Back to Journals » Drug Design, Development and Therapy » Volume 14
Effect of Platelet-Activating Factor on Barrier Function of ARPE-19 Cells
Authors Zhang F, Liu L , Zhang H , Liu ZL
Received 29 February 2020
Accepted for publication 25 September 2020
Published 12 October 2020 Volume 2020:14 Pages 4205—4214
DOI https://doi.org/10.2147/DDDT.S251941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Fan Zhang,1 Lei Liu,2 Han Zhang,2 Zhe-Li Liu2
1Department of Ophthalmology, The Fourth Affiliated Hospital of China Medical University, Eye Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang, Liaoning, People’s Republic of China; 2Department of Ophthalmology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
Correspondence: Han Zhang
Department of Ophthalmology, The First Affiliated Hospital of China Medical University, 155 Nanjing North St, Heping District, Shenyang 110001, Liaoning, People’s Republic of China
Tel +86-24-83283590
Fax +86-24-83282997
Email [email protected]
Aim: To examine the effects of platelet-activating factor (PAF) on the barrier functions of cultured retinal pigment epithelial (RPE) cells.
Methods: A human RPE cell line (ARPE-19) was cultured on microporous filter supports and treated with PAF and WEB 2086, a specific PAF-receptor (PAF-R) antagonist. The permeability of the RPE monolayer was measured using transepithelial electrical resistance (TER) and sodium fluorescein flux. The expression of the tight junction protein zonula occludens (ZO)-1 and the adherens junction protein N-cadherin was assessed using immunohistochemistry and Western blotting. We also measured the vascular endothelial growth factor (VEGF) concentrations in PAF-treated cultures and re-measured RPE monolayer permeability in the presence of VEGF-neutralizing antibodies.
Results: PAF significantly decreased the TER and enhanced the sodium fluorescein flux of the RPE monolayer and downregulated the expression of ZO-1 and N-cadherin. These effects were abolished by WEB 2086-mediated blockage of the PAF-R. PAF stimulation increased VEGF expression in RPE cells, and the antibody-mediated neutralization of VEGF caused a partial recovery of the barrier properties.
Conclusion: The barrier functions of ARPE-19 cells were altered by PAF, and these effects were partly mediated by an upregulation of VEGF expression in these cells. Our results contribute to the growing body of evidence supporting the role of PAF in choroidal neovascularization. Our findings suggest that PAF is a novel target in the development of therapies for increased permeability of the RPE monolayer.
Keywords: platelet-activating factor, ARPE-19 cells, barrier function, vascular endothelial growth factor
Introduction
The retinal pigment epithelium (RPE) is a single monolayer of epithelial cells at the back of the eye that forms the outer blood–retinal barrier (BRB). This barrier is fundamentally important for the health and integrity of the distal retina. The RPE plays important roles in the maintenance of the visual cycle and phagocytosis of the photoreceptor outer segment. Additionally, it is the main transport conduit for nutrients, metabolic waste products, ions, and fluids between the distal retina and choriocapillaris.1 Adjacent RPE structures are connected by tight junctions and adherens junctions to maintain intercellular barrier continuity, and these junctions are critical for maintaining the normal polarized functions of the RPE monolayer.2 The BRB may break down consequent to ocular trauma and many ocular diseases, such as proliferative vitreoretinopathy, diabetic retinopathy, and neovascular age-related macular degeneration (AMD). This breakdown increases the permeability of the barrier to serum components. In neovascular AMD, choroidal neovascularization (CNV) initially occurs under Bruch’s membrane and the RPE monolayer and then advances to the subretinal space, leading to subretinal hemorrhage, exudative lesions, serous retinal detachment and, ultimately, disciform scarring. The barrier properties of the RPE monolayer ensure equilibrium in the polarized secretion of antiangiogenic and proangiogenic factors and maintenance of the resulting chemotactic gradient, which play critical roles in the prevention of CNV development in the neurosensory retina.3 Outer BRB dysfunction may lead to photoreceptor degeneration and blindness.4 However, the potential mechanisms implicated in RPE barrier regulation have been poorly explored.
Platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is considered the first identified bioactive lipid. This potent proinflammatory mediator is involved in cellular activation, intracellular signaling, apoptosis, diverse inflammatory reactions, and angiogenesis.5–9 The biological actions of PAF are mediated through the activation of a G protein-coupled PAF-receptor (PAF-R).10 In a previous study, PAF-R expression was detected in RPE cells and choroidal endothelial cells, and PAF was shown to increase the production of vascular endothelial growth factor (VEGF) in RPE cells.11 In addition, our previous data indicated that PAF-R expression is upregulated locally in the subretinal space during experimental CNV development and that PAF-R antagonist treatment potently reduced the CNV lesion size.12 These findings suggest that PAF might play a role in the pathogenesis of CNV and neovascular AMD. Previous studies have shown that a PAF challenge can induce actin cytoskeletal rearrangement in endothelial cells and marked dysfunction of the endothelial barrier.13 However, the role of PAF in RPE barrier regulation is unclear.
The present study aimed to examine the effects of PAF on the barrier function of cultured RPE cells. Our findings will contribute to a growing body of evidence on the role of PAF in choroidal neovascularization and may suggest PAF as a novel therapeutic target for the increased permeability of RPE cells.
Materials and Methods
RPE Cell Culture
ARPE-19 cells (American Type Culture Collection, Manassas, VA, USA) were cultured as described previously.14 Briefly, ARPE-19 cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma Chemical Co., St. Louis, MO, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, 1% L-glutamine, and 0.1 mM nonessential amino acids in a humidified incubator with 5% CO2 at 37°C. After cell attachment, the medium was modified to contain only 1% FBS and was changed every 2 to 3 days.
Measurement of Transepithelial Electrical Resistance (TER)
To establish cell monolayers, ARPE-19 cells were grown on microporous filter membranes (0.4-μm pore size; Corning Inc., Corning, NY, USA), and the membranes were placed in 24-well culture plates. The TER was measured using an epithelial voltmeter (EVOM; World Precision Instruments, Sarasota, FL, USA), according to the manufacturer’s instructions. The TER values (Ω/cm2) were determined by subtracting the resistance of the filter alone (background) from the values obtained with the combined filters and RPE cells. Measurements were performed every 3 days during the first 16-day period and every day thereafter. After TER stabilization, the ARPE-19 monolayer was incubated with an exogenous stable derivative of PAF (carbamyl-PAF [cPAF], 100 nM; Enzo Biochemical, Inc., New York, NY, USA) or cPAF with the specific PAF-R antagonist WEB 2086 (10 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Medium with or without stimulants was changed every other day. Measurements were repeated at least five times per well, and each experiment was repeated in at least four different wells.
Permeability Assay
RPE cell permeability was assessed by measuring the apical-to-basolateral movements of fluorescein isothiocyanate (FITC)-dextran (4 kDa; Sigma Chemical Co.), as previously described.14 Briefly, 3 weeks after culture initiation, the ARPE-19 monolayers were treated with cPAF (100 nM) alone or with WEB 2086 (10 μg/mL) for 6 days. The medium was changed every 2 days, and 1000 μg/mL of FITC-dextran was added to the upper chamber on day 6 following stimulation. Samples (100 μL) were obtained from the upper and lower chambers at 24 h after the addition of FITC-dextran. The concentrations of FITC-dextran in these samples were quantified using spectrophotometry. The diffusion rate was expressed as a percentage and calculated as follows: (amount of dextran in the lower chamber) × 100/(amount of dextran in the upper chamber). Each experiment was repeated four times.
