Back to Journals » Drug Design, Development and Therapy » Volume 9
Efficacy and safety of a herbal mixture (Viron® tablets) in the treatment of patients with chronic hepatitis C virus infection: a prospective, randomized, open-label, proof-of-concept study
Authors Shawkat H, Yakoot M , Shawkat T, Helmy S
Received 7 November 2014
Accepted for publication 3 December 2014
Published 11 February 2015 Volume 2015:9 Pages 799—804
DOI https://doi.org/10.2147/DDDT.S77168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shu-Feng Zhou
Video presented by Hisham Shawkat and Mostafa Yakoot.
Views: 566
Hisham Shawkat,1 Mostafa Yakoot,2 Tarek Shawkat,1,3 Sherine Helmy4
1KEMET Clinic, Cairo, Egypt; 2Green Clinic and Research Center, Alexandria, Egypt; 3Internal Medicine Department, Cape Canaveral Hospital, Cocoa Beach, FL, USA; 4Pharco Corporation, Alexandria, Egypt
Background: Development of an optimal interferon-free regimen for chronic hepatitis C virus infection is believed to require the combination of different drug classes to provide good antiviral efficacy, clinical and quality of life benefits, as well as a high barrier to resistance. Viron® is a new herbal drug in film-coated tablet form, and is based on a mixture of herbs with known hepatoprotective and antiviral properties. We conducted this study to explore the safety and the potential clinical and quality of life benefits of this product in patients with chronic hepatitis C infection.
Methods: Eighty-two consecutive patients presenting to our outpatient clinics as already-known or newly-diagnosed cases of chronic hepatitis C virus (HCV) infection, were entered into the study and randomized to three groups to receive escalating doses of Viron for 6 months. Virological, clinical, and enzyme responses, as well as quality of life index scores for chronic liver disease were compared between the groups.
Results: Of the 20 patients treated with the highest dose of Viron (three tablets twice daily), two (10%) had a complete virological response at the end of treatment (ETR) and two (10%) had a partial ETR, defined as a decrease in viral load of at least 2-log10 at the end of 6 months of treatment, whereas patients treated with the medium dose (two tablets twice daily) and the lowest dose (one tablet twice daily) showed a significantly lower ETR (P=0.043). Alanine aminotransferase levels and scores on the Chronic Liver Disease Questionnaire improved to a significantly greater extent in the highest dose group (P=0.007 and P=0.021, respectively). No serious adverse effects attributable to the herbal formulation were reported in any of the groups, apart from mild transient nausea, bloating, giddiness, and headache in two patients in the group receiving two tablets twice daily and in three patients in the group receiving three tablets twice daily.
Conclusion: We conclude that this herbal formulation is potentially safe and may offer some added clinical and quality of life benefits when used in the treatment of patients with chronic hepatitis C virus infection. Larger studies could be warranted to evaluate the effects of using this formulation as an add-on therapy to an all-oral combination of a directly acting antiviral drug protocol in the treatment of chronic hepatitis C.
Keywords: chronic hepatitis C virus, proof-of-concept study, safety, efficacy
Introduction
Hepatitis C virus (HCV) was identified in 1989 as a major cause of parenterally transmitted non-A non-B hepatitis. Chronic HCV infection is characterized by varying degrees of inflammation and hepatic fibrosis.1 The World Health Organization estimates that about 3% of the world’s population is infected with HCV and that there are more than 170 million chronically HCV-infected patients, with about 3–4 million persons newly infected each year.1–3 Prevalence rates vary widely, ranging from 0.15% in Scandinavia to about 15% in Egypt.4,5
In newly infected patients, chronic infection develops in about 80% of cases, with 10%–20% developing cirrhosis and 1%–5% developing liver cancer over a period of 20–30 years.5,6 Until the very recent discovery of direct-acting antiviral drugs, and up to the time of writing this report, the gold standard therapy for chronic HCV in Egypt (approximately 90% of which is genotype 4) is a combination of PEGylated-interferon alfa and ribavirin, which results in a sustained virological response rate in about 50%–60% of patients after 48 weeks of therapy.6–9
In addition to its several contraindications, poor tolerability, and fairly common adverse effects, the current interferon-based treatment for chronic HCV is inadequately effective and also expensive for most patients in developing countries.6,7
Patients with genotype 1 infection have a 42%–51% probability of achieving a sustained virological response after a 48-week course of therapy, while about 78%–82% of those infected with genotype 2 or 3, excluding those with a very high virus load, have been shown to respond to 24 weeks of treatment.10,11 One paper reported that about 13% of nonresponders to prior standard interferon-based therapy and 58.5% of patients who relapsed in general, responded to retreatment.12
Increased understanding of the lifecycle of HCV has resulted in the discovery of several targets for antiviral therapy, including the NS3/4A (protease), the NS5B (polymerase), NS5A, and HCV-hepatocyte cell entry mechanisms. The possibility of developing an interferon-free regimen for difficult-to-treat genotypes is believed to need a combination of different drug classes to provide high antiviral efficacy, good clinical and quality of life benefits, and a high barrier to resistance.
