Back to Journals » Drug Design, Development and Therapy » Volume 14
Effects of Saikosaponin D on CYP1A2 and CYP2D6 in HepaRG Cells
Authors Li H, Tang Y, Wang Y, Wei W, Yin C, Tang F
Received 18 June 2020
Accepted for publication 3 November 2020
Published 26 November 2020 Volume 2020:14 Pages 5251—5258
DOI https://doi.org/10.2147/DDDT.S268358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Tuo Deng
Hongfang Li,1– 3,* Yunyan Tang,1,4,* Yang Wang,1– 3 Weipeng Wei,1– 3 Chengchen Yin,1– 3 Fushang Tang1– 3
1Department of Clinical Pharmacy, Key Laboratory of Basic Pharmacology of Guizhou Province and School of Pharmacy, Zunyi Medical University, Zunyi 563000, People’s Republic of China; 2Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi 563000, People’s Republic of China; 3Key Laboratory of Clinical Pharmacy of Zunyi City, Zunyi Medical University, Zunyi 563000, People’s Republic of China; 4Department of Pharmacy, Meitan People’s Hospital, Zunyi 564100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fushang Tang
Department of Clinical Pharmacy, Key Laboratory of Basic Pharmacology of Guizhou Province and School of Pharmacy, Zunyi 563000, People’s Republic of China
Tel +86 851 2864 2337
Fax +86 851 2864 2334
Email [email protected]
Background: Bupleurum is one of the most important traditional Chinese medicines and an ingredient in many compound preparations. It is widely used together with other drugs in clinical practice, and thus there is great potential for drug–drug interactions. Saikosaponin D (SsD) is a major bioactive triterpenoid saponin extracted from Bupleurum with anti-inflammatory, anticancer, antioxidative, and antihepatic fibrosis effects. Effects of the main components of Bupleurum on cytochromes P450 (CYPs) need to be clarified in the clinical application of combination therapies of formulations containing SsD or Bupleurum.
Purpose: This study aimed to investigate the effects of SsD on the CYP1A2 and CYP2D6 mRNAs, protein expression, and relative enzyme activities in HepaRG cells.
Methods: HepaRG cells were cultured with SsD at concentrations of 0.5, 1, 5 and 10 μM for 72 hours. mRNA and protein expression of CYP1A2 and CYP2D6 were analyzed with real-time PCR and Western blot analysis. Relative enzyme activities were analyzed with HPLC based on consumption of the specific probe substrate.
Results: SsD significantly induced expression of mRNA and increased relative activity of CYP1A2 in HepaRG cells after the cells had been treated with SsD at concentrations of 1, 5 and 10 μM. SsD also induced protein expression of CYP1A2 at concentrations of 5 and 10 μM. SsD exhibited an inductive effect on CYP2D6 mRNA and protein expression, while increasing the relative activity of CYP2D6 at concentrations of 5 and 10 μM.
Conclusion: This study is the first to investigate the effect of SsD on CYP1A2 and CYP2D6 in HepaRG cells, and the results may provide some useful information on potential drug–drug interactions related to clinical preparations containing SsD or Bupleurum.
Keywords: saikosaponin D, HepaRG cells, drug–drug interactions
Introduction
Traditional Chinese medicines (TCMs) are widely administered concomitantly with Western therapeutic drugs for the treatment of major ailments, due to their easy availability, cost-effectiveness, and a general perception that they are safer than Western drugs. Their versatile prevention and treatment effects are combined with Western drugs for various ailments.1,2 However, drug interactions between TCM and other drugs occur from time to time, due to the complex components of TCM. Bupleurum is a perennial herbaceous plant of the family Umbelliferae that is found in some regions of China (mainly in the provinces of Liaoning, Jiangsu, and Anhui) and other Asian countries (like Japan and South Korea), and has been used to treat fever, tumor, and inflammation diseases.3 It is also one of the most typical TCM ingredients in a variety of clinical preparations approved by the Chinese National Medical Products Administration.4 Saikosaponin D (SsD) is a major triterpenoid saponin extracted from Bupleurum, and its chemical structure is shown in Figure 1. With its relatively high content and strongest activity in Bupleurum,5 SsD has been proven by many studies to have antitumor,6 anti-inflammatory,7 anti–liver damage, and anti-infection effects.4,8 As such, it is a candidate drug worthy of being developed into clinical preparations. The development of SsA–SsD compound liposomes9 and SsD liposomes10 has been reported. However, it is interesting that though SsD is the main bioactive component of Bupleurum, its effect on cytochromes P450 (CYPs) has rarely been investigated.
