Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 16
Effect of Occupational Stress on Periodontitis According to the Salivary RANKL Level Among Iraqi Employees
Authors Mahmood AA , Al-Obadi HOM, Hussein HM
Received 20 December 2023
Accepted for publication 4 March 2024
Published 12 March 2024 Volume 2024:16 Pages 53—60
DOI https://doi.org/10.2147/CCIDE.S455831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Christopher E. Okunseri
Athraa Ali Mahmood,1 Hussain Owaid Muhammed Al-Obadi,1 Hashim Mueen Hussein2
1Department of Oral Surgery and Periodontics, College of Dentistry, Mustansiriyah University, Baghdad, Iraq; 2Department of Conservative Dentistry, College of Dentistry, Mustansiriyah University, Baghdad, Iraq
Correspondence: Athraa Ali Mahmood, Department of Oral Surgery and Periodontics, College of Dentistry, Mustansiriyah University, Baghdad, Iraq, Email [email protected]; [email protected]
Background: Findings show that periodontitis does not affect all populations; similarly, some individuals present risk conditions such as occupational stress, making them more susceptible to developing periodontitis through unhealthy habits like poor oral hygiene and immune suppression. Periodontitis triggers an inflammatory host immune response; “Receptor Activator Nuclear Factor KB ligand (RANKL)” is the primary regulator of osteoclast differentiation and activity. It was found that osteoclastic bone damage caused by periodontitis depends on the RANKL produced by osteoblastic and periodontal ligament cells.
Objective: This study aimed to assess the effect of occupational stress on employees with periodontitis using salivary RANKL marker.
Material and Methods: A case–control analysis was done at my clinic with 90 male employees aged 30– 50. The participants completed self-administered questionnaires and had periodontal exams. Employee occupational stress was estimated using a life events scale questionnaire. Calibrated dentists performed the parameters used in the periodontal assessment after collecting whole unstimulated salivary samples from each employee to measure salivary RANKL using ELISA technique.
Results: The present finding revealed a statistically significant difference among groups in “probing pocket depth, plaque index, bleeding on probing, clinical attachment level, and salivary RANKL level”. They were higher in the stressed employees’ group, which is not statistically significant.
Conclusion: The findings of this investigation observed that occupational stress increased clinical periodontal parameters and salivary RANKL of periodontitis in employees.
Keywords: periodontitis, occupational stress, salivary RANKL, employees
Introduction
Periodontitis is a destructive inflammatory disease that can cause loss of attachment apparatus, alveolar bone, and pathological pockets around the teeth.1–3 Several studies findings have found that periodontitis does not influence all populations similarly; some people have risk conditions or factors that make them susceptible to developing periodontal diseases, such as the virulence of the microorganism, immunological, and genetic mechanisms, and host environmental conditions, mainly smoking, psychosocial stress, and lifestyle factors.4–11
Stress is a psychological and physiological response effect of environmental variation and noxious stimulation, leading to disease if extensive.12 Stress is not harmful if a person has active coping behaviours and can control his environment.4
Several studies have linked stress factors to periodontal disease. Stress can indirectly affect the health of periodontal status by activating harmful factors like poor oral hygiene, an imbalanced diet, heavy smoking, and more doses of drugs or alcohol; In addition, periodontal health can be affected directly through a biological source that occurs by changes in the saliva amount and constituents, and changes in the gingival blood circulation that can be regulated by the immune system of the patient itself.13–16
Work-related stress, “Occupational stress”, has been reported as a predisposing factor to periodontal disease in staff working.17–19 It’s becoming more serious globally due to job involvement, notable changes in the working period, and workplace environment.20,21 Thus, “World Health Organization” defines occupational stress as a worldwide pandemic and is studying its severity.22 It adversely affects employment and contributes to physical and mental diseases.23 For example, studies show that occupational stress is linked to specific oral health diseases like caries,24 periodontal disease,16,25 temporomandibular disorder, and halitosis.26 This association is biologically plausible with studies demonstrating that exposure to stress agents with inadequate coping could modify the immune response by acting on the central nervous system to release immunoregulatory neurotransmitters, neuropeptides, inflammatory cytokines, and glucocorticoid hormones such as cortisol.