Immunohistochemistry
The formation of tight junctions and adherens junctions in ARPE-19 cell monolayers was examined by immunohistochemical staining to detect ZO-1 and N-cadherin, which are respectively a tight junction protein and adherens junction protein critical to barrier integrity. ARPE-19 cells cultured under conditions identical to those in the permeability assay were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature (RT) and rinsed thrice. The ARPE-19 monolayers on filters were permeabilized with 0.2% Triton X-100 in PBS for 20 min and blocked with 10% goat serum at RT for 30 min. A rabbit anti-ZO-1 (dilution, 1:100; Invitrogen, Carlsbad, CA, USA) or mouse anti-N-cadherin antibody (1:100; Cell Signaling Technology, Danvers, MA, USA) was added, and the cells were incubated overnight at 4°C. After washing thrice with PBS, the cells were further incubated with a FITC-conjugated goat anti-rabbit immunoglobulin (1:200; Invitrogen) or FITC-conjugated goat anti-mouse immunoglobulin (1:200; Invitrogen) for 30 min at RT. The slides were then treated with a nuclear stain and examined under a fluorescent microscope (BZ-9000, Keyence, Osaka, Japan).
Western Blot
Western blotting was used to further examine the expression of tight junction and adherens junction proteins in ARPE-19 cells. ARPE-19 cells cultured under conditions identical to those described for the permeability assay were washed with precooled PBS and then lysed for 30 min at 4°C with lysis buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.25% Na-deoxycholate, 1 mM EDTA, and protease inhibitor cocktail [B14001, Bimake, Houston, TX, USA]). The total proteins were harvested by centrifugation at 13,000 rpm and 4°C for 20 min, and the protein concentration in each sample was measured using G250. Next, 30–50 μg of total protein per sample was loaded into a well of an 8% polyacrylamide gel, separated by SDS-PAGE, and transferred to polyvinylidene fluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany). The membranes were blocked with 5% nonfat milk in 20 mM Tris (pH 7.4) with 137 mM NaCl and 0.05% Tween 20 for 1 h at RT. The membranes were then probed with a rabbit anti-ZO-1 (dilution, 1:1000; Invitrogen), mouse anti-N-cadherin (1:1000; Cell Signaling Technology), or mouse anti-GAPDH antibody (1:1000; Proteintech, Rosemont, IL, USA) overnight at 4°C. After washing, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h at RT. All the PVDF membranes were analyzed using chemiluminescence (Tanon, Shanghai, China).
VEGF Enzyme-Linked Immunosorbent Assay (ELISA)
The modulation of VEGF production by exogenous PAF was assessed using an ELISA kit (R&D Systems, Minneapolis, MN, USA). RPE cells were incubated in the presence of cPAF (100 nM) alone or with WEB 2086 (10 μg/mL) for 24 h. The supernatants were collected, and the VEGF concentrations produced under various conditions were determined according to the manufacturer’s protocol. Each assay was repeated thrice.
Neutralization of VEGF
Three weeks after culture initiation, the ARPE-19 monolayer was treated with bevacizumab (0.25 mg/mL, Avastin; Hoffmann La Roche, Basel, Switzerland), a recombinant humanized monoclonal antibody that targets all VEGF isoforms, in the presence of cPAF (100 nM) for 6 days. TER measurements, permeability assays, ZO-1 and N-cadherin staining, and Western blot analyses were performed to determine the role of VEGF in RPE barrier dysfunction induced by PAF.
Statistical Analysis
Each result is representative of at least three independent experiments. All values are presented as means ± standard errors of the means (SEM). The statistical analysis was performed using an analysis of variance (ANOVA) with the Newman–Keuls multiple comparison test (SPSS, Chicago, IL, USA). A p-value < 0.05 was considered to indicate statistical significance.
