Back to Journals » Drug Design, Development and Therapy » Volume 11
Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: a systematic review and meta-analysis
Authors Yu R, Yang B, Chi X, Cai L, Liu C, Yang L, Wang X, He P, Lu X
Received 11 October 2016
Accepted for publication 14 February 2017
Published 17 March 2017 Volume 2017:11 Pages 851—864
DOI https://doi.org/10.2147/DDDT.S124399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Ruili Yu,1 Bo Yang,2 Xiaohua Chi,3 Lili Cai,4 Cui Liu,5 Lei Yang,6 Xueyan Wang,1 Peifeng He,7 Xuechun Lu2
1Department of Allergy, Beijing Shijitan Hospital, Affiliated to Capital Medical University, 2Department of Geriatric Hematology, Chinese PLA General Hospital, 3Department of Pharmacy, Chinese PLA Rocket Force General Hospital, 4Department of Geriatric Laboratory Medicine, 5Department of Geriatric Ultrasound, 6Medical Department, Nanlou Clinic, Chinese PLA General Hospital, Beijing, 7School of Medical Information Management, Shanxi Medical University, Taiyuan, People’s Republic of China
Abstract: This study was designed to evaluate the efficacy and safety of cytokine-induced killer (CIK) cell-based immunotherapy as an adjuvant therapy for hepatocellular carcinoma (HCC). Published studies were identified by searching Medline, Cochrane, EMBASE, and Google Scholar databases with the keywords: cytokine-induced killer cell, hepatocellular carcinoma, and immunotherapy. The outcomes of interest were overall survival, progression-free survival, and disease-free survival. Eight randomized controlled trials (RCTs), six prospective studies, and three retrospective studies were included. The overall analysis revealed that patients in the CIK cell-treatment group had a higher survival rate (pooled hazard ratio (HR) =0.594, 95% confidence interval [CI] =0.501–0.703, P<0.001). Patients treated with CIK cells in non-RCTs had a higher progression-free survival rate (pooled HR =0.613, 95% CI =0.510–0.738, P<0.001). However, CIK cell-treated patients in RCTs had progression-free survival rates similar to those of the control group (pooled HR =0.700, 95% CI =0.452–1.084, P=0.110). The comparison between pooled results of RCTs and non-RCTs regarding the progression-free survival rate did not reach statistical significance. Patients in the CIK cell-treatment group had lower rates of relapse in RCTs (pooled HR =0.635, 95% CI =0.514–0.784, P<0.001). Similar results were found when non-RCT and RCTs were pooled (pooled HR =0.623, 95% CI =0.516–0.752, P<0.001). Adjuvant CIK cell-based immunotherapy is a promising therapeutic approach that can improve overall survival and reduce recurrence in patients with HCC.
Keywords: cytokine-induced killer cells, hepatocellular carcinoma, survival, relapse, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) accounts for 95% of primary liver cancer1 and is the second most common cause of cancer-associated death worldwide.2 Liver resection and liver transplantation are the only curative treatments for HCC. The majority of patients, however, are not eligible for either resection or transplantation because of advanced tumor stage, underlying liver dysfunction, and lack of donor organs. Additionally, postoperative recurrence is frequent and can be as high as 25% per year, leading to death of ~80% of patients within 12 months of diagnosis.3,4 Other therapeutic options, such as percutaneous chemical, thermal, or radiofrequency ablation (RFA); transarterial chemoembolization (TACE); chemotherapy; and targeted therapy, also have limited efficacy.5 Therefore, finding effective methods to increase efficacy of treatment and reduce recurrence rate is of utmost importance in the therapy of HCC.