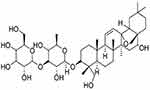 |
Figure 1 Chemical structure of SsD (molecular formula C42 O13 H68). |
The cytochrome P450 family mediates metabolism of the majority of important drugs,11,12 and most drug interactions are related to CYPs.13 At present, CYPs and its isoforms assays are currently used for the screening and metabolism research of new drugs candidates in Europe and the United States, and these are compulsory experiments to be performed for the development of new drug.14
In research by Gripon et al, HepaRG cells were isolated from the tissue of a female patient suffering from hepatocarcinoma in 2002. The cells were then differentiated into canaliculus-like and hepatocyte-like cells in the presence of 2% dimethyl sulfoxide (DMSO) and enzyme expression of CYP1A2, 2D6, 2B6, 2C9, and 2E1 examined for drug metabolism and drug interaction.15 CYP1A2 in HepaRG cells can be induced by omeprazole,16 and metoprolol can be used to evaluate the activity of CYP2D6 in HepaRG cells.17 HepaRG cells are widely considered a suitable model for studying drug metabolism and drug–drug interactions due to their gene expression of cellular function consistent with primary human hepatocytes.18,19,20 We aimed to investigate the effect of SsD on mRNA and protein expression, as well as the enzyme activity of CYP1A2 and CYP2D6, in HepaRG cells. We believe that the results of this study will provide more evidence for drug interactions related to clinical formulations containing SsD or Bupleurum.
Methods
Reagents and Cells
HepaRG cell lines were supplied by Guandao Biological Engineering (Shanghai, China). RPMI 1640 medium and FBS were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Trypsin–EDTA 0.25% solution and 1% antibiotics (100 × streptomycin–penicillin) were purchased from Biosharp (Shanghai, China) and HyClone (Logan, UT, USA), respectively. SsD (S114050, 20 mg, ≥98% purity), phenacetin, and dextromethorphan hydrobromide were purchased from Aladdin Bio-Chem Technology (Shanghai, China). MTT, PBS, and DMSO were provided by Solarbio Technology (Beijing, China). GAPDH rabbit polyclonal antibody, CYP1A2-specific rabbit polyclonal antibody, HRP-conjugated AffiniPure goat antirabbit IgG (H+L), and CYP2D6 rabbit polyclonal antibody were obtained from Proteintech (Wuhan, China).
Cell Culture
Undifferentiated HepaRG cells were cultured in RPMI 1640 medium containing 10% FBS, 1% antibiotics (100 × streptomycin–penicillin), and 50 µM hydrocortisone sodium hemisuccinate (basal growth medium) and incubated at a constant 37°C, 5% CO2, and 95% humidity for 2 weeks. Then, they were incubated in the same culture medium supplemented with 2% DMSO (differentiation medium) for 2 weeks,15 and then were differentiated into canaliculus-like and hepatocyte-like cells expressing liver-specific functions. Next, differentiated HepaRG cells were seeded at a density of 4.5×105 cells/cm2 in 96-well plates or six-well plates for follow-up research.15,21 The medium was renewed every 2–3 days.
