Back to Journals » Drug Design, Development and Therapy » Volume 13
Effect of pravastatin treatment on circulating adiponectin: a meta-analysis of randomized controlled trials
Received 10 September 2018
Accepted for publication 22 January 2019
Published 13 May 2019 Volume 2019:13 Pages 1633—1641
DOI https://doi.org/10.2147/DDDT.S186992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Xiangrong Shu,1 Liqun Chi2
1Department of Pharmacy, Tianjin Huanhu Hospital, Tianjin 300050, China; 2Department of Pharmacy, Haidian Maternal & Child Health Hospital of Beijing, Beijing 100080, China
Objective: Pravastatin has been suggested to increase circulating adiponectin in humans. However, results of randomized controlled trials (RCTs) are inconsistent. We aimed to systematically evaluate the influence of pravastatin on circulating adiponectin in humans by performing a meta-analysis of RCTs.
Materials and methods: Studies were identified via systematic searching of PubMed, Embase, and Cochrane’s Library databases. A random effect model was used to pool the results. Meta-regression and subgroup analyses were applied to explore the source of heterogeneity.
Results: Eight RCTs with nine comparisons of 595 participants were included. Pravastatin treatment was associated with a significant increased level of circulating adiponectin as compared with controls (weighted mean difference [WMD] =0.63 µg/mL; 95% CI, 0.17–1.09 µg/mL; P=0.007) with moderate heterogeneity (I2=28%). These results were confirmed by meta-analysis of double-blinded placebo-controlled RCTs (WMD =0.82 µg/mL; P=0.01). Meta-regression analyses indicated that proportions of males in each study were positively correlated with the effect of pravastatin on adiponectin (coefficient: 0.015, P=0.03). Subgroup analyses confirmed that pravastatin significantly increased adiponectin in studies of males (WMD =1.41 µg/mL; P=0.008), but not in those of females (WMD =-0.04 µg/mL; P=0.94).
Conclusion: Pravastatin treatment is associated with increased circulating adiponectin. Gender difference may exist regarding the effect of pravastatin treatment on adiponectin.
Keywords: pravastatin, adiponectin, meta-analysis, randomized controlled trials
Introduction
Pravastatin is a representative medication belonging to the class of 3-hydroxy-3-methylglutaryl coenzyme reductase inhibitors.1 Similar to other statins, pravastatin is proved to be effective for the primary and secondary prevention of cardiovascular diseases (CVDs),2,3 mainly depending on its lowering effect on low-density lipoprotein cholesterol. Different from other statins, pravastatin is suggested to be of a favorable influence on glucose metabolism and better safety profiles.4–6 Indeed, some cohort studies indicated that unlike other statins, long-term pravastatin treatment was not associated with an increased risk of new-onset diabetes.7–10 Moreover, pravastatin is recently proved to be preventative of preeclampsia, reflecting the potential benefits of pravastatin on endothelial function.11 Therefore, elucidation of the potential pharmacological mechanisms of pravastatin is important for the understanding the unique effects of pravastatin.
Adiponectin is an adipocyte-synthesized protein, which confers multiple benefits in cardiovascular and metabolic systems.12 It has been confirmed that adiponectin is an anti-inflammatory factor, which prevents the progression of atherosclerosis via its inhibitory effects on oxidation, platelet aggregation, and thrombosis formation.13 As for the metabolic system, adiponectin may exert its benefit via improving insulin resistance.14 Epidemiological studies suggest that higher circulating level of adiponectin is associated with lower risk of CVDs15 and diabetes.16 Interestingly, evidence from observational studies in humans has suggested that pravastatin treatment may lower circulating adiponectin.17 However, results of subsequent randomized controlled trials (RCTs) were inconsistent.18–25 A previous meta-analysis in 2016 evaluated the overall effect of statins on adiponectin.26 Although subgroup analysis in studies with pravastatin was performed, a non-RCT study27 and a study comparing pravastatin with fenofibrate28 were included and the results showed insignificant effect of pravastatin treatment on circulating adiponectin. Moreover, two qualified RCTs19,21 were not included in the previous meta-analysis, which may lead to the bias of the insignificant results. Therefore, the aim of current study was to evaluate the effect of pravastatin treatment on circulating adiponectin in a meta-analysis of RCTs. Results of our study may provide further information for understanding the benefits of pravastatin in cardiovascular and metabolic system.