Immunotherapy has been considered as a potential treatment option for HCC for a number of years.6,7 Several approaches to immunotherapy for HCC have shown promise in early clinical trials. These treatments can be divided into four main categories: immune checkpoint inhibitors, monoclonal antibodies, adoptive cell transfer, and oncolytic virus therapy.7 Adoptive cytokine-induced killer (CIK) cell transfer is one of the promising avenues of immunotherapy for HCC. CIK cells are non-major histocompatibility complex–restricted cells that exhibit strong cytolytic activities against susceptible tumors8 and express both T- and natural killer (NK) cell markers, CD3 and CD56, respectively.9 CIK cells can be generated from human peripheral blood mononuclear cells through induction with interferon-γ, anti-CD3 antibody, and interleukin-2.8 There are a number of advantages of CIK cells compared with other immune cells. CIK cells have a higher proliferation rate and can be obtained directly from cancer patients.10 Additionally, CIK cells have strong cytolytic activities and recognize a number of tumors, including those that are resistant to lymphokine-activated killer cells or NK cells.11 Furthermore, CIK cells were not shown to cause graft-versus-host disease.7,8 Therefore, CIK cells present a promising immunotherapy approach that could be used for HCC patients.12
And indeed, a number of recent clinical trials have demonstrated that adoptive infusion of CIK cells was associated with a substantial antitumor effect in HCC patients.13–19 CIK cell transfer was shown to decrease the rate of relapse after TACE and RFA therapy and increase disease-free survival and overall survival for HCC patients after liver resection or TACE.13–19
However, despite the increasing evidence pointing to CIK cells as a viable option for HCC treatment, more translational research and clinical trials are needed to provide convincing evidence regarding the efficacy of CIK cell immunotherapy. The aim of the present meta-analysis was to assess the efficacy and safety of CIK cell-based immunotherapy as an adjuvant therapy for HCC.
Materials and methods
Search strategy
We followed the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidance for systematic reviews of observational and diagnostic studies20 and searched the published literature using the Medline, Cochrane, EMBASE, and Google Scholar databases through November 6, 2015, with various combinations of the following keywords: cytokine-induced killer, CIK, hepatocellular carcinoma, HCC, liver neoplasm, and immunotherapy. The specific search terms were the following: (((hepatocellular carcinoma) OR HCC) OR liver neoplasm) AND ((cytokine-induced killer cell) OR CIK), with the following filters: Humans, Abstract available, Clinical study, Clinical trial, Meta-analysis, Review, and Systematic review. We manually searched references in relevant publications to identify additional eligible trials. The inclusion criteria were as follows: 1) randomized controlled trials (RCTs) and prospective or retrospective studies; 2) patients who were initially diagnosed with HCC and allocated to either an adoptive immunotherapy group or a control group; and 3) quantitative outcomes (overall survival, progression-free survival, and disease-free survival). The exclusion criteria were as follows: 1) format of cohort study, letter, comment, editorial, case report, proceeding, or personal communication; 2) patients without a diagnosis of HCC; 3) study designed for adoptive immunotherapy with other cell types (eg, NK cells, dendritic cells); and 4) no quantitative outcomes.
Study selection and data extraction
Data were extracted independently by two reviewers. A third reviewer was consulted in case of disagreements. We extracted data on study population (number, age, and gender of patients in each group), study design, length of follow-up time, Child–Pugh Class, cancer stage, viral hepatitis profile, and data for overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS).
Quality assessment
We assessed study quality using the Cochrane Risk of Bias Tool.21 The quality assessment was performed by two independent reviewers; the third reviewer was consulted if no consensus could be reached. The quality assessment of included studies is presented in Figure 1.
  | Figure 1 Quality assessment. (A) Risk of bias summary; (B) Risk of bias graph. |
Statistical analysis
The outcomes of interest were OS, PFS, and DFS. Hazard ratios (HR) and 95% confidence intervals (95% CI) reported by individual studies were used as the outcome measures. If not provided in individual studies, the HR and 95% CI were calculated from summary statistics of time-to-event analyses with the methods proposed by Tierney et al.22
Heterogeneity among the studies was assessed by the Cochran Q and the I2 statistic. The Q statistic was defined as the weighted sum of the squared deviations of the estimates of all studies; P<0.10 was considered statistically significant for heterogeneity. For the I2 statistic, which indicated the percentage of the observed between-study variability due to heterogeneity, the ranges used were the following: no heterogeneity (I2=0%–25%), moderate heterogeneity (I2=25%–50%), large heterogeneity (I2=50%–75%), and extreme heterogeneity (I2=75%–100%).