In Vitro Cell-Viability Assays
Evaluation of SsD activity in HepaRG cells was undertaken to find a suitable range of concentration for studying drug–drug interaction. Differentiated HepaRG cells were seeded in 96-well plates, incubated for 24 hours, treated with different concentrations of SsD (0.1, 1, 10, 20, 30, and 40 µM) and incubated for 72 hours at 37°C. Meanwhile, untreated cells incubated in medium and the medium without cells were used as the control and the blank, respectively. After incubation, 20 µL MTT solution (5 mg/mL) prepared in RPMI 1640 medium (without FBS, antibiotics, or hydrocortisone sodium hemisuccinate) was added to each well. After 4 hours’ incubation, the MTT containing medium was removed and DMSO (150 µL/well) added to dissolve the formazan crystals. The absorbance of each well was detected with a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 490 nm.
Real-Time Polymerase Chain–Reaction Quantification
Differentiated HepaRG cells were seeded in six-well plates, incubated for 24 hours, treated with different concentrations of SsD (0, 0.5, 1, 5 and 10 µM [this range was chosen based on MTT results to achieve cell viability for research needs]) and incubated for 72 hours at 37°C. Total RNA was acquired from HepaRG cells using RNAiso Plus reagent according to the manufacturer’s instructions, and total RNA concentration was quantified by absorbance at a wavelength of 260 nm. Total RNA (500 ng) was reverse-transcribed using the PrimeScript RT reagent kit (perfect real-time), and cDNA obtained from reverse transcription was diluted ten times with RNase-free distilled water. TB Green Premix Ex Taq II (TIi RNaseH Plus) was used for quantification. Primers sequences of related genes are shown in Table 1.
 |
Table 1 Primer Sequences for Real-time RT-PCR |
Western Blot Assays
The effect of SSd on the protein expression of CYPs in HepaRG cells was determined by Western blot analysis. Differentiated HepaRG cells were seeded, incubated and treated as per the the previous section. Total protein was extracted from HepaRG cells using RIPA cell-lysis buffer containing protease and phosphatase inhibitors on the basis of instructions.
After protein concentration had been measured using the BCA assay, proteins were loaded on sodium dodecyl sulfate–polyacrylamide gels, transferred onto a polyvinylidene fluoride membranes, and blocked with 5% skimmed milk for 2 hours (phosphorylated protein was incubated with 5% BSA). The primary antibodies GAPDH (1:5,000), CYP1A2 (1:2,000), and CYP2D6 (1:1,000) were incubated at 4°C overnight. The secondary antibody HRP-conjugated AffiniPure goat antirabbit IgG (H+L, 1:5,000) was incubated at room temperature for 1 hour. Membranes were washed three times with TBST. Chemiluminescent detection was performed using ECL reagents, and Image Lab Analysis software was used for quantitative analysis of the blots.
Detection of Enzyme Activity
Relative enzyme activity was reflected in the percentage of phenacetin (CYP1A2) and dextromethorphan hydrobromide (CYP2D6) consumption. Previous studies have frequently used 50 μM phenacetin and dextromethorphan for CYP1A2 and CYP2D6 enzyme-activity assays.22,23 As such, differentiated HepaRG cells were treated the same as outlined in the “Real-time polymerase chain–reaction quantification” section. Later, serum-free medium containing 50 µM phenacetin and dextromethorphan hydrobromide was added and incubated for 12 hours at 37°C. After that, reactions were stopped on ice and cell supernatant (medium) samples stored at −80°C before determining concentrations of phenacetin and dextromethorphan hydrobromide by HPLC. The HPLC method used was adapted and optimized from a previously established method.24,25 Briefly, chromatographic analysis was performed using an HPLC-DAD system (Agilent 1260 Infinity), and chromatographic separation was achieved on a 150×4.6 mm, 5 μm particle Agilent Extend C18 column at 25°C.
Statistical Analysis
SPSS 20.0 was used for statistical analyses, and results are expressed as means ± SD (n=3). Differences were analyzed with one-way ANOVA or unpaired Student’s t-test, and P<0.05 was considered statistically significant.