Herbal medicine is a popular complementary or alternative therapy in people with chronic liver disease. Many herbal products had been studied in vitro and in animal models, and been demonstrated to have hepatoprotective effects, and there have been reports of their clinical benefits in humans from many countries, from the Far East to the West.13–19 Glycyrrhizin extract, as an example, has been shown to have good effects in the treatment of chronic viral hepatitis.20–24 Turmeric (curcumin) was found to inhibit entry of HCV into human hepatocytes independent of genotype by affecting membrane fluidity, thus impeding virus binding and fusion.25 Curcumin was also found to decrease HCV gene expression via suppression of Akt-SREBP-1 activation.26
Eclipta alba extract was demonstrated to strongly inhibit the RNA-dependent RNA polymerase activity of HCV replicase. It effectively inhibited HCV replication, resulting in a reduced HCV RNA titer and lower translation levels of viral proteins.27 Further, treatment with Tinospora cordifolia extract was found to have immunomodulatory activity in many studies, and to protect the liver, as indicated by a significant reduction in serum levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin in CCl4-intoxicated rats.28,29
Viron® is a herbal drug manufactured by European Egyptian Pharmaceutical Industries (Alexandria, Egypt) as a film-coated tablet formulation. It is a mixture of herbs with known hepatoprotective and antiviral properties. Each 1 g film-coated tablet contains dried powdered T. cordifolia (whole plant) 200 mg, Glycyrrhiza glabra (root) 100 mg, Elettaria cardamomum (seeds) 200 mg, Curcuma longa(rhizome) 200 mg, E. alba (whole plant) 200 mg, and Rumex crispus (whole plant) 100 mg.
Viron has been studied for acute, subacute, and chronic toxicity in three species of laboratory animals. The results confirmed the absence of toxic, teratogenic, mutagenic, and carcinogenic effects or other adverse effects, including effects on fertility, after repeated administration in mice, rats, and dogs for 3 months at very high doses up to several times the recommended maximum dose for humans (El-Eshmawy, unpublished data, 2007). A small, unpublished pilot study in human volunteers with chronic HCV has shown improved clinical status, quality of life, and serum markers.
Our research group is committed to scientific evaluation of the benefits and risks of agents commonly used as complementary and alternative medicines. We have designed a common protocol to study the safety and efficacy of some of these botanical agents in the treatment of chronic HCV. This study was conducted according to the same study design, eligibility criteria, and outcome measures that we applied and described in our previously published research5 to explore the safety and any clinical or quality of life benefits offered by Viron tablets in patients with chronic HCV infection.
Materials and methods
This clinical trial was conducted according to a prospective, randomized, open-label, comparative design with a mean follow-up duration of 6 months. It was carried out in two outpatient clinics in Cairo and Alexandria, and in accordance with the recommendations of the Declaration of Helsinki and the International Conference on Harmonisation guidelines for good clinical practice. The protocol was approved by the local research ethics committee of the Green Clinic and Research Center, Alexandria, Egypt and all patients signed informed consents before any intervention. Eighty-two consecutive patients presenting to our outpatient clinics from May 2007 to March 2009 with documented chronic HCV infection or newly diagnosed HCV were entered into the study.
The inclusion criteria5 were: male or female sex; age 18–60 years; chronic HCV infection with a polymerase chain reaction-positive test with or without elevated liver enzymes; interferon-naïve (not previously treated with interferon alone or in combination with ribavirin therapy); relapse of HCV (patients with a transient biochemical or virological response to previous interferon or combined therapy); nonresponse (patients who did not respond to previous interferon or combined therapy). The exclusion criteria5 were: hepatitis B or other type of hepatitis; coinfection with human immunodeficiency virus or active schistosomiasis; alcohol or drug dependence or use during at least a year before the study; critical illness with severe hepatic decompensation or multiorgan failure; inability to swallow tablets or to comply with the study protocol for another reason; refusal to participate or sign informed consent; and pregnancy or lactation.
Eligibility criteria were verified from the patient history, clinical examination, and the following confirmatory tests: detection of HCV-RNA by qualitative and quantitative polymerase chain reaction measurements; screening test for HBV surface antigen, schistosome antigens, and human immunodeficiency virus by enzyme-linked immunosorbent assay; upper abdominal and liver ultrasonography; and liver and kidney function tests, a pregnancy test, and complete blood count.