Materials and methods
Database searching
We followed the instructions of the PRISMA statement29 and the Cochrane Handbook for Systematic Review,30 when performing this meta-analysis. PubMed, Embase, and Cochrane’s Library databases were systematically searched with the terms “pravastatin” combined with “adiponectin.” We limited the searching to studies in humans, and the last database searching was performed on December 10, 2018. The reference lists of the original articles and reviews were also manually screened for potential inclusion.
Study selection
The following inclusion criteria were applied in this meta-analysis: 1) published as full-length articles in English or Chinese; 2) designed as parallel RCTs; 3) included subjects that were randomly assigned to a pravastatin treatment group or a control group; 4) with the treatment of pravastatin for at least 3 days because we did not aim to evaluate the acute effect of pravastatin; 5) reported the outcomes of changes of circulating adiponectin (total, low-molecular weight [LMW], or high-molecular weight [HMW] adiponectin) from baseline in each group as means and SDs, or these data could be calculated. Reviews, abstracts, editorials, and studies that are not RCTs were excluded.
Data extraction
The processes of database searching, study selection, data extraction, and quality assessment were independently performed by two authors according to the predefined criteria. If discrepancies occurred, they were solved by consensus. For studies with different doses of pravastatin, multiple comparisons were considered. The sample size of the controls was equally split accordingly to overcome a unit of analysis error as indicated by the Cochrane Handbook for Systematic Review.30 Data regarding the characteristics of study design (blinded or open label, placebo-controlled [PC] or not), participants (disease status, sample size, mean age, gender, and mean body mass index [BMI]), pravastatin treatment (dose and treatment duration), and adiponectin measurement methods were extracted.
Quality evaluation
The Cochrane Risk of Bias Tool was applied to evaluate the quality of the included studies.30 This tool included seven domains regarding the following aspects of the studies, including criteria concerning sequence generation, allocation concealment, participant and personnel blinding, outcome assessor blinding, incomplete outcome data, selective outcome reporting, and other potential threats to validity.
Statistical analysis
We used RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation, College Station, TX, USA) to perform the meta-analysis and statistical analysis. The primary outcome of the study was the changes of circulating adiponectin levels between the baseline and the endpoint in response to pravastatin treatment, as compared with controls. We used weighted mean difference (WMD) with 95% CIs to describe the outcome. Heterogeneity among the included studies was tested using Cochrane’s Q test, and significant heterogeneity was identified at P-values <0.10.30 We also included the I2 statistics to evaluate the heterogeneity, presenting the percentages of total variation across studies that are due to heterogeneity rather than chance. Significant heterogeneity was considered if I2>50%.31 A random effect model rather than a fixed effect model was applied to pool the results, since the random effect model incorporates the study heterogeneity and thereby providing more generalizable results. Sensitivity analysis by excluding one study at a time was performed to evaluate the potential influence of single study on the meta-analysis results.30 The contributions of the predefined study characteristics (including number of participants, age, gender, mean BMI, pravastatin doses, and treatment duration) to the heterogeneity among the studies were analyzed via univariate meta-regression and subgroup analyses, with the medians of the continuous variables as cutoffs. Potential publication bias was assessed by a funnel plot and Egger’s regression asymmetry test.32 P-values were two-tailed, and statistical significance was set at 0.05.