Results
Cell-Viability Tests
The effect of SsD on the viability of HepaRG cells was tested mainly to determine the appropriate concentration without significant toxicity to the cells for subsequent drug-interaction studies. The results of in vitro cell-viability assays shown in Figure 2 suggested that SsD had no significant effects on cell viability at a dose <10 µM. SsD concentrations of ≤10 µM were used for subsequent drug-interaction studies.
 |
Figure 2 Cytotoxicity assays of SsD after 72 hours of incubation in HepaRG cells. Data expressed as means ± SD (n=3). **p<0.01 compared with control. |
Effects of SsD on Expression Levels of CYP mRNA
Real-time PCR assays were implemented to detect whether SsD were able to induce or inhibit CYPs expression at the mRNA level in HepaRG cells with the treatment of SsD. As illustrated in Figure 3, compared with the control, SsD at concentrations of 1, 5, and 10 µM significantly induced CYP1A2 mRNA expression in a concentration-dependent manner (Figure 3A), while CYP2D6 was observed to be markedly induced by SsD at concentrations of 5 and 10 µM (Figure 3B).
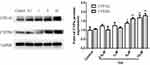
Effects of SsD on Protein Expression of CYPs
Protein expression of CYPs in HepaRG cells treated with SsD for 72 hours is shown in Figure 4. The results demonstrated that treatment of HepaRG cells with SsD significantly upregulated protein-expression levels of CYP1A2 and CYP2D6 at concentrations of 5 and 10 µM compared with the control.
Effects of SsD on Relative Activity of CYPs
As demonstrated in Figure 5, the effect of SsD on the relative enzyme activity of CYP1A2 and CYP2D6 in HepaRG cells was consistent with the trend of mRNA and protein expression. In brief, it significantly induced relative activity of CYP1A2 and CYP2D6 at 1, 5, 10 µM and 5 and 10 µM, respectively compared with the control.
Discussion
It has been reported that CYPs are involved in >90% of clinical drug metabolism26 and are one of the major indicators in predicting drug–drug interactions recommended by the FDA.27 Directly and indirectly, TCM affects the efficacy and safety of other drugs through drug–drug interactions because a large number of active ingredients in TCM are metabolic substrates of CYPs.28 Bupleurum is the dry root of Bupleurum chinense (Apiaceae) or Bupleurum scorzonerifolium according to the Chinese Pharmacopoeia, and has been used clinically for >2,000 years to treat fever, hepatitis, and inflammation in China, Japan, South Korea, and other Asian countries.29 So far, 43 kinds of saikosaponins have been isolated and identified from Bupleurum, which has the highest content of SsA, SsC, and SsD, among which SsD is considered the most bioactive component,8 with multiple pharmacological effects of anti-inflammation, antioxidation, and liver protection.30 Formulations containing Bupleurum have been widely used in clinical practice, but their effects on CYPs have not been generally studied.
Though rodents and monkeys have been used as experimental models to study drug metabolism for many years, the application of animal models in this area is limited. The reason for the limited drug-metabolism information from animal experimental models may be the limited reference value of the results due to species differences, as well as concerns related to animal protection and ethics. As such, the application of animal models, such as dogs and monkeys, are increasingly restricted in medical research.31 Although various models for the study of drug metabolism or drug–drug interactions have been developed, these models, including animals, primary human hepatocytes, and HepG2 and Huh7 cells have various limitations. Specifically, there are species differences from humans when animal experimental models are applied in this area, frequently resulting in failure to predict drug–drug interactions.32 HepG2 and Huh7 cells only express relatively low functions of human liver–specific CYPs.21,33,34 Primary human hepatocytes are considered the gold standard of in vitro for drug-metabolism studies, but difficulty in culture, poor stability of functions in culture, and differences among donors limit their application as an ideal model for in vitro drug-metabolism studies,35,36 while the HepaRG cell line can exhibit CYP activities (CYP3A4, 1A2, 2D6, 2C9, 2E1) free of the disadvantages of difficulty in culture and unstable functional expression in primary human hepatocytes.37 As such, HepaRG cell is a better and promising model for in vitro drug-metabolism and drug–drug interaction research. In the present study, HepaRG cells were used to determine the effect of SsD on mRNA and protein expression of major CYPs to understand drug interactions related to clinical formulations containing SsD or Bupleurum.