The random-effect model (DerSimonian–Laird method) was used to generate pooled estimates across studies for each outcome. A two-sided P-value <0.05 was considered statistically significant. All analyses that were performed were stratified by study design (ie, randomized and nonrandomized trials). To assess whether a single study impacts the pooled results, a sensitivity analysis was performed using the leave-one-out approach. All statistical analyses were performed with the statistical software Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Basic characteristics of included studies
After considering the inclusion and exclusion criteria, eight randomized trials,13,14,18,23–27 six prospective studies,15,28–33 and three retrospective studies16,17,32 were eligible for this review (Figure 2). The eligible studies analyzed a total of 1,979 patients with HCC, 1,029 of whom underwent adjuvant immunotherapy with CIK cells. The number of patients ranged from 38 to 410 per study. The patients’ age ranged from 43 to 56 years. The proportion of male patients ranged from 52.4% to 97.8%. Information regarding patient demographics, liver function, stage of HCC, hepatitis infection, and treatment regimens is summarized in Table 1. The treatment used by the majority of studies was TACE, either alone (six studies) or in combination with RFA (five studies), percutaneous ethanol injection (one study), or surgery (one study). Surgery was the second most common treatment, used alone (two studies) or with RFA and percutaneous ethanol injection (one study). For all studies, patients who received CIK cell immunotherapy also received the same treatment as the control group.
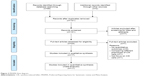  | Figure 2 PRISMA flow diagram. |
Outcome measures
A summary of the data for OS, PFS, and DFS is shown in Table 2. A total of 7 RCTs14,18,23–27 and 9 non-RCTs14–17,28–33 reported the HR for overall survival. There was significant heterogeneity among the studies (RCTs: I2=50.4%, P=0.060; non-RCTs: I2=43.3%, P=0.079; overall: I2=47.2%, P=0.019). The overall analysis revealed that patients in the CIK cell-treatment group had a higher survival rate (pooled HR =0.594, 95% CI =0.501–0.703, P<0.001). The results were similar for both RCTs (pooled HR =0.644, 95% CI =0.506–0.820, P<0.001) and non-RCTs (pooled HR =0.548, 95% CI =0.432–0.695, P<0.001) (Figure 3A).
One RCT23 and 4 non-RCTs15,16,28,33 analyzed the HR for PFS and were included in the meta-analysis. There was no evidence of heterogeneity across individual non-RCT studies (I2=0%, P=0.642). Analysis of RCTs revealed that there was no significant difference between the PFS rate in patients with or without CIK treatment (HR =0.700, 95% CI =0.452–1.084, P=0.110). The overall analysis of the non-RCTs indicated a higher rate of PFS in patients treated with CIK cells (pooled HR =0.613, 95% CI =0.510–0.738, P<0.001). The comparison between the pooled results from RCTs and that of non-RCTs (Figure 3B) regarding the PFS rate did not reach statistical significance.
One non-RCT30 and 4 RCTs13,14,18,24 reported the HR for disease-free survival. There was no evidence of heterogeneity across the four RCTs that were included in the meta-analysis of the disease-free survival rate (I2=0%, P=0.781). The overall analysis of RCTs revealed that patients treated with CIK cells had lower rates of relapse or recurrence (pooled HR =0.635, 95% CI =0.514–0.784, P<0.001). Similar results were also found when non-RCT and RCTs were pooled (pooled HR =0.623, 95% CI =0.516–0.752, P<0.001) (Figure 3C).
Sensitivity analysis and publication bias
Sensitivity analyses were performed using the leave-one-out approach (Figure 4). For all outcomes, the direction and magnitude of the combined estimates did not vary markedly with the removal of one of the studies, indicating that the data were not overly influenced by each study. Publication bias was not assessed due to small sample size.34
Quality assessment
We assessed the study quality of the prospective studies included in this meta-analysis using the Cochrane Risk of Bias Tool (Figure 1). There were eight studies with potential selection bias for random sequence generation, and two studies had potential for allocation concealment bias. None of the included studies was double-blinded, and only two studies were blinded for outcome assessment (Figure 1A). There were 11 studies with low risk in attrition bias and 15 studies with low risk of reporting bias. Overall, the quality of included studies was limited due to the study design and difficulty with blinding (Figure 1B). The described issues were partially related to the procedure used to administer the CIK cell therapy as well as the ethics behind patient allocation to treatment groups.
Discussion
The effectiveness of the current therapies for advanced HCC is limited, and the incidence of treatment-related adverse reactions is high, particularly in elderly patients with underlying liver conditions.4 Therefore, new treatment modalities, capable of prolonging survival in patients with advanced HCC while minimizing the risk of adverse reactions, are urgently needed. Immunotherapy has a potential to offer systemic, nontoxic, and durable antitumor effects, and therefore is highly attractive as a treatment option for HCC. HCC tumor cells can be targeted by various immune effector mechanisms,7 including by using CIK cells as effector cells. CIK cells belong to the T-cell population, display a T-cell- and NK cell-like phenotype, and are characterized by a non-major histocompatibility complex–restricted tumor killing activity.7 Recently, a number of clinical trials have been undertaken to evaluate CIK cell-based immunotherapy in the treatment of HCC. To summarize and evaluate the most recent findings regarding the efficacy and safety of CIK cell immunotherapy as an adjuvant treatment for HCC, we performed the current meta-analysis.