Results
Literature searching results
The flowchart of database searching is summarized in Figure 1. Overall, 141 articles were identified after the initial database searching, and 125 were excluded after primary screening with titles and abstracts, mainly because they were irrelevant to the aim of the current meta-analysis. The remaining 16 articles underwent full-text review, and seven articles were further excluded because three of them were irrelevant studies, three were not RCTs, one was the duplicated presentation of an already included study, and the other one was without any available outcome data. Finally, eight RCTs were included in our meta-analysis.18–25
  | Figure 1 Flowchart of database searching and study selection. |
Study characteristics and quality evaluation
Since one of the studies23 included two pravastatin treatment groups with different doses, two comparisons were considered. Overall, eight RCTs18–25 with nine comparisons of 595 participants were included. The baseline characteristics of the included studies are presented in Table 1. Briefly, all of the included studies were RCTs, and four comparisons were derived from three RCTs18,21,23 with double-blinded (DB) and PC design. Patients with various disease statuses were included, such as those with hyperlipidemia,21,22,24,25 type 2 diabetes mellitus (DM),23 metabolic syndrome (MetS),19 and coronary artery disease.20 The sample sizes of the included comparisons varied from 31 to 152. The mean ages of the included patients ranged between 47.0 and 67.0 years, with varying proportions of male participants. Pravastatin was prescribed 40 mg per day in seven comparisons,18,19,21–25 while 20 mg per day in the other two comparisons.20,23 The treatment durations varied from 8 to 48 weeks. In all of the included studies, total adiponectin rather than HMW adiponectin was measured. Two of the comparisons used radioimmunoassay to measure circulating adiponectin,18,19 while the other seven applied ELISA.20–25 The drop-out rates were within 10% for all of the included RCTs. The details of quality assessment via the Cochrane’s risk of bias tool are presented in Table 2. The quality of the included RCTs was generally modest, with a total score of 1–3 in most of the studies.
Meta-analysis for the effect of pravastatin on circulating adiponectin
By pooling the results of the nine included comparisons, our meta-analysis showed that pravastatin treatment was associated with a significant increased level of circulating adiponectin as compared with controls (WMD =0.63 μg/mL; 95% CI, 0.17–1.09 μg/mL; P=0.007; Figure 2A), with moderate heterogeneity among the included studies (P for Cochrane’s Q test =0.20, I2=28%). Subsequent analyses by including four comparisons from RCTs of DB and PC design18,21,23 further confirmed the results, which showed an even larger increment of circulating adiponectin after pravastatin treatment (WMD =0.82 μg/mL; 95% CI, 0.17–1.47 μg/mL; P=0.01; Figure 2B) with considerable heterogeneity (I2=62%). We performed sensitivity analysis by excluding one study at a time to evaluate the potential influence of single study on the meta-analysis results. While excluding other studies did not significantly affect results, excluding the study by Takagi et al21 retrieved an insignificant result (WMD =0.27 μg/mL; 95% CI, −0.26–0.80 μg/mL; I2=0%; P=0.31). These results indicated that the positive results of the current meta-analysis were mainly driven by the study of Takagi et al.21 Since the health/disease status of patients may affect the results, we performed subgroup analysis in patients with MetS, impaired glucose tolerance (IGT), or DM, and the hyperlipidemic (HL) or hypercholesterolemic (HC) patients. The results showed that the effects of pravastatin on adiponectin were not significantly different between the two groups (P for subgroup difference =0.49; Figure 2C).