Notably, we found that SsD significantly induced mRNA and protein expression of CYP1A2 and CYP2D6 in HepaRG cells in a concentration-dependent manner (Figures 3 and 4). Their relative enzyme activity was also induced, as shown in Figure 5. A previous study demonstrated Bupleurum and vinegar-baked Bupleurum had strong induction effects on CYP2D6 in rats,38 while our results indicated that SsD plays an important role in the induction effect of CYP2D6, which may be one of the imperative factors for Bupleurum and vinegar-baked Bupleurum in that it can induce the CYP2D6 enzyme. For CYP1A2, SsD has been reported to have a slight inhibition effect as a compromise to its induction effect in rat-liver microsomes,39 while our study indicated that SsD can significantly induce CYP1A2, probably owing to the fact that when the activity of CYP enzymes was measured through microsomal in vitro incubation, the actual concentration of substrate available to them depends on processes missing in subcellular models, covering transport mechanisms, cytosolic enzymes, and intracellular protein binding, while intact cells can better simulate the disposal environment of drugs in the liver.40 Disadvantages of the liver microsomal drug-metabolism model in vitro include but are not limited to destruction of the complete structure during the preparation process and aspecific reactions being more likely to occur in the in vitro incubation system and the lack of a complete enzyme-reaction system being required for metabolism, which needs to have the right amount of NADPH added. Some drug-metabolizing enzymes are removed during the preparation process, such as the metabolic enzymes located in the cytoplasm. Also, screening of CYP inducers cannot be done in microsomes, as it requires a cellular system fully capable of expressing CYP genes.34,40 Further research is required. Bupleurum contains a complex mixture of components (such as flavonoids, phenyl propanol derivatives, triterpenoid saponins, and volatile oils), so to highlight the necessity of determining which ingredients are responsible for the effect on 1A2 and 2D6 enzymes is indispensable.
It is interesting that the mRNA level of CYP1A2 (~80-fold, Figure 3A) was not absolutely consistent with the protein level (~1.6-fold, Figure 4). The lower posttranscription level may be due to degradation of the CYP1A2 protein.36 Protein ubiquitination is an imperative pathway in a variety of degradation of proteins and performs an indispensable role in posttranslational modification.41 Additionally, the ubiquitin–26S proteasome pathway is the most important and highly selective protein-degradation pathway. It has been demonstrated that CYP3A1 and CYP3A2 can be ubiquitinated and then degraded by proteasomes.42
In clinical practice, 10% of clinical drugs are metabolized by CYP1A2, including propranolol, clomipramine, phenacetin, mexiletine, propanol, fluamine, verapamil, and nifedipine.43,44 Though CYP2D6 accounts for only 2%–4% of the total CYPs in the liver, it is involved in 20%–25% of drug metabolism,45,46 including in antidepressants, antiarrhythmics, antipsychotics, β-blockers, and analgesics,47 which may cause the blood concentration of these drugs to decrease when combined with formulations containing SsD or Bupleurum. Therefore, the mechanisms need more investigation. To some extent, the results of this study are of great significance for the prediction of CYP-mediated drug metabolic interactions and clinically relevant drug interactions of preparations containing SsD or Bupleurum early in the drug-discovery process. This study focused only on one of the most important ingredients in Bupleurum, and other components may also have certain effects on CYPs. As such, further studies need to be carried out on other major ingredients of Bupleurum to provide more comprehensive and reliable evidence for clinical medication combinations related to Bupleurum.
