Back to Journals » Drug Design, Development and Therapy » Volume 10
Efficacy of BIBF 1120 or BIBF 1120 plus chemotherapy on nasopharyngeal carcinoma in vitro and in vivo
Authors Xue C , Tian Y, Zhang J, Zhao Y , Zhan J, Fang W, Zhang L
Received 18 September 2015
Accepted for publication 5 January 2016
Published 15 March 2016 Volume 2016:10 Pages 1173—1180
DOI https://doi.org/10.2147/DDDT.S96634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Cong Xue,1 Ying Tian,2 Jing Zhang,3 Yuanyuan Zhao,1 Jianhua Zhan,2 Wenfeng Fang,1 Li Zhang1
1Department of Medical Oncology, 2Department of Research, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 3Department of Medical Oncology, The First Affiliated Hospital of Guangzhou Traditional Chinese Medicine University, Guangzhou, People’s Republic of China
Purpose: BIBF 1120 is a potent triple angiokinase inhibitor now being evaluated in many types of tumors. We examine the antitumor effects of BIBF 1120 on nasopharyngeal carcinoma (NPC) in vitro and in vivo.
Materials and methods: The effect of BIBF 1120 on NPC cell proliferation was evaluated using the Cell Counting Kit 8 assay. The activities of BIBF 1120 as a single agent and in combination with cisplatin (DDP) in NPC tumor xenografts were evaluated by measuring microvessel density and expression of vascular endothelial growth factor signaling.
Results: BIBF 1120 exhibited limited inhibition of the growth of three NPC cell lines. Concurrent administration of BIBF 1120 and DDP provided greater antitumor effects compared to that observed with the use of either inhibitor as a single agent in the NPC xenograft model. Microvessel density and expression of vascular endothelial growth factor signaling were significantly reduced.
Conclusion: BIBF 1120, either as a single agent or in combination with DDP, demonstrates significant antitumor and antiangiogenic effects in the NPC xenograft model. Our results indicate that BIBF 1120 administered in conjunction with chemotherapy might provide an effective treatment method for NPC.
Keywords: BIBF 1120, nasopharyngeal carcinoma, antiangiogenesis, microvessel density
Introduction
Nasopharyngeal carcinoma (NPC) is the most common head and neck cancer in Southern China, where its incidence rate ranks the highest worldwide (20–30 per 100,000).1–3 In spite of the relatively high response rates to chemotherapy (~60%)4,5 and emerging new drugs, survival in advanced disease cases is poor (progression-free survival of 5–10 months and overall survival [OS] of 7–19 months).6–8 It is imperative to explore novel treatments, especially targeted therapies, for patients with advanced NPC.
The vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) system is a potential target for antiangiogenic therapy as angiogenesis constitutes an important pathway for NPC progression and metastasis. Overexpression of VEGF/VEGFR is observed in most patients with NPC and is related to an increased risk of metastasis and shortened OS,9–13 providing a rationale for investigating antiangiogenic agents for NPC.
A number of antiangiogenic inhibitors have been studied in clinical settings. However, acquired resistance was commonly observed in anti-VEGF/VEGFR treatments.14,15 Previous studies have revealed that the inhibition of the VEGF/VEGFR pathway results in the activation of alternative pathways to maintain neo-angiogenesis in the tumor. Fibroblast growth factor (FGF)/FGF receptor and platelet-derived growth factor (PDGF)/PDGF receptor pathways are acknowledged as the main pathways involved in the potential alternative mechanisms.16 Fernando et al17 found that tumor cells could escape angiogenesis inhibition by upregulating various pro-angiogenic factors, including FGF and PDGF. Multiple blockage of these escape mechanisms might delay the revascularization of the tumor and its progression.16
BIBF 1120, a structurally optimized oxindole derivative, competitively inhibits ATP binding to VEGFR2 and other angiogenic receptor tyrosine kinases. BIBF 1120 powerfully blocks VEGFR, PDGF receptor, and FGF receptor kinase activity in enzymatic assays (IC50 [half maximal inhibitory concentration] values were 13 nM, 59 nM, and 37 nM, respectively). Moreover, BIBF 1120 has been shown to inhibit the growth of various human tumor xenografts and prevent tumor angiogenesis in vivo in a dose-dependent manner. Pharmacokinetic studies in mice showed the highest and lowest plasma concentrations, of ~1,000 nmol/L and 8 nmol/L, at 1 hour and 24 hours postadministration, respectively.18–20 Results of a Phase III randomized clinical trial, Lume-Lung 1, revealed that progression-free survival of patients with non-small cell lung cancer who failed previous chemotherapy, when treated with docetaxel plus BIBF 1120, was significantly longer than that of patients treated with docetaxel alone (3.4 vs 2.7 months, P=0.0019). OS was improved from 6.3 months to 9.8 months in patients with adenocarcinoma. Due to its efficacy, BIBF 1120 plus docetaxel has been currently approved as a second-line treatment for advanced lung adenocarcinoma in Europe.21
Based on its novel characteristics, we assumed that BIBF 1120 would exhibit antiangiogenic activity in preclinical models of NPC. As cisplatin (DDP) is the foundational chemotherapy agent in treating NPC,4,5 we hypothesized that the combination of BIBF 1120 with DDP might show greater antitumor activity than when these two inhibitors are administered as single agents. In vivo experiments were performed to verify the earlier hypothesis.