Meta-regression and subgroup analyses
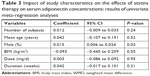
Subsequently, we performed univariate meta-regression and subgroup analyses to evaluate whether predefined study characteristics have a significant influence on the effect of pravastatin treatment on circulating adiponectin. Results of meta-regression showed that proportions of male participants in each study were positively correlated with the effect of pravastatin treatment on circulating adiponectin (coefficient: 0.015, P=0.03; Table 3; Figure 3), while not for other characteristics, including number of participants, age, mean BMI, pravastatin doses, or treatment duration (P all >0.05; Table 3). These results were further confirmed by stratified analyses comparing the effect of pravastatin treatment on circulating adiponectin in male and female participants, which showed significantly increased adiponectin after pravastatin in studies of male participants (WMD =1.41 μg/mL; 95% CI, 0.58–2.23 μg/mL; P=0.008; Figure 4), but not in studies of female participants (WMD =−0.04 μg/mL; 95% CI, −1.01 to 0.93 μg/mL; P=0.94; Figure 4). Consistently, subgroup analyses using the median of the proportion of males in each study as the cut-off value indicated that pravastatin significantly increased circulating adiponectin in studies with male participants of >50% (WMD =1.01 μg/mL; 95% CI, 0.43–1.59 μg/mL; P<0.001; Table 4), but not in studies with male participants of ≤50% (WMD =0.01 μg/mL; 95% CI, −0.73 to 0.76 μg/mL; P=0.97; Table 4). The difference between the two stratums was significant (P=0.04).
  | Figure 4 Forest plot for the meta-analysis of effect of pravastatin treatment on circulating adiponectin stratified by genders of the participants. |
Publication bias
No significant publication biases were indicated by the funnel plot (Figure 5) or the results of Egger’s significance test for the effect of pravastatin on circulating adiponectin (P=0.47).
  | Figure 5 Funnel plot for the meta-analysis of effect of pravastatin treatment on circulating adiponectin. |
Discussion
Our meta-analysis by including eight RCTs showed that pravastatin treatment is associated with increased circulating adiponectin. Moreover, subsequent analyses showed that gender of the participants may be an important determinant for the effect of pravastatin treatment on circulating adiponectin. Specifically, pravastatin may significantly increase the circulating adiponectin in male participants, but not in female participants. These results suggest that pravastatin treatment is associated with an increment of circulating adiponectin, which may be alternative mechanisms underlying the benefits of pravastatin to cardiovascular and metabolic systems. Moreover, gender difference may exist regarding the influence of pravastatin on circulating adiponectin.
Previous studies suggest that increased circulating adiponectin level is an independent marker of reduced risk of CVDs.15,33 Our meta-analysis showed that pravastatin may increase the circulating adiponectin level, which may be an important mechanism underlying the preventative effects of pravastatin for CVDs. More importantly, an increased circulating adiponectin level has been suggested to confer a protective effect against the development of diabetes. Indeed, a previous meta-analysis of 13 prospective studies showed that every increment of 1 μg/mL adiponectin was associated with a 28% reduction in new-onset diabetes.16 Although the underlying mechanisms remain unknown, the diabetogenicity has been considered as an inherited adverse effect of statins. We hypothesized that the enhancement of circulating adiponectin by pravastatin may substantially overcome the inherited diabetogenicity of the medications.34 This is consistent with the previous findings from cohort studies that long-term treatment with pravastatin is not associated with increased risk of new-onset diabetes.7–9 Obviously, these hypotheses should be further investigated.
The underlying mechanisms contributing to the enhancement by pravastatin on circulating adiponectin remain unknown at current stage. Although experimental studies have been performed to confirm the stimulatory effect of pravastatin on adiponectin in animal models,35,36 the potential molecular signal pathways involved were rarely observed. Moreover, these studies were focused on the acute effect of pravastatin treatment on adiponectin. Therefore, further studies are needed to elucidate the potential molecular pathways involved in the chronic effect of pravastatin on circulating adiponectin.