Conclusion
Our results indicated that SsD has induction effects on CYP1A2 and CYP2D6 in HepaRG cells. When drugs metabolized by CYP1A2 and CYP2D6 are coadministered with formulations containing SsD or Bupleurum in clinical practice, blood concentrations and effects of these drugs should be observed carefully to avoid or make use of the potential drug interactions.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (31460246), Guizhou Provincial Science and Technology Foundation (Qiankehe J [2013] 2323), Project of Special Funds for Science and Technology Cooperation in Guizhou Province and Zunyi City (Shengshikehe [2015] 53), Collaborative Innovation Center of Guizhou Traditional Chinese Medicine and Ethnic Medicine (Qianjiaokeyanfa [2012] 311).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Shaikh AS, Thomas AB, Chitlange SS. Herb-drug interaction studies of herbs used in treatment of cardiovascular disorders-a narrative review of preclinical and clinical studies. Phytother Res. 2020;34(5):1008–1026. doi:10.1002/ptr.6585
2. Niu L, Ding L, Lu C, et al. Flavokawain a inhibits cytochrome P450 in in vitro metabolic and inhibitory investigations. J Ethnopharmacol. 2016;191:350–359. doi:10.1016/j.jep.2016.06.039
3. Li X, Liu R, Zhang L, et al. The emerging role of AMP-activated protein kinase in cholestatic liver diseases. Pharmacol Res. 2017;125:105–113. doi:10.1016/j.phrs.2017.09.002
4. Yuan B, Yang R, Ma Y, et al. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm Biol. 2017;55(1):620–635. doi:10.1080/13880209.2016.1262433
5. Wong VK, Zhou H, Cheung SSF, et al. Mechanistic study of saikosaponin-d (Ssd) on suppression of murine T lymphocyte activation. J Cell Biochem. 2009;107(2):303–315. doi:10.1002/jcb.22126
6. Zhong D, Zhang HG, Jiang YD, et al. Saikosaponin-d: a potential chemotherapeutics in castration resistant prostate cancer by suppressing cancer metastases and cancer stem cell phenotypes. Biochem Biophys Res Commun. 2016;474(4):722–729. doi:10.1016/j.bbrc.2016.05.017
7. Lu GN, Yuan ZG, Zhang XL, et al. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int Immunopharmacol. 2012;14(1):121–126. doi:10.1016/j.intimp.2012.06.010
8. Li XQ, Song YN, Wang SJ, et al. Saikosaponins: a review of pharmacological effects. J Asian Nat Prod Res. 2018;20(5):399–411. doi:10.1080/10286020.2018.1465937
9. Zhang GS, Hu PY, Li DX, et al. Formulations, hemolytic and pharmacokinetic studies on saikosaponin a and saikosaponin d compound liposomes. Molecules. 2015;20(4):5889–5907. doi:10.3390/molecules20045889
10. Ding WX, Qi XR, Chen YW, et al. Cholesteryl hemisuccinate as liposomal membrane stabilizer and its use in the preparation of saikosaponin-D liposomes. Yao Xue Xue Bao. 2005;40(7):623–627.