Results
PAF Reduces TER and Increases ARPE-19 Monolayer Permeability
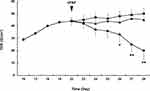
ARPE-19 monolayers were established on permeable membrane filters. The TER of the ARPE-19 monolayer increased rapidly during the initial 18 days of culture and reached a plateau within the following 3 days. The TER values were similar to those reported previously for this cell line.14–16 A mean TER of 44.0 ± 3.5 Ω/cm2 was recorded on day 22, after which cPAF was added. When compared with the control group, the incubation of ARPE-19 monolayers with cPAF-induced a gradual decrease in TER, and a significant effect was noted 4 days after stimulation (p < 0.05). Continuous decreases were also observed 5 and 6 days after stimulation (p < 0.01). The effect of cPAF was inhibited by the specific PAF-receptor antagonist WEB 2086 (Figure 1). The permeability of the ARPE-19 monolayer was further evaluated by measuring the transepithelial diffusion rate of FITC-dextran through the monolayer. Stimulation of the ARPE-19 monolayer with cPAF for 6 days induced a higher FITC-dextran diffusion rate at 24 h when compared to that in the control group (p < 0.05). Additionally, this effect of cPAF was inhibited by WEB 2086 (Figure 2).
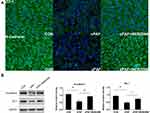
PAF Disrupts the Distribution and Decreases the Expression of ZO-1 and N-Cadherin in the ARPE-19 Monolayer
Next, ZO-1 and N-cadherin staining and Western blotting were performed to evaluate the barrier functions of RPE cell monolayers. Immunostaining for ZO-1 and N-cadherin showed continuous labeling in the regions of cell–cell contact in the untreated ARPE-19 monolayer. The exposure of these cells to cPAF for 6 days caused a markedly disturbed distribution of ZO-1 and N-cadherin (Figure 3A). Furthermore, a Western blot analysis demonstrated significant decreases in ZO-1 and N-cadherin expression in response to cPAF (p < 0.01 for both; Figure 3B).
PAF Increases VEGF Expression in the ARPE-19 Monolayer
The VEGF level was significantly higher in the supernatants of RPE cells preincubated with cPAF than in the supernatants of control cells (p < 0.05). Furthermore, treatment with WEB 2086 reduced the expression of VEGF (Figure 4).
Neutralization of VEGF Led to a Partial Recovery of PAF-Induced Barrier Dysfunction
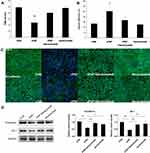
VEGF blockade significantly reversed the cPAF-induced reduction in TER (p < 0.01) and increase in permeability (p < 0.05) in the ARPE-19 monolayer (Figure 5A and B). Similarly, VEGF blockade attenuated the cPAF-induced disruption of ZO-1 and N-cadherin, as indicated by immunohistochemical staining (Figure 5C), and inhibited the cPAF-induced decreases in ZO-1 and N-cadherin expression, as indicated by a Western blot analysis (Figure 5D). Treatment with bevacizumab alone did not affect the cPAF-induced changes in TER, permeability, and ZO-1 and N-cadherin distribution or expression in the ARPE-19 monolayer.
Discussion
In this study, we determined that PAF significantly decreased the TER and enhanced sodium fluorescein flux across a cultured RPE monolayer. The expression of ZO-1 and N-cadherin was downregulated in cells cultured in PAF-supplemented medium, and these effects were abolished by PAF-R blockade. PAF stimulation increased the production of VEGF in RPE cells, and exposure to VEGF-neutralizing antibodies caused a partial recovery of the RPE barrier properties. To our knowledge, our study is the first to characterize the relationship between PAF and the barrier function of cultured RPE cells.
In this study, we used the ARPE-19 cell line to study the mechanisms responsible for PAF-mediated changes in RPE permeability. ARPE-19 is a line of human RPE cells with differentiated properties, and these cells exhibit morphological polarization when cultured as a monolayer on microporous filter supports.17 To evaluate the integrity of the RPE barrier, we used the TER assay, a classic method for measuring the resistance to an electrical current across a monolayer.18,19 Under the conditions mentioned above, the tight junctions of the RPE cells exhibited a maximum TER of 50 to 100 Ω/cm2.14–16 In our study, the TER of the ARPE-19 monolayer gradually increased and reached a plateau of 44 Ω/cm2 after 3 weeks of culture, consistent with the results of previous studies.14–16 The addition of PAF resulted in a decrease in the TER of the ARPE-19 monolayer. We also analyzed the transepithelial fluxes in FITC-dextran, a method used to evaluate the structural integrity of cultured epithelium monolayers.14,19,20 Exposure to PAF for 6 days resulted in a significantly increased diffusion rate, compared to that observed in unstimulated controls. These results suggest that PAF could significantly compromise the barrier function of the ARPE-19 monolayer and could affect the structural integrity of the monolayer.