We found that patients who underwent CIK cell-based immunotherapy had a higher rate of overall survival compared to patients who did not receive CIK cell-based therapy. The observed results were similar in both RCTs and non-RCTs. Additionally, patients who underwent CIK cell-based immunotherapy had lower rates of tumor recurrence. While we did not observe a statistically significant difference between the patients in the intervention and control groups regarding the PFS rate, the observed trend was in favor of CIK cell-based immunotherapy.
The studies included in this meta-analysis did not report adverse and unexpected side effects of the treatment. Several studies16,18,26,33 reported constitutional symptoms, such as fever and chills, in some patients who received therapy with CIK cells. Yu et al,23 reported nausea in 4 patients in the CIK cell group and in 5 patients in the non-CIK cell group. One patient was allergic to CIK cells in the 2014 study by Guo et al.33
Our meta-analysis is the most current, with rather broad inclusion parameters. We included RCTs and non-RCTs, as well as studies published in English and Chinese. Overall, our results are in agreement with previous studies. Ma et al35 analyzed 13 articles reporting phase II and III clinical trials of CIK cell-based therapy in the treatment of HCC. This meta-analysis revealed a significant advantage of CIK cell-combined therapy in prolonging the overall survival of patients. Pooled analysis showed that treatment with CIK cells was associated with significantly improved 1-year survival (odds ratio [OR] =0.25, 95% CI =0.12–0.52, P<0.001) and 2-year survival (OR =0.17, 95% CI =0.07–0.43, P<0.001), but not half-year survival (77% in the CIK cell group versus 67% in the non-CIK cell group; OR =0.43, 95% CI =0.05–3.94, P=0.45). CIK cell-based treatment was also associated with a significantly prolonged half-year and 1-year PFS (OR =0.29, 95% CI =0.16–0.52, P<0.001; OR =0.35, 95% CI =0.22–0.53, P<0.001, respectively).35 We did not observe a significant improvement of PFS in the CIK cell treatment group in our meta-analysis, possibly due to differences in the study designs of the included studies, eg, number of RCTs versus non-RCTs. Recently, another meta-analysis assessed the efficacy of CIK cell therapy after TACE or TACE plus RFA and showed that CIK cell therapy combined with TACE plus RFA treatment was associated with a higher 1-year recurrence-free survival rate and 1- and 2-year OS rates.36 While subgroup analysis based on the prior treatment was beyond the scope of our review, it should be further investigated in future studies. Furthermore, subgroup analysis based on other parameters, such as stage of cancer and exact therapeutic regimen, would be beneficial in providing a better understanding of the effectiveness of immunotherapy and determining optimal therapeutic approaches for treatment of HCC.
The conclusions of this meta-analysis are subject to several limitations. Despite inclusion of non-English publications, the number of analyzed studies is limited, potentially leading to random errors. Another major drawback of the study is the moderate to large heterogeneity among the studies included for analysis of overall survival.
Conclusion
Our results highlight that adjuvant CIK cell-based immunotherapy is a promising therapeutic modality that can improve OS and reduce recurrence in patients with HCC. Future studies with subgroup analyses including etiologic factors, liver function, previous treatments, and disease stage should help to identify groups of HCC patients who would benefit the most from CIK cell-based immunotherapy.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no 81273597, no 81302801), Clinical Support Foundation of Chinese PLA General Hospital (no 2016FC-ZHCG-1004), the Innovation and Nursery Foundation of Chinese PLA General Hospital (no 11KMM24, no 15KMM21), Capital Characteristic Key Project of Beijing Municipal Science and Technology Commission (no Z161100000516006), and Medical Science and Technology Document Sharing Service Platform Project of Shanxi Province (no 201605D121012). Ruili Yu, Bo Yang, Xiaohua Chi, Lili Cai, Cui Liu, and Lei Yang contributed equally as co-first authors. All authors contributed equally to this work.