Cortisol upregulates “Receptor Activator Nuclear Factor KB ligand (RANKL)” expressions in osteoblasts and stimulates osteoclast differentiation, activation, and survival; thus, negative bone turnover and loss may result through increased bone resorption, making the person more susceptible to developing disease and affecting periodontal health. So, the RANKL can play an essential work in periodontal destruction, while the RANKL inhibition can stop the destruction of the periodontium of the patients.13,19,27,28
As far as we know, no study has investigated the effect of occupational stress among employees on periodontal clinical parameters of periodontitis compared to healthy control employees according to the salivary RANKL level. Thus, the rationale of this study was to assess these effects among Iraqi employees.
Materials and Methods
Study Population
The study design was observational case-control conducted at a private clinic from March 2023 to September 2023. The study population included 90 male employees aged 30 to 50 years with the presence of ≥ twenty teeth. They were categorized into three groups: healthy periodontium “probing pocket depth (PPD) ≤3 mm without clinical attachment loss (CAL) and bleeding on probing (BOP) <10%” non-stressed control (30 subjects), periodontitis “(PPD) ≥ 5 mm or PPD ≥ 4 mm with BOP” non-stressed (30 patients), and periodontitis with occupational stress (30 patients).
The Mustansiriyah University College of Dentistry ethics committee approved this study. Our study excluded smokers, alcohol drinkers, obese, diabetics, immune system disorders, antibiotics, anti-inflammatory, immunosuppressive drug users, and unemployed participants.
Procedure
Participants signed a written consent form that explained the study’s purpose. Then, each participant was asked to answer self-administered questions about the subject’s name, age, employment status, and information about dental care. Occupational stress was estimated using “a life events scale questionnaire”.13,29,30 Participants answered “yes” or “no” to agree or disagree with a statement. All procedures in this study followed ethical principles, including the ‘World Medical Association Declaration of Helsinki’ and its later amendments for human research.
After collecting whole unstimulated salivary samples from each participant, periodontal examination “plaque index (PLI), BOP, PPD, and CAL” were assessed, as the following: The percentage of surfaces with visible plaque after staining with disclosing agents was measured at each tooth’s mesial, distal, lingual, and facial aspects to assess dental plaque (occlusal surfaces being excluded). Then, BOP was measured immediately after PPD. A site was scored positively if bleeding occurred within 30 seconds of probing and negatively if not. They calculated the bleeding percentage of total surfaces.
In addition, sites with PPD ≥ 4mm were measured using a “Williams probe” inserted into the gingival crevice near the tooth’s long axis, counting from the gingival margin to the apex in millimeters. The measurement sites were mid-facial, mid-lingual, mesiofacial, distofacial, mesiolingual, and distolingual. The probe fell by its weight without pressure. It was measured to the millimetre. CAL represents the millimeter vertical distance from the cementoenamel junction to the probable gingival pocket bottom. The third molar was not recorded.
An enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of RANKL in the collected salivary samples in this study.
Statistical Analysis
Database (Excel for Windows) stored data and the results were subjected to statistical analysis using the software SPSS for Windows version 28.00. The histogram, quantile–quantile plot, and the Shapiro–Wilk test were used to identify the normality of the data distribution.
For the descriptive analysis, the mean, standard Deviation (SD) and median values of PLI, BOP, PPD, CAL, and salivary RANKL were calculated for each subject and compared among groups by Kruskal–Wallis H. In addition, it was compared for each group using a Mann–Whitney U-test; Spearman’s coefficient correlation determined the statistically significant correlations between clinical periodontal parameters and RANKL salivary level among employee groups. The comparison was 5% significant.
Results
The sample included 90 employees who completed the periodontal clinical examination and a self-reported questionnaire. Sixty employees had periodontitis with and without occupational stress groups were regarded as (cases groups), while thirty were regarded as healthy-periodontium (control group) without occupational stress.
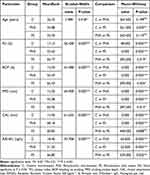
(Table 1) shows the total mean PLI percentage in the periodontitis non-stressed and stressed groups were 57.334% and 69.157%, respectively, while it was 10.424% for the control group, with a significant difference (P < 0.001), as illustrated in (Table 2).
 |
Table 1 The Mean of PLI, BOP, PD, and CAL for All Sites and the Mean of Salivary RANKL |
In addition, Table 1 describes the distribution of BOP percentage in each group. The mean percentages of probing site bleeding in the periodontitis non-stressed and stressed groups were 41.638% and 52.062%, respectively. In comparison, it is 3.969% for the control group, with a significant difference (P < 0.001), as illustrated in (Table 2).
