Back to Journals » Drug Design, Development and Therapy » Volume 11
Efficacy and tolerability of two different formulations of atorvastatin in Korean patients with hypercholesterolemia: a multicenter, prospective, randomized clinical trial
Authors Lee JH, Kim SH, Choi DJ , Tahk SJ, Yoon JH, Choi SW, Hong TJ, Kim HS
Received 7 May 2016
Accepted for publication 25 November 2016
Published 2 August 2017 Volume 2017:11 Pages 2277—2285
DOI https://doi.org/10.2147/DDDT.S112241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Sukesh Voruganti
Ju-Hee Lee,1,2 Sang-Hyun Kim,3,4 Dong-Ju Choi,3,5 Seung-Jea Tahk,6 Jung-Han Yoon,7 Si Wan Choi,8 Taek-Jong Hong,9 Hyo-Soo Kim3,10
1Division of Cardiology, Department of Internal Medicine, Chungbuk National University College of Medicine, 2Department of Cardiology, Chungbuk National University Hospital, Cheongju, 3Division of Cardiology, Department of Internal Medicine, Seoul National University College of Medicine, 4Department of Cardiology, Seoul Metropolitan Government – Seoul National University Boramae Medical Center, Seoul, 5Department of Cardiology, Seoul National University Bundang Hospital, Seongnam, 6Department of Cardiology, Ajou University Hospital, Suwon, 7Department of Cardiology, Yonsei University Wonju College of Medicine, Wonju Severance Christian Hospital, Wonju, 8Department of Cardiology, Chungnam National University Hospital, Daejeon, 9Department of Cardiology, Pusan National University Hospital, Pusan, 10Department of Cardiology, Seoul National University Hospital, Seoul, South Korea
Purpose: This study was designed to compare the efficacy and tolerability of the generic formulation (Atorva®) and the reference formulation (Lipitor®) of atorvastatin, both at a dosage of 20 mg once daily.
Methods: This study was a prospective open-label, randomized controlled study. Hypercholesterolemic patients who had not achieved low-density lipoprotein (LDL) cholesterol goals according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) guideline were randomized to generic formulation or reference formulation of atorvastatin. The primary end point was the percent change of blood LDL cholesterol at 8 weeks from the baseline. The secondary end points included the percent changes of total cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride (TG), apolipoprotein B (ApoB), and apolipoprotein A1 (ApoA1) levels, the percent changes of ApoB/ApoA1 and total cholesterol/HDL cholesterol ratios, and the change in high-sensitivity C-reactive protein (hsCRP) levels. The LDL cholesterol goal achievement rate according to the NCEP-ATP III guideline was also evaluated.
Results: Three hundred and seventy-six patients were randomized, and 346 patients (176 in the generic group and 170 in the reference group) completed the study. After the 8 weeks of treatment, LDL cholesterol level was significantly decreased in both the groups, and the decrement was comparable between the two groups (−43.9%±15.3% in the generic group, −43.3%±17.0% in the reference group, P=0.705). The percent changes of total cholesterol, HDL cholesterol, TG, ApoB, ApoA1, ApoB/ApoA1 ratio, total cholesterol/HDL cholesterol ratio, and hsCRP showed insignificant difference between the two groups. However, LDL cholesterol goal achievement rate was significantly higher in the generic group compared to the reference group (90.6% vs 83.0%, P=0.039) in per-protocol analysis. Adverse event rate was comparable between the two groups (12.0% vs 13.7%, P=0.804).
Conclusion: The generic formulation of atorvastatin 20 mg was not inferior to the reference formulation of atorvastatin 20 mg in the management of hypercholesterolemia.