Materials and methods
Cell lines
Three poorly differentiated human NPC cell lines were studied: HNE-1, CNE-2, and HONE-1. HNE-1 was a gift from Professor Kaitai Yao (Southern Medical University, Guangzhou, People’s Republic of China). CNE-2 and HONE-1 were gifts from Professor Musheng Zeng (Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China). Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 units/mL streptomycin at 37°C in a 5% CO2 humidified atmosphere. Logarithmically growing cells were used in the experiments. No ethics statement was required from the institutional review board for the use of these cell lines.
Drugs, chemicals, and antibodies
BIBF 1120 was kindly provided by Boehringer Ingelheim (Ingelheim, Germany). DDP (Hansoh Pharmaceuticals, Lianyungang, People’s Republic of China) was obtained as a commercial product from our hospital pharmacy. Captisol was obtained from CyDex Pharmaceuticals, Inc. (Lenexa, KS, USA). Fetal bovine serum was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies for VEGF, VEGFR1, and VEGFR2 were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Antibodies against CD31 were obtained from Beijing Zhongshan Jinqiao Biotechnology (Beijing, People’s Republic of China).
BIBF 1120 was dissolved in dimethylsulfoxide at a stock concentration of 10 mM, and stored at −20°C, diluting in culture medium just before use. For the in vivo study, BIBF 1120 was dissolved in 0.3% Captisol to a concentration of 10 mg/mL.
Proliferation assay
Tumor cells were cultured in 96-well plates at an appropriate density per well. Varying concentrations of BIBF 1120 were added to the cells 24 hours after plating and incubated for 72 hours, followed by Cell Counting Kit 8 (Dojindo, Tokyo, Japan) assay. The optical density was measured at 450 nm on an enzyme-linked immunosorbent assay reader (SpectraMax M5; Molecular Devices LLC, Sunnyvale, CA, USA). The IC50 value was defined as the concentration resulting in 50% lower cell growth compared to that in untreated control cells. The assay was repeated in triplicate in more than three independent experiments.
Tumor xenograft studies
Six- to eight-week-old male BALB/c nude mice, weighing ~16–18 g each, were supplied by Vital River Laboratory Animal Technology Co. Ltd (Beijing, People’s Republic of China). All animal experiments were conducted in accordance with “Guidelines for the Welfare of Animals in Experimental Neoplasia” and approved by the Experimental Animal Ethical Committee of Sun Yat-sen University. HNE-1 cells (1×107 cells resuspended in 0.2 mL of 0.9% sodium chloride solution, NaCl) were inoculated subcutaneously into the right flank of the nude mice. When tumors reached ~70 mm3 in volume, animals were randomized into four groups (n=8/group): BIBF 1120 group, DDP group, combination group, and control group. BIBF 1120 (10 mL/kg) was administered by oral gavage for 5 days a week. Intraperitoneal injection of DDP (10 mg/kg) was dosed with 0.9% NaCl every week. Captisol (10 mL/kg), by oral gavage for 5 days per week, and intraperitoneal injection of 0.9% NaCl every week were administered to the controls. The body weight of the mice and tumor sizes were measured and recorded every 3 days. Tumor volume was calculated using the equation volume (mm3) = length × width2 ×0.5. After 4 weeks of treatment, mice were euthanized, and tumors were harvested, fixed in 10% buffered formalin, and embedded in paraffin.