The epithelial barrier function depends on intercellular tight junctions and adherens junctions, which respectively involve several tight junction and adherens junction proteins. ZO-1 has been shown to localize exclusively in the tight junction strands of various types of epithelial cells and is considered one of the most important tight junction proteins in the RPE cell layer.13 N-cadherin is a major cadherin cell adhesion protein in RPE cells.21 We showed that the expression of ZO-1 and N-cadherin was lower in the presence of PAF through immunohistochemistry and Western blotting analyses. The low expression and abnormal distribution of ZO-1 and N-cadherin in response to PAF may have led to the decreased TER and increased FITC-dextran diffusion rate. A previous study showed that PAF could induce severe leakiness of the endothelial barrier by causing the redistribution of ZO-1 and other junctional proteins inside endothelial cells.13 Similarly, Xu et al reported that PAF played an important role in the regulation of mucosal permeability, and these effects were correlated with structural alterations in the F-actin-based cytoskeleton and tight junction proteins, including ZO-1, in intestinal epithelial cells.22 Moreover, PAF treatment led to a dose- and time-dependent reduction in the expression of the main components of the adherens junctions in Kaposi’s sarcoma cells.23
VEGF has been implicated in the disruption of RPE barrier function. Ablonczy et al demonstrated that VEGF administration caused a significant drop in TER in an ARPE-19 cell monolayer.16 On the other hand, RPE cells express the PAF-R, and PAF increased VEGF production in these cells.11 Considering these findings, we propose that VEGF is a likely candidate associated with PAF-induced RPE barrier dysfunction. We confirmed that PAF increased VEGF expression in the ARPE-19 monolayer and determined that the neutralization of VEGF caused a partial reversal of the indicators of barrier dysfunction, including a decreased TER, enhanced sodium fluorescein flux, and lower ZO-1 and N-cadherin expression induced by PAF. These results suggest that PAF-induced barrier dysfunction in RPE cells might be partly mediated by the upregulation of VEGF expression. The partial effectiveness of VEGF antibodies suggests that factors other than VEGF might be involved in PAF-induced RPE barrier dysfunction. Our results contradicted the findings of Ghassemifar et al, who reported that VEGF slightly (approximately 10%) increased the TER of ARPE-19 cells and tightened the RPE junctions.24 This discrepancy between study results might be associated with the route or dose of VEGF administration.