Interestingly, results of sensitivity analysis indicated that the positive results of the current meta-analysis were mainly driven by the study of Takagi et al.21 Since the present study is the only study that included hyperlipidemic patients, these results may indicate that pravastatin may reduce circulating adiponectin in hyperlipidemic patients. However, subgroup analysis did not indicate that the effects of pravastatin on adiponectin were significantly different between patients with MetS, IGT, or DM, and those with HL and HC. In view of the limited comparisons included in each stratum, influence of health/disease status of the patient on the results should be evaluated in the future. Moreover, results of subsequent meta-regression and subgroup analyses suggest that gender difference may exist regarding the influence of pravastatin on circulating adiponectin. These results should be interpreted cautiously because limited numbers of RCTs were available for the subgroup analysis. However, some previously published studies seem to support the potential gender difference regarding the pharmacological characteristics of pravastatin. Results of a pilot study of healthy volunteers indicated that sex may affect the pharmacokinetics of pravastatin.37 In addition, an early prospective study showed that the inhibitory effect of pravastatin on thrombin generation seems to be more remarkable in male participants as compared with females,38 which is also reflective of the sex difference of the pharmacokinetics of pravastatin. Based on above evidence, it is hypothesized that gender difference may influence the stimulatory effect of pravastatin on adiponectin. Further studies are warranted to confirm these findings.
Limitations
Our study has limitations. First, as previously mentioned, due to the limited number of the RCTs, results of our meta-analysis and subgroup analysis should be confirmed by future studies with adequate statistical power. Moreover, the quality of the included RCTs is modest, which highlights the needs of high-quality RCTs regarding the influence of pravastatin on adiponectin. In addition, our study included patients with different disease statuses, which may also contribute to the potential heterogeneity. Furthermore, whether pravastatin increases LMW or HMW adiponectin remains undetermined according to the current meta-analysis. Finally, the treatment durations in our study were within 48 weeks. The long-term influence of pravastatin treatment on circulating adiponectin beyond 48 weeks remains to be determined.
Conclusion
This meta-analysis showed that pravastatin treatment is associated with increased circulating adiponectin. Gender difference may exist regarding the effect of pravastatin treatment on adiponectin.
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Peters B, Maitland-van der Zee AH. Pharmacogenomic importance of pravastatin. Pharmacogenomics. 2008;9(9):1207–1210. | ||
Gotto AM. Review of primary and secondary prevention trials with lovastatin, pravastatin, and simvastatin. Am J Cardiol. 2005;96(5A):34–38. | ||
del Sol AI, Nanayakkara PW. Pravastatin: an evidence-based statin? Expert Opin Drug Metab Toxicol. 2008;4(6):821–825. | ||
McGovern ME, Mellies MJ. Long-term experience with pravastatin in clinical research trials. Clin Ther. 1993;15(1):57–64. | ||
Byington RP, Sacks FM. Lessons learned from the prospective pravastatin Pooling Project. Curr Atheroscler Rep. 2004;6(5):366–374. | ||
Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757–769. | ||
Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. | ||
Zaharan NL, Williams D, Bennett K. Statins and risk of treated incident diabetes in a primary care population. Br J Clin Pharmacol. 2013;75(4):1118–1124. | ||
Danaei G, García Rodríguez LA, Fernandez Cantero O, Hernán MA. Statins and risk of diabetes: an analysis of electronic medical records to evaluate possible bias due to differential survival. Diabetes Care. 2013;36(5):1236–1240. | ||
Agarwala A, Kulkarni S, Maddox T. The association of statin therapy with incident diabetes: evidence, mechanisms, and recommendations. Curr Cardiol Rep. 2018;20(7):50. | ||
Esteve-Valverde E, Ferrer-Oliveras R, Gil-Aliberas N, Baraldès-Farré A, Llurba E, Alijotas-Reig J. Pravastatin for preventing and treating preeclampsia: a systematic review. Obstet Gynecol Surv. 2018;73(1):40–55. | ||
