-Tenon sustained controllable delivery of dexamethasone for treating severe acute experimental uveitis. Ocul Immunol Inflamm;2019; 1–10. doi:10.1080/09273948.2019.1643027
26. Cheng CK, Berger AS, Pearson PA, Ashton P, Jaffe GJ. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest Ophthalmol Vis Sci. 1995;36:442–453.
27. Jaffe GJ, Yang CS, Wang XC, Cousins SW, Gallemore RP, Ashton P. Intravitreal sustained-release cyclosporine in the treatment of experimental uveitis. Ophthalmology. 1998;105:46–56. doi:10.1016/S0161-6420(98)91176-9
28. Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi:10.1016/S0161-6420(85)34001-0
29. Papangkorn K, Prendergast E, Higuchi JW, Brar B, Higuchi WI. Noninvasive ocular drug delivery system of dexamethasone sodium phosphate in the treatment of experimental uveitis rabbit. J Ocul Pharmacol Ther. 2017;33(10):753–762. doi:10.1089/jop.2017.0053
30. Inoki T, Yamagami S, Sakai R, Isobe M, Tsuru T, Kawashima H. Suppression of experimental autoimmune uveoretinitis by anti-alphabeta TCR monoclonal antibody. Jpn J Ophthalmol. 2002;46:518–524. doi:10.1016/S0021-5155(02)00538-5
31. Luo L, Yang J, Oh Y, et al. Controlled release of corticosteroid with biodegradable nanoparticles for treating experimental autoimmune uveitis. J Control Release. 2019;296:68–80. doi:10.1016/j.jconrel.2019.01.018
32. Okada AA, Wakabayashi T, Morimura Y, et al. Trans-Tenon’s retrobulbar triamcinolone infusion for the treatment of uveitis. Br J Ophthalmol. 2003;87(8):968–971. doi:10.1136/bjo.87.8.968
33. Kumar DA, Agarwal A, Karnathi S, Patadiya R. Anterior segment optical coherence tomography for imaging the sub-Tenon space. Ophthalmic Res. 2013;50(4):231–234. doi:10.1159/000354381
34. Li SK, Hao J. Transscleral passive and iontophoretic transport: theory and analysis. Expert Opin Drug Deliv. 2018;15(3):283–299. doi:10.1080/17425247.2018.1406918
35. Barar J, Aghanejad A, Fathi M, Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts. 2016;6(1):49–67. doi:10.15171/bi.2016.07
36. Li J, Lan B, Li X, Sun S, Lu P, Cheng L. Effect of intraocular pressure (IOP) and choroidal circulation on controlled episcleral drug delivery to retina/vitreous. J Control Release. 2016;243:78–85. doi:10.1016/j.jconrel.2016.10.001
37. Shen J, Lu GW, Hughes P. Targeted ocular drug delivery with pharmacokinetic/pharmacodynamic considerations. Pharm Res. 2018;35(11):217. doi:10.1007/s11095-018-2498-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.