We calculated the mean of sites with a PPD ≥ 4mm. Table (1) indicates a mean PPD rate of ≥ 4mm in the periodontitis non-stressed and stressed groups was 4.452mm and 4.795mm, respectively, while there was no pocket in the control group with significant difference (P < 0.001), as illustrated in (Table 2).
The total mean CAL for the periodontitis non-stressed and stressed groups were 3.448mm and 3.461mm, respectively, while there was no attachment loss in the control group. A statistically significant difference was found among these groups (P < 0.001), as illustrated in (Table 2).
The present study showed a significant positive association between periodontal parameters and salivary RANKL levels among groups, as illustrated in (Table 3).
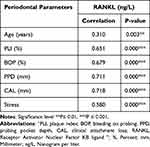 |
Table 3 The Spearman Correlation of the Periodontal Parameters and Salivary RANKL Levels Among Groups |
Discussion
The global incidence of occupational stress, which harms employers’ social psychology, is high.22 This study is the first to examine if occupational stress factors affect periodontal variables of periodontitis and compare it to healthy controls according to the salivary RANKL level in 90 Iraqi employees aged 30 to 50. Other authors worldwide conducted other studies using various target communities, indices, and scores to determine the relationship.19,24,31,32 In addition, studies measured psychological variables using various self-report scales “Minnesota Multiphasic Personality Inventory, Modifiers and Perceived Stress Scale, Brief Symptom Inventory, as well as stress, anxiety, and depression”.33–35 These variations may restrict investigation comparisons.
This study measured participants’ oral hygiene by the percentage of surfaces with plaque after disclosure. A total mean plaque score is slightly higher, with about (69) % of employees feeling occupational stress compared to employees without such stress. Furthermore, BOP was found in over 50% of sites, consistent with other studies’ results. More gingival inflammation among employees with occupational stress than those without stress might explain the increased BOP site percentage. These findings suggest that the impact of occupational stress on the parameters of periodontitis, which can be explained by unhealthy habits like poor oral hygiene and malnutrition, can drastically change oral health.36–38 Concurrently, occupational stress can lead to immune suppression and aggravating chronic inflammatory diseases like periodontitis.39 Findings uncorroborated this in a previous study, which exacerbated experimental gingivitis in a randomized split-mouth experiment and measured stress differently. The study found no association between stress, plaque accumulation, and gingival inflammation.40
Throughout our study, employees with occupational stress had a higher mean PPD and CAL, indicating a greater severity of periodontitis. Previous studies have found similar results for comparable groups. Occupational stress can be caused by bad working environments, workload over or under, long hours (more than 40 hours per week), role in the organization, career advancement, work relationships, and work environment. Stress in employees may depend on social support or how an individual manages occupational stress because everybody experiences it differently.29
Several studies show workers inhibit good emotions, leading to increased psychosocial stress and lower occupational enjoyment. In some workers, overwork-related stress was connected to systemic diseases like cardiovascular attacks, myocardial infarction, diabetes, and hypertension.41–43 Our study added to the evidence suggesting that occupational stress possibly influences the presence of periodontitis and may be a risk indicator of periodontitis development; this finding agrees with several studies using a wide range of questionnaires and clinical measures have found that people with periodontal disease were more likely to report feeling stressed than healthy controls.19,35,44,45
The employees in the periodontitis with occupational stress group had significantly higher levels of salivary RANKL as an immunological mediator than those in the other groups; this proposed synergetic association between the elevated RANKL by stress and periodontal parameters, and thus an association between occupational stress and periodontal conditions.46,47 These results agree with other studies, which found increased RANKL concentrations in people with stress compared to healthy controls.12,28 Furthermore, a case–control study conducted by Mengel et al searched for the correlation between different levels of immunological mediators and glucocorticoids and subjective measures of stress. Overall, no statistically significant difference was found in stress levels between the controls and cases and the levels of inflammatory mediators and glucocorticoids had no positive correlation with stress recorded via the questionnaire.48
Some limitations of the study were identified. At first, no other possible confounders leading to periodontal disease, such as poor health knowledge, low socioeconomic status, and education level, were examined. An additional limitation is that the study’s observational design may have revealed stress and periodontal disease temporal relationships. Thus, longitudinal studies of the sequence of risk indicators of periodontitis beginning and progressing are needed to define causing links between possibility stressors and periodontitis.