Immunohistochemical staining of VEGF-VEGFRs
Immunohistochemical (IHC) staining was performed on the formalin-fixed, paraffin-embedded tumor tissue sections. Pathological changes were examined by hematoxylin and eosin staining. The standard avidin–biotin complex peroxidase method was used for IHC detection.22 H-score was used as a measure of IHC. VEGF and VEGFR1/2 were localized in the cytoplasm and cell membrane, respectively. The extension was scored as percentage of positive cells (0%–100%), and the intensity of staining was assessed in comparison to that of a known external positive control (1, weak or below detection; 2, moderate; and 3, strong). H-score was calculated by multiplying the staining intensity and extension at each intensity level.
Microvessel density
Microvessel density (MVD) in the tumor stroma was measured to quantitatively assess angiogenesis. CD31 is usually used as an indicator of angiogenesis, as CD31 is a marker of undifferentiated endothelial cells, which are found in the new blood vessels of malignant tumors. High density of undifferentiated blood vessels indicates a worse prognosis.21 MVD was quantified by measuring the number of CD31-positive endothelial cells in the tumors. Five random fields per tumor sample, at 200 magnifications, were captured. MVD was reported as the average number for each group.23
Statistics
Statistical analysis was performed using SPSS Version 16.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation. Comparisons in the in vivo studies were analyzed by the unpaired t-test with Welch correction. Two-sided P<0.05 was considered statistically significant.
Results
Proliferation-inhibiting effects of BIBF 1120 in NPC cell lines
Three human NPC cell lines, CNE-2, HNE-1, and HONE-1, were treated with BIBF 1120 (0–10 μM) for 72 hours. BIBF 1120 significantly inhibited the growth of these three cell lines in a dose-dependent manner (Figure 1). The IC50 values for the effect of BIBF 1120 on HNE-1, CNE-2, and HONE-1 were 4.16±0.04 μM, 5.62±2.64 μM, and 6.32±1.18 μM, respectively. The percentage of inhibition is shown in Table S1.
Antitumor activity of BIBF 1120 in vivo
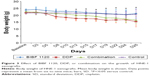
Since the IC50 value of BIBF 1120 was found to be the lowest for HNE-1 cells, we further evaluated the efficacy of BIBF 1120 in the HNE-1 xenograft model in nude mice, both as a single agent or in combination with cisplatin. The tumor sizes and weights were evaluated. As shown in Figure 2, BIBF 1120 demonstrated significant growth inhibition of NPC tumors in the HNE-1 xenograft model in nude mice as a single agent; the growth was significantly lower than that in the control group. At the same time, BIBF 1120 showed a greater inhibition of tumor growth in the human NPC cell line xenograft model in nude mice when combined with DDP than in the DDP single-agent group or the control group. Body weight reduction was observed in the DDP single-agent group and combination group, and temporary body weight reduction was observed in the BIBF 1120 single-agent group (Figure 3). No other obvious toxicity was observed in the mice.
BIBF 1120 decreased the expression of VEGF/VEGFRs in the NPC tumor xenograft model
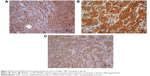
H-scores of VEGF were 107.5±30, 160±76.2, 106±43.4, and 207.5±65 in BIBF 1120, DDP, BIBF 1120 plus DDP, and control groups (P=0.059), respectively (Figure 4A). H-scores of VEGFR1 were 200±37.4, 165±38.7, 156±28.8, and 217.5±45 in BIBF 1120, DDP, BIBF 1120 plus DDP, and control groups (P=0.097), respectively (Figure 4B). H-score of VEGFR2 was 85±41.2, 145±50, 70±52, and 162.5±26.3 in BIBF 1120, DDP, BIBF 1120 plus DDP, and control groups (P=0.023), respectively (Figure 4C). We found that there was a strong tendency with regard to the expression of VEGF and VEGFR1, despite no statistical significance (P=0.059 for VEGF and 0.097 for VEGFR1). Results showed that BIBF 1120 resulted in significantly lower expression of VEGF/VEGFRs (especially for VEGFR2) in the single-agent group and in the combination group compared to that in the control group.
BIBF 1120 reduced MVD in NPC tumor xenograft model
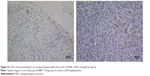
CD31 staining was used to evaluate MVD. MVDs were 17.9±2.13, 22.6±6.0, 16.7±1.6, and 21.1±3.0 in the BIBF 1120, DDP, BIBF 1120 plus DDP, and control groups (P=0.002), respectively (Figure 5). BIBF 1120 treatment resulted in significantly lower MVD in the HNE-1 xenograft model compared to that in the control group.