Keywords: atorvastatin, hypercholesterolemia, low-density lipoprotein cholesterol
Introduction
Atherosclerosis is the main cause of cardiovascular diseases in the world.1 Although the pathogenesis of atherosclerotic disease depends on many factors,2 it is well established that elevated serum cholesterol levels and the risk of atherosclerotic disease are closely related.3–5 As the pivotal role of lowering plasma low-density lipoprotein (LDL) cholesterol levels in the prevention of adverse cardiovascular events is well demonstrated in many clinical trials,6–8 strict lipid-lowering therapy is now strongly recommended in patients with hyperlipidemia.7–10
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, the so-called statins, effectively reduce cholesterol biosynthesis in liver through the competitive inhibition of the reductase enzyme,11 and they are the first-choice drugs for lowering cholesterol in the current guidelines.12 Lipitor® (atorvastatin calcium trihydrate; Pfizer Inc., New York, NY, USA), one such HMG-CoA reductase inhibitor (Figure 1A), has been widely used to lower LDL cholesterol levels and has proven its effect on improving cardiovascular outcomes in many clinical trials.6,8,13,14 Atorva® (atorvastatin calcium anhydrous; Yuhan Corp., Seoul, Korea) is one of the new formulations of atorvastatin calcium for generic use (Figure 1B) and has been approved by the Korean Food and Drug Administration for the treatment of dyslipidemia based on bioequivalence study since 2008.
We conducted this study to compare the efficacy and tolerability of the generic formulation (Atorva®) and the reference formulation (Lipitor®) of atorvastatin in hypercholesterolemic patients who have not achieved LDL cholesterol goals.
Methods
Recruitment of patients
Patients were initially enrolled from eight university hospitals in Korea from March 2010 to January 2011. We included hypercholesterolemic patients aged between 20 and 79 years who had not achieved LDL cholesterol goals according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) guideline10 which recommends an LDL cholesterol goal of <100 mg/dL for patients with coronary heart disease (CHD) or CHD-equivalent disease (10-year CHD risk >20%), <130 mg/dL for patients with two or more risk factors (CHD risk ≤20%), and <160 mg/dL for patients with no or one risk factor.
Exclusion criteria were as follows: current use of any kind of antihyperlipidemic drug (within 4 weeks before enrollment); hypersensitivity or intolerance to atorvastatin or other HMG-CoA reductase inhibitor; newly diagnosed (within 3 months before enrollment) or uncontrolled diabetes (hemoglobin A1C >9%); uncontrolled hypertension (>160/100 mmHg); an unexplained elevation of serum creatinine kinase (CK) of more than two times the upper limit of normal (ULN), chronic kidney disease (a serum creatinine concentration of >2.5 mg/dL); elevated liver enzymes (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] levels two or more times the ULN); a major operation at the time of screening, with the result of lipid profiles after 24 hours or within 6 weeks of index operation; a history of malignancy or cervical dysplasia; pregnancy and lactation; childbearing age with the need to take adequate contraception; a history of drug abuse or alcoholism; and participation in other investigational studies within 4 weeks prior to screening.
Design of study
This was a multicenter, prospective, randomized, open-label, parallel-group study conducted to compare the efficacy and tolerability of generic formulation and reference formulation of atorvastatin, both at a dose of 20 mg once daily. The protocol is illustrated in Figure 2. All patients underwent a complete physical examination, history taking (eg, medical conditions, smoking status, and current medications), laboratory assessment, and 10-year risk assessment for CHD at the initial screening visit. Eligible patients underwent a therapeutic lifestyle modification for 4 weeks of pretrial run-in period. The patients who had not achieved their LDL cholesterol goals after 4 weeks of lifestyle modification were randomized to two groups in a 1:1 ratio by computerized allocation. The patients received 8-week treatment with generic formulation of atorvastatin 20 mg once daily or branded formulation of atorvastatin 20 mg once daily. The following medications were prohibited during the entire period of study: foods or medications affecting lipid profiles (eg, fish oil, phytosterol margarines, progestins, fiber-based laxatives, bile acid sequestrants, fibrates, niacin, systemic steroids, cholestine), medications affecting thyroid function (eg, antithyroid drugs or levothyroxine), and medications that can cause significant drug interaction with study drugs (eg, macrolide antibiotics, diltiazem, cyclosporine, niacin, azole antifungals, protease inhibitors, fibrate derivatives). The compliance was calculated from the difference in the number of tablets given and number of tablets returned to the pharmacist during the study period, divided by the number of tablets which the patient had to take, and then the number was multiplied by 100.
  | Figure 2 Study design. |
The protocol was approved by the Seoul National University IRB/ethics committee and each participating institution’s IRB and independent ethics committee also gave approval. Written informed consent was obtained from all patients (ClinicalTrials.gov registration number: NCT01624207).