11. Lewis DF. P450 structures and oxidative metabolism of xenobiotics. Pharmacogenomics. 2003;4(4):387–395. doi:10.1517/phgs.4.4.387.22752
12. Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97(3):125–134. doi:10.1111/j.1742-7843.2005.pto973160.x
13. Sychev DA, Ashraf GM, Svistunov AA, et al. The cytochrome P450 isoenzyme and some new opportunities for the prediction of negative drug interaction in vivo. Drug Des Devel Ther. 2018;12:1147–1156. doi:10.2147/DDDT.S149069
14. Huang SM, Temple R, Throckmorton DC, et al. Drug interaction studies: study design, data analysis, and implications for dosing and labeling. Clin Pharmacol Ther. 2007;81(2):298–304. doi:10.1038/sj.clpt.6100054
15. Gripon P, Rumin S, Urban S, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99(24):15655–15660. doi:10.1073/pnas.232137699
16. Anthérieu S, Chesné C, Li R, et al. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 2010;38(3):516–525. doi:10.1124/dmd.109.030197
17. Berger B, Bachmann F, Duthaler U, et al. Cytochrome P450 enzymes involved in metoprolol metabolism and use of metoprolol as a CYP2D6 phenotyping probe drug. Front Pharmacol. 2018;9:774. doi:10.3389/fphar.2018.00774
18. Wu Y, Geng XC, Wang JF, et al. The HepaRG cell line, a superior in vitro model to L-02, HepG2 and hiHeps cell lines for assessing drug-induced liver injury. Cell Biol Toxicol. 2016;32(1):37–59. doi:10.1007/s10565-016-9316-2
19. Leite SB, Wilk-Zasadna I, Zaldivar JM, et al. Three-dimensional HepaRG model as an attractive tool for toxicity testing. Toxicol Sci. 2012;130(1):106–116. doi:10.1093/toxsci/kfs232
20. Wang Z, Luo X, Anene-Nzelu C, et al. HepaRG culture in tethered spheroids as an in vitro three-dimensional model for drug safety screening. J Appl Toxicol. 2015;35(8):909–917. doi:10.1002/jat.3090
21. Aninat C, Piton A, Glaise D, et al. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34(1):75–83. doi:10.1124/dmd.105.006759
22. Jiang B, Meng L, Zhang F, et al. Enzyme-inducing effects of berberine on cytochrome P450 1A2 in vitro and in vivo. Life Sci. 2017;15(189):1–7. doi:10.1016/j.lfs.2017.09.011
23. Van LM, Sarda S, Hargreaves JA, et al. Metabolism of dextrorphan by CYP2D6 in different recombinantly expressed systems and its implications for the in vitro assessment of dextromethorphan metabolism. J Pharm Sci. 2009;98(2):763–771. doi:10.1002/jps.21455
24. Wang XM, Peng YR, Jing XY, et al. HPLC determination of phenacetin and its metabolite in rat plasma and microsome and its application. Chin Pharm Bull. 2013;29(4):591–592. doi:10.3969/j.issn.1001-1978.2013.04.034
25. Hui JM, Guo TL, Yang Z, et al. Determination of CYP2D6 activity in rat microsomes and study on its kinetics with dextromethorphan in vitro. China J New Drugs Clin Res. 2013;32(9):744–748.
26. Shi M, Cui Y, Liu C, et al. CYPs-mediated drug-drug interactions on psoralidin, isobavachalcone, neobavaisoflavone and daidzein in rats liver microsomes. Food Chem Toxicol. 2020;136:111027. doi:10.1016/j.fct.2019.111027
27. Wang L, Yue H, Huang N, et al. Human cytochrome P450 enzyme inhibition profile of three flavonoids isolated from Psoralea corylifolia: in silico predictions and experimental validation. New J Chem. 2018;42(13):10922–10934. doi:10.1039/C7NJ00884H
28. Wu ML, Li YP, Wei YL, et al. Calycosin influences the metabolism of five probe drugs in rats. Drug Des Devel Ther. 2020;14:429–432. doi:10.2147/DDDT.S236221
29. Li XJY, Li XY, Huang N, et al. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine. 2018;15(50):73–87. doi:10.1016/j.phymed.2018.09.174
30. Tian YD, Lin S, Yang PT, et al. Saikosaponin-d increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer. 2019;10(20):4947–4953. doi:10.7150/jca.30286