Lee S, Kwak HB. Role of adiponectin in metabolic and cardiovascular disease. J Exerc Rehabil. 2014;10(2):54–59. | ||
Ebrahimi-Mamaeghani M, Mohammadi S, Arefhosseini SR, Fallah P, Bazi Z. Adiponectin as a potential biomarker of vascular disease. Vasc Health Risk Manag. 2015;11:55–70. | ||
Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol. 2016;8(2):101–109. | ||
Kanhai DA, Kranendonk ME, Uiterwaal CS, van der Graaf Y, Kappelle LJ, Visseren FL. Adiponectin and incident coronary heart disease and stroke. A systematic review and meta-analysis of prospective studies. Obes Rev. 2013;14(7):555–567. | ||
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–188. | ||
Saito S, Fujiwara T, Matsunaga T, et al. Increased adiponectin synthesis in the visceral adipose tissue in men with coronary artery disease treated with pravastatin: a role of the attenuation of oxidative stress. Atherosclerosis. 2008;199(2):378–383. | ||
Gannagé-Yared MH, Azar RR, Amm-Azar M, et al. Pravastatin does not affect insulin sensitivity and adipocytokines levels in healthy nondiabetic patients. Metabolism. 2005;54(7):947–951. | ||
Trøseid M, Lappegård KT, Mollnes TE, Arnesen H, Seljeflot I. Changes in serum levels of E-selectin correlate to improved glycaemic control and reduced obesity in subjects with the metabolic syndrome. Scand J Clin Lab Invest. 2005;65(4):283–290. | ||
Sugiyama S, Fukushima H, Kugiyama K, et al. Pravastatin improved glucose metabolism associated with increasing plasma adiponectin in patients with impaired glucose tolerance and coronary artery disease. Atherosclerosis. 2007;194(2):e43–e51. | ||
Takagi T, Matsuda M, Abe M, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196(1):114–121. | ||
Koh KK, Quon MJ, Han SH, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis. 2009;204(2):483–490. | ||
Kim JH, Lee MR, Shin JA, et al. Effects of pravastatin on serum adiponectin levels in female patients with type 2 diabetes mellitus. Atherosclerosis. 2013;227(2):355–359. | ||
Koh KK, Lim S, Choi H, et al. Combination pravastatin and valsartan treatment has additive beneficial effects to simultaneously improve both metabolic and cardiovascular phenotypes beyond that of monotherapy with either drug in patients with primary hypercholesterolemia. Diabetes. 2013;62(10):3547–3552. | ||
Koh KK, Quon MJ, Sakuma I, et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int J Cardiol. 2013;166(2):509–515. | ||
Chruściel P, Sahebkar A, Rembek-Wieliczko M, et al. Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208. | ||
Yokoyama H, Saito S, Daitoku K, et al. Effects of pravastatin and rosuvastatin on the generation of adiponectin in the visceral adipose tissue in patients with coronary artery disease. Fundam Clin Pharmacol. 2011;25(3):378–387. | ||
Fichtenbaum CJ, Yeh TM, Evans SR, Aberg JA. Treatment with pravastatin and fenofibrate improves atherogenic lipid profiles but not inflammatory markers in ACTG 5087. J Clin Lipidol. 2010;4(4):279–287. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. | ||
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available from: https://training.cochrane.org/handbook. Accessed on January 15, 2019. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Woodward L, Akoumianakis I, Antoniades C. Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol. 2017;174(22):4007–4020. | ||
Arnaboldi L, Corsini A. Could changes in adiponectin drive the effect of statins on the risk of new-onset diabetes? The case of pitavastatin. Atheroscler Suppl. 2015;16:1–27. | ||
Chen Y, Ohmori K, Mizukawa M, et al. Differential impact of atorvastatin vs pravastatin on progressive insulin resistance and left ventricular diastolic dysfunction in a rat model of type II diabetes. Circ J. 2007;71(1):144–152. | ||
Araki K, Masaki T, Katsuragi I, Kakuma T, Yoshimatsu H. Effects of pravastatin on obesity, diabetes, and adiponectin in diet-induced obese mice. Obesity. 2008;16(9):2068–2073. | ||
Niemi M, Pasanen MK, Neuvonen PJ. SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin but not fluvastatin. Clin Pharmacol Ther. 2006;80(4):356–366. | ||
Dangas G, Smith DA, Badimon JJ, et al. Gender differences in blood thrombogenicity in hyperlipidemic patients and response to pravastatin. Am J Cardiol. 1999;84(6):639–643. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.