Patients fulfilling the inclusion/exclusion criteria were enrolled in the study and randomly allocated to 6 months of treatment with Viron one tablet twice daily (group 1), two tablets twice daily (group 2), or three tablets twice daily (group 3). All patients were followed up monthly with a physical examination, assessment of quality of life (Chronic Liver Disease Questionnaire [CLDQ] score), hematological studies, hepatic and renal function tests, and ultrasonography of the liver. Quantitative polymerase chain reaction RNA tests were performed at 3 and 6 months.
Endpoints
The study endpoints were:
- Early and end of treatment virological response (ETR; defined as complete [loss of detectable HCV-RNA] or partial [>2 log virus load reduction]) at months 3 and 6.
- Biochemical response (normalization or significant reduction of transaminases) at the end of treatment.
- Improvement of health-related quality of life scores using the CLDQ developed by Younossi et al.30,31 The CLDQ includes 29 items in the following domains: abdominal symptoms, fatigue, systemic symptoms, activity, emotional function, and worry. Responses on the CLDQ were graded on a scale of 1 (“all of the time” [worst]) to 7 (“none of the time” [best]). The change in the final CLDQ overall score from baseline was compared. The CLDQ overall score is calculated by dividing the total score by the total number of items (29).30,31
- Incidence of adverse events and toxicity.5
Statistical analysis
The data were analyzed using Statistical Package for the Social Sciences version 12 software (SPSS Inc, Chicago, IL, USA). Paired quantitative variables were compared before and after using the paired-samples t-test. The three independent treatment groups were compared by one-way analysis of variance, Tukey’s test for post hoc analysis, and analysis of covariance for adjustment for covariates. The Chi-squared/Fisher’s Exact tests were used for analysis of categorical variables.
Results and discussion
Sixty consecutive eligible assessable patients were included in the study analysis. Eligible patients were randomly allocated to three treatment groups using a block randomization technique. Group 1 received a low dose of Viron (one tablet twice daily), group 2 received a medium dose (two tablets twice daily), and group 3 received a high dose (three tablets twice daily). All patients were followed up for 6 months. Figure 1 shows the patient disposition from screening to analysis (Figure 1). Our variables were mostly quantitative, so the analysis was performed on a per protocol basis, given the small and balanced number of patients who dropped-out from each group. Baseline characteristics were comparable between the three groups (Table 1).
  | Figure 1 Patient flow chart. |
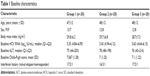  | Table 1 Baseline characteristics |
Among the 20 patients who completed 6 months of treatment with the high dose of Viron, two (10%) had a complete ETR, and two (10%) had a partial ETR. No virological response (no ≥2-log10 reduction of baseline virus load) was reported in the remaining 80% in group 3 (Table 2). Among the 20 patients who completed the full protocol with the medium dose, only one (5%) had a complete ETR. No patient in the group treated with the low dose showed a complete or partial ETR. There was a statistically significant linear association (P=0.043) between the higher dose and virological response.
  | Table 2 Virological response at the end of 6 months of treatment according to Viron® dose strength |
Alanine aminotransferase levels decreased in group 3 (the high dose) at the end of 6 months of treatment by a mean −30.85 U/L (19.09) from baseline. This decrease was significantly greater than that in groups 2 and 3 (F=5.74, P=0.007, Table 3).
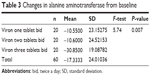  | Table 3 Changes in alanine aminotransferase from baseline |
Health-related quality of life, as measured by the CLDQ score, was improved significantly only in the high dose group at the end of 6 months (P<0.001, paired-sample t-test). The mean difference in scores from baseline was found to be significantly greater in the high dose group than in the low dose group (F=4.142, P=0.021, one-way analysis of variance, Table 4, and Tukey’s post hoc analysis, Table 5). This significant improvement in CLDQ score was held true even after adjusting for the effect of both the baseline CLDQ score and the Child–Pugh score as two covariates by an ANCOVA model (F=2.9, P=0.029, Table 6).
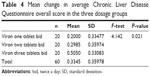  | Table 4 Mean change in average Chronic Liver Disease Questionnaire overall score in the three dosage groups |
  | Table 5 Tukey’s honestly significant differencea |
No serious adverse effects attributable to therapy were reported in any of the three groups, apart from mild transient nausea, bloating, giddiness, and headache in two patients in the medium dose group and in three patients in the high dose group.