Discussion
The efficacy of five antiangiogenic agents including sorafenib, sunitinib, pazopanib, axitinib, and bevacizumab has been investigated in NPC.24–29 The administration of sorafenib, sunitinib, and pazopanib as single agents has been shown to be clinically efficacious in treating advanced NPC. The clinical benefit rates ranged from 28% to 54%, most of which reached stable disease, indicating that VEGFR inhibitors might show better efficacy when combined with chemotherapy or radiotherapy.25,26,28,30 A Phase II study of bevacizumab combined with chemoradiation revealed good efficacy in treating locally advanced NPC.27 A Phase II study of sorafenib combined with DDP and 5-fluorouracil showed that it was a tolerable and feasible regimen as a first-line therapy in advanced NPC.29 The earlier results indicate that the combination of an antiangiogenic agent with chemotherapy or radiotherapy is mostly safe and more efficacious than treatment with an inhibitor as a single agent.
This is the first preclinical study to investigate the efficacy of BIBF 1120 in NPC. We evaluated the growth inhibitory activity of BIBF 1120 as a single agent and in combination with DDP in human NPC cell lines and in a human NPC xenograft model. Results from the in vitro study showed that the IC50 values of BIBF 1120 ranged from 4 μM to 7 μM in three poorly differentiated human NPC cell lines. The data indicated that the efficacy of BIBF 1120 in inhibiting proliferation in NPC cell lines was not impressive. Although BIBF 1120 could inhibit NPC cell proliferation at high doses, the average steady-state maximum concentration (Cmax,ss) was 68.6 ng/mL (0.12 μM, molecular weight 539.62 g/L) in Phase I clinical trials.30 The IC50 values of BIBF 1120 for NPC could not be achieved in an in vivo study. As the target of antiangiogenic inhibitors is not the tumor cell itself, we surmised that BIBF 1120 might produce a more powerful effect in xenograft models.
The human NPC xenograft model demonstrated good sensitivity to BIBF 1120 treatment at 100 mg/kg similar to the results of previous studies in other tumor xenograft models.18–20 BIBF 1120 in combination with DDP resulted in a significant inhibition of tumor growth. However, weight loss was only observed in the combination group and DDP group. We attributed the weight loss to the toxicity of DDP, indicating a good tolerability of BIBF 1120 at 100 mg/kg repeat dosing in mice. Further studies are warranted to explore the best administration dosage of chemotherapy to fully utilize this potential synergistic effect of BIBF 1120, without increasing toxicity.
Furthermore, we demonstrated that BIBF 1120 resulted in significantly lower expression of VEGF/VEGFRs and MVD compared to that in the control, suggesting that antiangiogenesis represented a pivotal mechanism of action of BIBF 1120 in vivo. The usage of numerous biomarkers of antiangiogenesis remains controversial, including plasma proteins, angiogenesis signaling pathways, MVD, circulating endothelial progenitor/cells, and functional imaging technology.31,32 Among these, biomarkers of VEGF/VEGFR pathway have received particular attention, as they enable direct detection of antiangiogenic activity of VEGFR agents. We found that BIBF 1120 mainly inhibits VEGFR2, which was consistent with results from earlier studies.18,19 The observed MVD also confirmed the antiangiogenic activity of BIBF 1120, both in the single-agent group and in the combination groups and compared it to that in the mono-chemotherapy and control groups.
Conclusion
In conclusion, we showed that BIBF 1120 induces mild inhibition of proliferation in NPC cell lines and a moderate inhibitory effect on tumor growth in an NPC xenograft model. Moreover, data from the NPC xenograft model suggested that concurrent administration of BIBF 1120 with DDP might lead to significantly improved efficacy, but with higher toxicity attributable to DDP. With a modified chemotherapy dosage, we expect that BIBF 1120 plus chemotherapy might be an effective and tolerable regimen for the treatment of NPC.
Acknowledgments
Cong Xue and Ying Tian are joint first authors. This study was supported by the Chinese National Natural Science Foundation project (Grant Nos 81372502 and 81201917), the Specialized Research Fund for the Doctoral Program of Higher Education (20120171120116), the Young Teacher Training Program of Sun Yat-Sen University (14ykpy38), and the Outstanding Young Talent Cultivation Project of Sun Yat-Sen University Cancer Center (04140701).