ARPE-19 cells cannot fully differentiate into RPE-like layers. For example, ARPE-19 cells have a low capacity to express the critical proteins that regulate and maintain a barrier with strong tight junctions.15 Therefore, the use of ARPE-19 cells as a model of RPE function in vivo might be a limitation of the present study. Nevertheless, ARPE-19 cells remain valuable, as the observed changes in the barrier function appear to be quantitative rather than qualitative. Moreover, some characteristics of ARPE-19 cells, such as hypersensitivity to VEGF, loss of pigmentation, and weakness of tight junctions, somewhat resemble the characteristics of an aged eye or an eye with a pathologic condition.15
WEB 2086, a thieno-triazolodiazepine, is a potent and specific antagonist of PAF-R both in vitro and in vivo. A previous study suggested that the affinity of WEB 2086 for PAF binding sites is similar to that of PAF itself.25 However, a study of the effect of WEB 2086-mediated PAF-R pathway blockade on macrophage recruitment in vivo demonstrated that treatment with this agent did not improve macrophage infiltration to the degree observed in PAF-R-deficient mice. The authors hypothesized that WEB 2086 might not suppress the PAF-R pathway completely because of unfavorable pharmacokinetics and pharmacodynamics.26 In another study of the role of PAF in traumatic shock, PAF blockade with WEB 2086 induced unexpected and fundamentally opposite properties before and after trauma, suggesting that PAF pathway blockade may have a positive or a negative action depending on the administration timing and preexisting condition.27 PAF-R is the only known receptor for PAF and is thus important for most PAF functions. However, the role of this receptor was questioned in previous studies that demonstrated eosinophil degranulation, cytokine release, and inflammasome activation in response to PAF and lysoPAF (a biologically inactive de-acetylated precursor of PAF) independently of the PAF-R, as revealed in assays using PAF-R antagonists (including WEB 2086) or PAF-R gene deletion.28,29 Taken together, these previous results suggest that further studies involving lysoPAF stimulation, PAF-R gene deletion, and PAF-R small interfering RNA (siRNA) are required to identify the role of PAF in the barrier function of cultured RPE cells and the effect of PAF-R blockage.
Conclusion
In summary, the tight junctions of ARPE-19 cells are altered by PAF, and these effects are partly mediated by the upregulation of VEGF expression in RPE cells. Our results contribute to a growing body of evidence supporting the role of PAF in choroidal neovascularization. Furthermore, our findings suggest that PAF is a novel target for the treatment of increased RPE cell permeability.
Statement of Ethics
This research was carried out on the ARPE-19 cell line in vitro and did not involve live human or animal subjects. The authors have no ethical conflicts to disclose.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Liaoning Science and Technology Project (No. 2013225303, H. Z.) and Fund for Scientific Research of the First Hospital of China Medical University (No.2014-04, H. Z.).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Adijanto J, Philp NJ. Cultured primary human fetal retinal pigment epithelium (hfRPE) as a model for evaluating RPE metabolism. Exp Eye Res. 2014;126:77–84. doi:10.1016/j.exer.2014.01.015.
2. Chen M, Muckersie E, Robertson M, Fraczek M, Forrester JV, Xu H. Characterization of a spontaneous mouse retinal pigment epithelial cell line B6-RPE07. Investigative Opthalmology & Visual Science. 2008;49(8):3699–3706. doi:10.1167/iovs.07-1522.
3. Wittchen ES, Hartnett ME. The small GTPase Rap1 is a novel regulator of RPE cell barrier function. Invest Ophthalmol Vis Sci. 2011;52(10):7455–7463. doi:10.1167/iovs.11-7295.
4. Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1–18. doi:10.1016/j.preteyeres.2008.10.001.
5. Fukuda AI, Breuel KF. Effect of platelet activating factor on embryonic development and implantation in the mouse. Human Reproduction. 1996;11(12):2746–2749.
6. Nilsson G, Metcalfe DD, Taub DD. Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology. 2000;99(2):314–319.
7. Braquet P, Paubert-Braquet M, Bourgain RH, Bussolino F, Hosford D. PAF/cytokine auto-generated feedback networks in microvascular immune injury: consequences in shock, ischemia and graft rejection. J Lipid Mediat. 1989;1(2):75–112.
8. Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation. 2008;31(2):112–120. doi:10.1007/s10753-007-9056-9.
9. Hu-Lowe DD, Chen E, Zhang L, et al. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res. 2011;71(4):1362–1373. doi:10.1158/0008-5472.CAN-10-1451.
10. Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem. 2002;131(6):773–779. doi:10.1093/oxfordjournals.jbchem.a003164
11. He Y-G, Wang H, Zhao B, et al. Functional analysis of platelet-activating factor in the retinal pigment epithelial cells and choroidal endothelial cells. Curr Eye Res. 2009;34(11):957–965. doi:10.3109/02713680903231135.