Assessment of efficacy and tolerability
The primary end point of this study was the difference in percent change of serum LDL cholesterol concentration between generic atorvastatin and reference atorvastatin groups after 8 weeks of treatment. Secondary end points were the percent changes in other lipid profiles (total cholesterol, high-density lipoprotein [HDL] cholesterol, triglyceride [TG], apolipoprotein B [ApoB], and apolipoprotein A1 [ApoA1]) after 8 weeks of treatment. Percent changes of lipoprotein and apolipoprotein ratios, such as ApoB/ApoA1 ratio and total cholesterol/HDL cholesterol ratio, which reflect a balance between pro- and antiatherogenic properties and provide a reliable assessment of CHD risk15–19 and changes in high-sensitivity C-reactive protein (hsCRP) levels were also evaluated. After 8 weeks of treatment, LDL cholesterol goal achievement rate according to the NCEP-ATP III guideline10 was compared between the two groups as a secondary end point. Blood specimens were collected after a 12-hour fasting period for lipid analyses.
Tolerability was checked by self-reported adverse side effects, physical examination, and laboratory data. Any clinical event that occurred after taking the first dose of study drug was defined as a treatment-emergent adverse event (TEAE), and serious TEAE was reported separately during the entire study period. The investigators assessed the relationship between the TEAE and the study agent, and the intensity of TEAE. Safety assessments included laboratory data for blood chemistry (AST, ALT, CK, serum creatinine, blood urea nitrogen, and serum glucose), complete blood cell counts (white blood cell count with differential count, hemoglobin and hematocrit, and platelet count), and urinalysis. These tests were performed at the time of screening, randomization, and the end of the study (after 8 weeks of treatment).
Statistical analysis
The purpose of this study was to assess the percent change in serum LDL cholesterol from baseline to week 8 and to prove the non-inferiority of generic formulation of atorvastatin to branded formulation of atorvastatin. An estimated sample of 308 patients was calculated, assuming a power of 0.95 and an estimated standard deviation of 17%, to demonstrate the non-inferiority of generic form with a 7% of non-inferiority margin. Adjusting the sample size for a dropout rate of 20%, 384 patients (192 patients for each group) were required.
The efficacy was analyzed based on the full analysis (FA) set including all randomized patients taking at least one dose of study medication and having at least one efficacy assessment during the study. Confirmatory analysis was done with the per-protocol (PP) set including all participants in the FA set who completed this study without major violation of the study protocol (eg, violations of inclusion/exclusion criteria, use of prohibited foods or medications, and poor drug compliance [<80%]). Safety analysis was performed in the safety set with all patients taking at least one dose of study drug.
Independent sample t-test was used to calculate the mean percent changes in lipid profiles in the two groups from baseline to the end of the study. Other continuous variables were analyzed using the paired sample t-test for each group. Comparison of mean values for the continuous variables was performed with the independent sample t-test, and comparison of mean values for the categorical variables was done using χ2 tests or Fisher’s exact test. A P-value of <0.05 was considered statistically significant. SAS® version 8.2 software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Study patients and their baseline characteristics
After the screening of 397 patients, 376 patients were randomized to either generic atorvastatin group (n=191) or reference atorvastatin group (n=185). Among the randomized patients, 366 patients received the allocated intervention, and 346 patients completed the 8-week study (176 in the generic group, 170 in the reference group); seven patients in the generic group and two patients in the reference group withdrew informed consent, and eight patients in the generic group and 13 patients in the reference group were lost to follow-up and did not have any efficacy data (Figure 3). A total of 366 patients (183 in each group) who took at least one dose of study drug were included in the safety analysis.
  | Figure 3 Patient disposition by treatment group and analysis population. |
Mean age was 63.3±8.4 years, and 45.1% were males. Baseline LDL cholesterol level was 150.0±27.8 mg/dL. Overall drug compliance was 91.6%. The baseline characteristics of the two treatment groups showed no significant difference (Table 1).