Conclusion
We conclude that this herbal formula is potentially safe, and may offer some added clinical and quality of life benefits when used in the treatment of patients with chronic HCV infection. Further larger studies may be justified to evaluate the effects of this product as an add-on therapy to an all-oral combination of a directly acting antiviral drug protocol in the treatment of chronic HCV infection.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Hepatitis C. Fact sheet 164. Available from:http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed July 3, 2014. | ||
Patel K, Muir AJ, McHutchison JG. Diagnosis and treatment of chronic hepatitis C infection. BMJ. 2006;332:1013–1017. | ||
[No authors listed]. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. | ||
Sievert W, Altraif I, Razavi HA, et al. systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31:61–80. | ||
Yakoot M, Salem A. Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterol. 2012;12:32. | ||
Egyptian Ministry of Health. [Annual Report 2007]. Available from:http://www.mohp.gov.eg/sites/minister/Publications/Disindex.aspx. Accessed May 2, 2012. Arabic. | ||
El-Zayadi AR, Attia M, Barakat EM, et al. Response of hepatitis C genotype-4 naïve patients to 24 weeks of PEG-interferon-alpha2b/ribavirin or induction-dose interferon-alpha2b/ribavirin/amantadine: a non-randomized controlled study. Am J Gastroenterol. 2005;100:2447–2452. | ||
Kamal SM, Nasser IA. Hepatitis C genotype 4: what we know and what we don’t yet know. Hepatology. 2008;47:1371–1383. | ||
Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. | ||
Cacoub P, Ouzan D, Melin P, et al. Patient education improves adherence to peg-interferon and ribavirin in chronic genotype 2 or 3 hepatitis C virus infection: a prospective, real-life, observational study. World J Gastroenterol. 2008;14:6195–6203. | ||
Zeuzem S, Hultcrantz R, Bourliere M, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–999. | ||
Moucari R, Ripault MP, Oules V, et al. High predictive value of early viral kinetics in retreatment with peginterferon and ribavirin of chronic hepatitis C patients non-responders to standard combination therapy. J Hepatol. 2007;46:596–604. | ||
Luper S. A review of plants used in the treatment of liver disease: Part 1. Altern Med Rev. 1998;6:410–421. | ||
Qin XK, Han M, Liu JP. [Compound Chinese herbal medicines, Chinese herbal drugs and their active extracts for treatment of chronic hepatitis C: a systematic review and meta-analysis of randomized clinical trials]. Zhong Xi Yi Jie He Xue Bao. 2009;7:913–928. Chinese. | ||
Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. | ||
Deng D. [Thirty cases of hepatitis C treated with Song Zhi Mixture]. Hunan Journal of Traditional Chinese Medicine. 1997;13:27–28. Chinese. | ||
Li H, et al. [Qingtui Fang applied in treating 128 cases of chronic hepatitis C]. Chinese Journal of Integrated Traditional and Western Medicine for Liver Diseases. 1994;4:40. Chinese. | ||
Wu C, et al. [Thirty-three patients with hepatitis C treated by Chinese traditional medicine syndrome differentiation]. Chinese Journal of Integrated Traditional and Western Medicine for Liver Diseases. 1994;4:44–45. Chinese. | ||
Cohen MR. Herbal and complementary and alternative medicine therapies for liver disease. A focus on Chinese traditional medicine in hepatitis C virus. Clin Liver Dis. 2001;5:461–478. | ||
Matsunami H, Lynch SV, Balderson GA, Strong RW. Use of glycyrrhizin for recurrence of hepatitis B after liver transplantation. Am J Gastroenterol. 1993;88:152–153. | ||
Suzuki H, Ohta Y, Takino T, et al. Effects of glycyrrhizin on biochemical tests in patients with chronic hepatitis: double blind trial. Asian Med J. 1984;26:423–438. | ||
Matsumoto Y, Matsuura T, Aoyagi H, et al. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One. 2013;8:e68992. | ||
Manns MP, Wedemeyer H, Singer A, et al; European SNMC Study Group. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks. J Viral Hepat. 2012;19:537–546. | ||
Ashfaq UA, Masoud MS, Nawaz Z, Riazuddin S. Glycyrrhizin as antiviral agent against hepatitis C virus. J Transl Med. 2011;9:112. | ||
Anggakusuma, Colpitts CC, Schang LM, et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137–1149. | ||
Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N, Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707–712. | ||
Manvar D, Mishra M, Kumar S, Pandey VN. Identification and evaluation of antihepatitis C virus phytochemicals from Eclipta alba. J Ethnopharmacol. 2012;144:545–554. | ||
Aher V, Kumar Wahi A. Biotechnological approach to evaluate the immunomodulatory activity of ethanolic extract of Tinospora cordifolia stem. Iran J Pharm Res. 2012;11:863–872. | ||
Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–146. | ||
Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. | ||
Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96:579–583. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