12. Zhang H, Yang Y, Takeda A, et al. A novel platelet-activating factor receptor antagonist inhibits choroidal neovascularization and subretinal fibrosis. PLoS One. 2013;8(6):e68173. doi:10.1371/journal.pone.0068173.
13. Knezevic II, Predescu SA, Neamu RF, et al. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem. 2009;284(8):5381–5394. doi:10.1074/jbc.M808958200.
14. Chen Y, Yang P, Li F, Kijlstra A, Proost P. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011;6(3):e18139. doi:10.1371/journal.pone.0018139.
15. Ablonczy Z, Dahrouj M, Tang PH, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investigative Opthalmology & Visual Science. 2011;52(12):8614–8620. doi:10.1167/iovs.11-8021.
16. Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res. 2007;85(6):762–771. doi:10.1016/j.exer.2007.08.010.
17. Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1β and Barrier Function of Retinal Pigment Epithelial Cells (ARPE-19): aberrant Expression of Junctional Complex Molecules. Investigative Opthalmology & Visual Science. 2003;44(9):4097–4104. doi:10.1167/iovs.02-0867
18. Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Investigative Opthalmology & Visual Science. 2003;44(2):808–817. doi:10.1167/iovs.02-0473
19. Buzza MS, Netzel-Arnett S, Shea-Donohue T, et al. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A. 2010;107(9):4200–4205. doi:10.1073/pnas.0903923107.
20. Miura Y, Klettner A, Roider J. VEGF antagonists decrease barrier function of retinal pigment epithelium in vitro: possible participation of intracellular glutathione. Invest Ophthalmol Vis Sci. 2010;51(9):4848–4855. doi:10.1167/iovs.09-4699.
21. McKay BS, Irving PE, Skumatz CHRISTINEMB, Burke JM. Cell–Cell Adhesion Molecules and the Development of an Epithelial Phenotype in Cultured Human Retinal Pigment Epithelial Cells. Exp Eye Res. 1997;65(5):661–671. doi:10.1006/exer.1997.0374.
22. Xu LF, Xu C, Mao ZQ, Teng X, Ma L, Sun M. Disruption of the F-actin cytoskeleton and monolayer barrier integrity induced by PAF and the protective effect of ITF on intestinal epithelium. Arch Pharm Res. 2011;34(2):245–251. doi:10.1007/s12272-011-0210-4.
23. Boccellino M, Camussi G, Giovane A, et al. Platelet-Activating Factor Regulates Cadherin-Catenin Adhesion System Expression and β-Catenin Phosphorylation during Kaposi’s Sarcoma Cell Motility. The American Journal of Pathology. 1997;65(5):661–671.
24. Ghassemifar R, Lai CM, Rakoczy PE. VEGF differentially regulates transcription and translation of ZO-1alpha+ and ZO-1alpha- and mediates trans-epithelial resistance in cultured endothelial and epithelial cells. Cell Tissue Res. 2006;323(1):117–125. doi:10.1007/s00441-005-0046-7.
25. Casals-Stenzel J, Muacevic G, Weber KH. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharm Exp Therapeutics. 1987;241(3):974–981.
26. Doi K, Okamoto K, Negishi K, et al. Attenuation of folic acid-induced renal inflammatory injury in platelet-activating factor receptor-deficient mice. Am j Pathol. 2006;168(5):1413–1424. doi:10.2353/ajpath.2006.050634.
27. Schurr MJ, Fabian TC, Croce MA, Geraci SA, Proctor KG. Unexpected action of platelet activating factor antagonism after fluid resuscitation from traumatic shock. Surgery. 1997;121(5):493–500. doi:10.1016/s0039-6060(97)90102-1.
28. Dyer KD, Percopo CM, Xie Z, et al. Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor-independent mechanism: evidence for a novel receptor. J Immunol. 2010;184(11):6327–6334. doi:10.4049/jimmunol.0904043.
29. Deng M, Guo H, Tam JW, et al. Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J Exp Med. 2019;216(12):2838–2853. doi:10.1084/jem.20190111.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.