Efficacy
Mean LDL cholesterol level at baseline was 148.0±31.2 mg/dL in the generic group and 153.0±23.9 mg/dL in the reference group (P=0.096, Table 2). The percent change of LDL cholesterol, the primary end point of the study, was decreased significantly in those two groups (−43.9%±15.3% in the generic group vs −43.3%±17.0% in the reference group, P=0.705). These reductions were similar between the two groups (Figure 4). The difference in percent changes of LDL cholesterol between generic and reference groups was 0.66%, favoring generic group, and the two-sided 95% confidence interval (−2.76% to 4.08%) was higher than the prespecified non-inferiority margin (−7%). In PP analysis, percent changes of LDL cholesterol were −44.6%±14.7% and −43.5%±16.7% (P=0.516) in the generic and reference groups, respectively, and also showed non-inferiority of the generic formulation.
Other lipid parameters including total cholesterol, TG, and ApoB were significantly reduced in both the groups, but these changes were not significantly different between the two groups (Table 2). Percent changes of HDL cholesterol and ApoA1 showed no significance. ApoB/ApoA1 and total cholesterol/HDL were decreased significantly in both the groups, but those changes were comparable between the two groups. The change of hsCRP was also comparable between the two groups.
After 8 weeks of treatment, LDL cholesterol goal achievement rate according to the NCEP-ATP III guideline10 was comparable between the two groups (89.2% in the generic group vs 82.4% in the reference group, P=0.068; Table 3). However, in the PP analysis, a significantly higher number of patients achieved the LDL cholesterol goal in the generic atorvastatin group, when compared with those in the reference atorvastatin group (90.6% vs 83.0%, P=0.039). This difference mainly comes from target LDL cholesterol <100 mg/dL group (93.1% vs 79.4% goal achievement rate, P=0.003), whereas target LDL cholesterol <130 mg/dL group (89.2% vs 91.3% goal achievement rate, P=0.746) and <160 mg/dL group (100% vs 100% goal achievement rate) showed no significant differences (P=1.000).
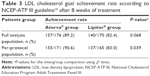  | Table 3 LDL cholesterol goal achievement rate according to NCEP-ATP III guideline10 after 8 weeks of treatment |
Tolerability
During the study period, TEAEs were reported by 22 patients (12.0%: 26 events) in the generic group and by 25 patients (13.7%: 29 events) in the reference group (P=0.639). TEAEs related to the study drug had occurred in nine patients (4.9%) in the generic group and eight patients (4.4%) in the reference group; the difference was not statistically significant (P=0.804). Three serious TEAEs were reported: one in the generic group (admission with benign prostate hyperplasia) and two in the reference group (admission with atrial fibrillation and death from cardiogenic shock due to myocardial ischemia). These serious TEAEs were not considered to be related to study drugs. The incidences of specific TEAEs are described in Table 4.
  | Table 4 Incidences of specific adverse events |
Discussion
This open-label, parallel-group, multicenter, prospective, randomized trial was designed to compare the efficacy and tolerability of the generic and branded formulation of atorvastatin in hypercholesterolemic patients who had not achieved LDL cholesterol goals. In the primary efficacy analysis, the generic atorvastatin was non-inferior to the branded atorvastatin regarding the percent change of LDL cholesterol from baseline to week 8, based on the prespecified non-inferiority margin of 7%, in both FA and PP populations. The percent reductions of LDL cholesterol, −43.9% and −43.3%, respectively, for the generic atorvastatin group and reference atorvastatin group, were compatible with results from previous studies.20–27
The changes of secondary end points including other lipid parameters and hsCRP were also comparable between the two groups. The 8-week LDL cholesterol target achievement rates recommended in the NCEP-ATP III guideline10 were 89.2% and 82.4% in FA population and 90.6% and 83.0% in PP population, respectively, in the generic and reference groups.