Disclosure
The authors report no conflicts of interest in this work.
References
Lau WY. Primary liver tumors. Semin Surg Oncol. 2000;19(2):135–144. | ||
IARC. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. IARC, 2012. | ||
Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231(4):544–551. | ||
Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. | ||
Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25(27):3866–3884. | ||
Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3(1):31–39. | ||
Hong YP, Li ZD, Prasoon P, Zhang Q. Immunotherapy for hepatocellular carcinoma: from basic research to clinical use. World J Hepatol. 2015;7(7):980–992. | ||
Introna M, Golay J, Rambaldi A. Cytokine Induced Killer (CIK) cells for the treatment of haematological neoplasms. Immunol Lett. 2013;155(1–2):27–30. | ||
Linn YC, Hui KM. Cytokine-induced killer cells: NK-like T cells with cytotolytic specificity against leukemia. Leuk Lymphoma. 2003;44(9):1457–1462. | ||
Alvarnas JC, Linn YC, Hope EG, Negrin RS. Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2001;7(4):216–222. | ||
Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11:83. | ||
Mesiano G, Todorovic M, Gammaitoni L, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12(6):673–684. | ||
Weng DS, Zhou J, Zhou QM, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31(1):63–71. | ||
Dong H, Li Q, Wang J, Zhang T, Kong DL. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41(1):36–41. | ||
Hao MZ, Lin HL, Chen Q, Ye YB, Chen QZ, Chen MS. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer. 2010;29(2):172–177. | ||
Huang ZM, Li W, Li S, et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother. 2013;36(5):287–293. | ||
Pan K, Li YQ, Wang W, et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20(13):4305–4311. | ||
Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–1391.e6. | ||
Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. | ||
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Yu X, Zhao H, Liu L, et al. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol. 2014;34(2):194–203. | ||
Deng WJ CJ, Luo YJ, Xiang GY. Efficacy and safety of min-imally invasive treatment combined with autologous CIK cellsinfusion. Ling Nan Xian Dai Ling Chuang Wai Ke. 2013;13:29–31. | ||
He XB, Wang J, Hu JH. CIK cells combined with TACE treatmentof primary liver cancer randomized controlled study. Si ChuanXi Xue. 2012;33:1696–1697. | ||
Huang LX, Zhou QM, Xia JC. Minimally invasive treatmentcombined with autologous CIK cell therapy for primary hep-atocellular carcinoma: safety and efficacy. Guang Dong Yi Xue. 2007;28:1466–1468. | ||
Zhang ZN, Xu YM, Chen FX, et al. Clinical study on the treatment of advanced hepatocellular car-cinoma by CIK cell. Dong Nan Guo Fang Yi Yao. 2006;8:84–87. | ||
Tong LQ, Zhao HF, You LG, et al. Transarterial chemoembolization combined with autologouscytokine-induced killer cells therapy for primary liver cancer. Zhong Guo Pu Tong Wai Ke Za Zhi. 2013;22:9. | ||
Hao MZ, Chen Q, Ye YB, et al. Transcatheter arterial chemoembolization combined with cytokine-induced killers in treatment of hepatocellular car-cinoma. Zhong Guo Zhong Liu Sheng Wu Zhi Liao Za Zhi. 2006;13:303–305. | ||
Wang JP, Li W, Huang ZL, et al. [Value of CIK in the treatment of TACE combined with RFA for HCC in long-term survival and prognostic analysis]. Zhonghua Yi Xue Za Zhi. 2012;92(43):3062–3066. | ||
Shi Y, Gao CJ, Chen FX, Xu YM, Dong SL. Cytokine-induced killer cell for interventional chemotherapy of hepatocellular carcinoma. J Interv Radiol. 2007;16(4):235–239. | ||
YU W, YE Y, Zhou D, et al. Effect of postoperative transcatheter arterial chemoembolization combined with cytokine-induced killer immunotherapy on recurrence and survival rate of hepatocellular carcinoma patients. J Min Invasive Med. 2009;4(5):459–461. | ||
Guo W, Liu L, Wu D. [Dendritic cell-cytokine induced killer cell immunotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: safety and efficacy]. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(5):674–678. | ||
Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. | ||
Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1(1):11. | ||
Li X, Dai D, Song X, Liu J, Zhu L, Xu W. A meta-analysis of cytokine-induced killer cells therapy in combination with minimally invasive treatment for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014;38(5):583–591. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