31. Xu ZK, Wei H. Comparison of pharmacokinetics between recombinant liver microsomes with CYP3A4 and CYP3A29. Her Med. 2015;31(1):15–21. doi:10.3870/yydb.2015.01.004
32. Zhang YW, Zheng XW, Liu YL, et al. Effect of oridonin on cytochrome P450 expression and activities in HepaRG cell. Pharmacology. 2018;101(5–6):246–254. doi:10.1159/000486600
33. Lübberstedt M, Müller-Vieira U, Mayer M, et al. HepaRG human hepatic cell line utility as a surrogate for primary human hepatocytes in drug metabolism assessment in vitro. J Pharmacol Toxicol Methods. 2011;63(1):59–68. doi:10.1016/j.vascn.2010.04.013
34. Pernelle K, Guevel RL, Glaise D, et al. Automated detection of hepatotoxic compounds in human hepatocytes using HepaRG cells and image-based analysis of mitochondrial dysfunction with JC-1 dye. Toxicol Appl Pharmacol. 2011;254(3):256–266. doi:10.1016/j.taap.2011.04.018
35. Andersson TB, Kanebratt KP, Kenna JG. The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin Drug Metab Toxicol. 2012;8(7):909–920. doi:10.1517/17425255.2012.685159
36. Ferreira A, Rodrigues M, Silvestre S, et al. HepaRG cell line as an in vitro model for screening drug-drug interactions mediated by metabolic induction: amiodarone used as a model substance. Toxicol in Vitro. 2014;28(8):1531–1535. doi:10.1016/j.tiv.2014.08.004
37. Young CKJ, Young MJ. Comparison of HepaRG cells following growth in proliferative and differentiated culture conditions reveals distinct bioenergetic profiles. Cell Cycle. 2019;18(4):476–499. doi:10.1080/15384101.2019.1578133
38. Cheng Y, Huang Y, Tian Y, et al. Assessment of the effects of Radix Bupleuri and vinegar-baked Radix Bupleuri on cytochrome 450 activity by a six-drug cocktail approach. Chin J Nat Med. 2013;113(3):0302–0308. doi:10.1016/S1875-5364(13)60033-3
39. Yu T, Chen X, Wang Y, et al. Modulatory effects of extracts of vinegar-baked Radix Bupleuri and saikosaponins on the activity of cytochrome P450 enzymes in vitro. Xenobiotica. 2014;44(10):861–867. doi:10.3109/00498254.2014.914600
40. Gómez-Lechón MJ, Donato MT, Castell JV, et al. Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab. 2004;5(5):443–462. doi:10.2174/1389200043335414
41. Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi:10.1126/science.1175371
42. Correia MA, Davoll SH, Wrighton SA, et al. Degradation of rat liver cytochromes P450 3A after their inactivation by 3, 5-dicarbethoxy-2, 6-dimethyl-4-ethyl-1, 4-dihydropyridine: characterization of the proteolytic system. Arch Biochem Biophys. 1992;297(2):228–238. doi:10.1016/0003-9861(92)90666-k
43. Zanger M, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–141. doi:10.1016/j.pharmthera.2012.12.007
44. Pragyan SS, Kesharwani PP, Nandekar PP, et al. Predicting drug metabolism by CYP1A1, CYP1A2, and CYP1B1: insights from MetaSite, molecular docking and quantum chemical calculations. Mol Divers. 2014;18(4):865–878. doi:10.1007/s11030-014-9534-6
45. Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5(1):6–13. doi:10.1038/sj.tpj.6500285
46. Kawakami M, Takenoshita-Nakaya S, Takeba Y, et al. Evaluation of CYP2D6 protein expression and activity in the small intestine to determine its metabolic capability in the Japanese population. Biol Pharm Bull. 2017;40(9):1344. doi:10.1248/bpb.b16-00370
47. Shiraga T, Matsuda H, Nagase K, et al. Metabolism of FK506, a potent immunosuppressive agent, by cytochrome P450 3A enzymes in rat, dog and human liver microsomes. Biochem Pharmacol. 1994;47(4):727–735. doi:10.1016/0006-2952(94)90136-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.