In PP analysis, the goal achievement rate was significantly higher in the generic group than in the reference group, and these rates were considerably higher in both the groups when compared to the previous studies.25,27,28
In a previous study comparing the efficacy and tolerability of another generic formulation of atorvastatin (Lipilou®; Chong Kun Dang Pharmacy Corp., Seoul, Korea) 20 mg once daily and branded formulation of atorvastatin 20 mg once daily,29 the percent reduction of LDL cholesterol (44% in generic formulation vs 46% in branded formulation) and the rates of achieving the LDL cholesterol targets (87.4% in generic formulation vs 84.5% in branded formulation) showed no statistically significant differences between the two groups; the absolute values were similar to the results in our study. However, the LDL cholesterol goal achievement rate was significantly higher in generic formulation according to PP analysis in our case.
Both treatments were well tolerated, and the adverse event profiles of generic group and reference group were similar. This is consistent with previous studies that have shown a favorable tolerability profile of atorvastatin in a broad range of patients.23,24,26 Of note, comparative improvements in lipid profiles including reduction of LDL cholesterol and higher LDL cholesterol goal achievement rate in the generic group were not associated with an increase in TEAEs. Thus, generic formulation of atorvastatin may be useful for the treatment of hypercholesterolemia in Korean patients.
Amid the increasing awareness of cost issues in health care, it is well established that generic substitution of statins can be cost-saving.30–32 The financial burden to individual patients can lead to poor medication adherence and may result in poorer health outcomes.33–35 Therefore, the use of generic statins seems logical and desirable if the same efficacy and safety of the branded formulations could be retained. In this study, we proved equal efficacy and tolerability between the generic and branded formulations of atorvastatin. Moreover, the generic atorvastatin showed significantly higher LDL cholesterol goal achievement rate than branded atorvastatin. These results support an evidence-based use of generic atorvastatin with substantial cost savings while maintaining the established efficacy and tolerability.30,34
This study has some limitations, and these need to be considered when evaluating the study results. 1) This was an open-label study. The open-label design could have caused bias in evaluation of efficacy results and assessment of adverse drug events. However, these potential biases could be reduced by selecting end points from laboratory tests. 2) Despite all our efforts to retain patients, the dropout and non-completion rate was relatively high (30 patients, 7.98%) in this study. However, because the adjusted sample size was calculated assuming a 20% dropout rate, this might not affect the results. 3) The long-term clinical outcomes were not evaluated in this study. Finally, because this study was not primarily powered to detect differences in safety profile, the comparable rates of TEAE in this study cannot exclude the possible undetected differences. Therefore, future post-marketing survey is warranted.
Conclusion
The generic formulation of atorvastatin 20 mg was not inferior to the branded formulation of atorvastatin 20 mg in this 8-week treatment of hyperlipidemic Korean patients. In PP analysis, the LDL cholesterol goal achievement rate was significantly higher in the generic atorvastatin group. Both formulations were well tolerated. Based on these results, generic formulation of atorvastatin might be suitable for the management of hyperlipidemia in Korea.
Acknowledgment
This research was financially supported by Yuhan Corp., Seoul, South Korea. The funding body had no role in the analysis and interpretation of the data.
Disclosure
The authors report no conflicts of interest in this work.