Disclosure
The authors report no conflicts of interest in this work.
References
Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114–119. | ||
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer. 2014;33(8):381–387. | ||
Zhang LF, Li YH, Xie SH, et al. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chin J Cancer. 2015;34(8):350–357. | ||
Au E, Ang PT. A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 1994;5(1):87–89. | ||
Wang TL, Tan YO. Cisplatin and 5-fluorouracil continuous infusion for metastatic nasopharyngeal carcinoma. Ann Acad Med Singapore. 1991;20(5):601–603. | ||
Ma BB, Hui EP, Wong SC, et al. Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma – correlation with excision repair cross-complementing-1 polymorphisms. Ann Oncol. 2009;20(11):1854–1859. | ||
Ngan RK, Yiu HH, Lau WH, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol. 2002;13(8):1252–1258. | ||
Zhang L, Zhang Y, Huang PY, Xu F, Peng PJ, Guan ZZ. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol. 2008;61(1):33–38. | ||
Guang-Wu H, Sunagawa M, Jie-En L, et al. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110(12):2066–2069. | ||
Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8(8):2595–2604. | ||
Krishna SM, James S, Balaram P. Expression of VEGF as prognosticator in primary nasopharyngeal cancer and its relation to EBV status. Virus Res. 2006;115(1):85–90. | ||
Li YH, Hu CF, Shao Q, et al. Elevated expressions of survivin and VEGF protein are strong independent predictors of survival in advanced nasopharyngeal carcinoma. J Transl Med. 2008;6:1. | ||
Sha D, He YJ. [Expression and clinical significance of VEGF and its receptors Flt-1 and KDR in nasopharyngeal carcinoma]. Ai Zheng. 2006;25(2):229–234. Chinese. | ||
Rini BI. Sorafenib. Expert Opin Pharmacother. 2006;7(4):453–461. | ||
Rini BI. Sunitinib. Expert Opin Pharmacother. 2007;8(14):2359–2369. | ||
Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. | ||
Fernando NT, Koch M, Rothrock C, et al. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res. 2008;14(5):1529–1539. | ||
Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–4782. | ||
Kudo K, Arao T, Tanaka K, et al. Antitumor activity of BIBF 1120, a triple angiokinase inhibitor, and use of VEGFR2+pTyr+ peripheral blood leukocytes as a pharmacodynamic biomarker in vivo. Clin Cancer Res. 2011;17(6):1373–1381. | ||
Poindessous V, Ouaret D, El Ouadrani K, et al. EGFR- and VEGF(R)-targeted small molecules show synergistic activity in colorectal cancer models refractory to combinations of monoclonal antibodies. Clin Cancer Res. 2011;17(20):6522–6530. | ||
Reck M, Kaiser R, Mellemgaard A, et al; LUME-Lung 1 Study Group. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–155. | ||
Zhao YY, Xue C, Jiang W, et al. Predictive value of intratumoral microvascular density in patients with advanced non-small cell lung cancer receiving chemotherapy plus bevacizumab. J Thorac Oncol. 2012;7(1):71–75. | ||
Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147(1):9–19. | ||
Chinese University of Hong Kong. Nasopharyngeal Carcinoma (NPC) Axitinib. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01249547. NLM identifier: NCT01249547. Accessed February 12, 2016. | ||
Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–3773. | ||
Hui EP, Ma BB, King AD, et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann Oncol. 2011;22(6):1280–1287. | ||
Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13(2):172–180. | ||
Lim WT, Ng QS, Ivy P, et al. A phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res. 2011;17(16):5481–5489. | ||
Xue C, Huang Y, Huang PY, et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 2013;24(4):1055–1061. | ||
Zips D, Eicheler W, Geyer P, et al. Enhanced susceptibility of irradiated tumor vessels to vascular endothelial growth factor receptor tyrosine kinase inhibition. Cancer Res. 2005;65(12):5374–5379. | ||
Brown AP, Citrin DE, Camphausen KA. Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis Rev. 2008;27(3):415–434. | ||
Sessa C, Guibal A, Del Conte G, Rüegg C. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat Clin Pract Oncol. 2008;5(7):378–391. |
Supplementary material
  | Table S1 The percentage of inhibition of BIBF 1120 on nasopharyngeal carcinoma cell lines |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.