References
Kastelein JJ. The future of best practice. Atherosclerosis. 1999;143 Suppl 1:S17–S21. | ||
Badimon JJ, Fuster V, Chesebro JH, Badimon L. Coronary atherosclerosis. A multifactorial disease. Circulation. 1993;87(3 Suppl):II3–II16. | ||
Iso H, Jacobs DR Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320(14):904–910. | ||
Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971;74(1):1–12. | ||
Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham heart study. N Engl J Med. 1990;322(23):1635–1641. | ||
Sever PS, Dahlöf B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. | ||
Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation. 1998;97(15):1453–1460. | ||
Waters DD, Guyton JR, Herrington DM, McGowan MP, Wenger NK, Shear C; TNT Steering Committee Members and Investigators. Treating to New Targets (TNT) Study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? Am J Cardiol. 2004;93(2):154–158. | ||
Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. | ||
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. | ||
Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–1164. | ||
European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, et al; ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–1818. | ||
Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. | ||
Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus “usual” care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18(4):220–228. | ||
Ballantyne CM, Hoogeveen RC. Role of lipid and lipoprotein profiles in risk assessment and therapy. Am Heart J. 2003;146(2):227–233. | ||
Kastelein JJ, van der Steeg WA, Holme I, et al; TNT Study Group; IDEAL Study Group. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117(23):3002–3009. | ||
van der Steeg WA, Boekholdt SM, Stein EA, et al. Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: a case-control analysis in EPIC-Norfolk. Ann Intern Med. 2007;146(9):640–648. | ||
Parish S, Peto R, Palmer A, et al; International Studies of Infarct Survival Collaborators. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. 2009;30(17):2137–2146. | ||
Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med. 2004;255(2):188–205. | ||
Lee SH, Chung N, Kwan J, et al. Comparison of the efficacy and tolerability of pitavastatin and atorvastatin: an 8-week, multicenter, randomized, open-label, dose-titration study in Korean patients with hypercholesterolemia. Clin Ther. 2007;29(11):2365–2373. | ||
Wang KY, Ting CT. A randomized, double-blind, placebo-controlled, 8-week study to evaluate the efficacy and safety of once daily atorvastatin (10 mg) in patients with elevated LDL-cholesterol. Jpn Heart J. 2001;42(6):725–738. | ||
Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005;149(3):464–473. | ||
Olsson AG, Istad H, Luurila O, et al; Rosuvastatin Investigators Group. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. Am Heart J. 2002;144(6):1044–1051. | ||
Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol. 1998;81(5):582–587. | ||
Jones PH, Davidson MH, Stein EA, et al; STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152–160. | ||
Goldberg RB, Guyton JR, Mazzone T, et al. Ezetimibe/simvastatin vs atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia: the VYTAL study. Mayo Clin Proc. 2006;81(12):1579–1588. | ||
Cho YK, Hur SH, Han CD, et al. Comparison of ezetimibe/simvastatin 10/20 mg versus atorvastatin 20 mg in achieving a target low density lipoprotein-cholesterol goal for patients with very high risk. Korean Circ J. 2011;41(3):149–153. | ||
Shepherd J, Hunninghake DB, Barter P, McKenney JM, Hutchinson HG. Guidelines for lowering lipids to reduce coronary artery disease risk: a comparison of rosuvastatin with atorvastatin, pravastatin, and simvastatin for achieving lipid-lowering goals. Am J Cardiol. 2003;91(5A):11C–17C; discussion 17C–19C. | ||
Kim SH, Park K, Hong SJ, et al. Efficacy and tolerability of a generic and a branded formulation of atorvastatin 20 mg/d in hypercholesterolemic Korean adults at high risk for cardiovascular disease: a multicenter, prospective, randomized, double-blind, double-dummy clinical trial. Clin Ther. 2010;32(11):1896–1905. | ||
Jackevicius CA, Chou MM, Ross JS, Shah ND, Krumholz HM. Generic atorvastatin and health care costs. N Engl J Med. 2012;366(3):201–204. | ||
Gumbs PD, Verschuren WM, Souverein PC, et al. Society already achieves economic benefits from generic substitution but fails to do the same for therapeutic substitution. Br J Clin Pharmacol. 2007;64(5):680–685. | ||
Corp EV, Antoniou S, Wright PG, Khachi H, Vercaeren S, Wald DS. Use and cost of branded and generic drugs in patients with coronary heart disease – results from a prospective survey of 1008 patients in two London hospitals. QJM. 2009;102(12):843–849. | ||
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. | ||
Gill L, Santa J, Peter DL, Keehn J. Lipitor goes generic: business as usual or more big business? Ann Intern Med. 2012;156(12):892–893, W313. | ||
Helin-Salmivaara A, Korhonen MJ, Alanen T, Huupponen R. Impact of out-of-pocket expenses on discontinuation of statin therapy: a cohort study in Finland. J Clin Pharm Ther. 2012;37(1):58–64. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.