Conclusion
The present study findings suggest that occupational stress may predispose indicators to increased risk levels of periodontitis among a group of Iraqi employees. Therefore, occupational stress should also be considered when managing periodontitis among employees.
Acknowledgments
The authors would like to thank Mustansiriyah University, College of Dentistry, Baghdad – Iraq (www.uomustansiriyah.edu.iq).
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gasner NS, Schure RS. Periodontal Disease. StatPearls. Treasure Island (FL) Ineligible Companies. Disclosure: Ryan Schure Declares No Relevant Financial Relationships with Ineligible Companies: StatPearls Publishing Copyright © 2023. StatPearls Publishing LLC.; 2023.
2. Hussein HM, Mahmood AA, Alberaqdar FA. The prevalence and relationship of root caries depth and gingival recession among different Iraqi groups. Mustansiria Dental Journal. 2015;12(1):144–155.
3. Matarese G, Isola G, Anastasi GP, et al. Transforming growth factor beta 1 and vascular endothelial growth factor levels in the pathogenesis of periodontal disease. Eur J Inflam. 2013;11(2):479–488. doi:10.1177/1721727X1301100217
4. Aleksejuniené J, Holst D, Eriksen HM, Gjermo P. Psychosocial stress, lifestyle and periodontal health. Journal of Clinical Periodontology. 2002;29(4):326–335. doi:10.1034/j.1600-051X.2002.290408.x
5. Sedghi LM, Bacino M, Kapila YL. Periodontal disease: the good, the bad, and the unknown. Front Cell Infect Microbiol. 2021;11:766944. doi:10.3389/fcimb.2021.766944
6. Mahmood AA, Abbas RF. Assessment of NLRP3 gene polymorphisms with periodontitis as compared with healthy periodontium in Iraqi Arabs patients. Eur J Dent. 2023;17(4):1338–1348. doi:10.1055/s-0043-1761185
7. Saeed NA, Hussein HM, Mahmood AA. Prevalence of dental anxiety in relation to sociodemographic factors using two psychometric scales in Baghdad. Mustansiria Dental Journal. 2017;14(1):38–50. doi:10.32828/mdj.v14i1.753
8. Kzar WA, Abbas RF, Hussein HM. The antimicrobial peptide ll-37 as a predictor biomarker for periodontitis with the presence and absence of smoking: a case-control study. Biomed Res Int. 2023;2023:5581267. doi:10.1155/2023/5581267
9. Mahmood AA, Abbas RF, Hussein HM. Novel association between single-nucleotide polymorphisms of IKKβ at rs17875746 and rs12676482 and periodontitis. Dental and Medical Problems. 2023;60(4):627–634. doi:10.17219/dmp/170879
10. Mahmood AA, AbdulAzeez AR, Hussein HM. The effect of smoking habit on apical status of adequate endodontically treated teeth with and without periodontal involvement. Clin Cosmetic Invest Dent. 2019;11:419–428. doi:10.2147/CCIDE.S236747
11. Isola G, Tartaglia GM, Santonocito S, Polizzi A, Williams RC, Iorio-Siciliano V. Impact of N-terminal pro-B-type natriuretic peptide and related inflammatory biomarkers on periodontal treatment outcomes in patients with periodontitis: an explorative human randomized-controlled clinical trial. Journal of Periodon. 2023;94(12):1414–1424. doi:10.1002/JPER.23-0063
12. Azuma K, Adachi Y, Hayashi H, Kubo KY. Chronic psychological stress as a risk factor of osteoporosis. J UOEH. 2015;37(4):245–253. doi:10.7888/juoeh.37.245
13. Solis A, Lotufo R, Pannuti C, Brunheiro E, Marques A, FJJocp L. Association of periodontal disease to anxiety and depression symptoms, and psychosocial stress factors. J Clinical Perio. 2004;31(8):633–638. doi:10.1111/j.1600-051X.2004.00538.x
14. Goyal S, Gupta G, Thomas B, Bhat KM, Bhat GS. Stress and periodontal disease: the link and logic!! Indust psy j. 2013;22(1):4–11. doi:10.4103/0972-6748.123585
15. Corridore D, Saccucci M, Zumbo G, et al. Impact of Stress on periodontal health: literature revision. Healthcare. 2023;11(10):1.
16. Hussein HM, AlAnsari SAS, Baldawi MKH, Mahmood AA. Association between health risk factors and apical periodontitis in fitted endodontically and non-endodontically treated teeth. Journal of Emergency Medicine, Trauma and Acute Care. 2023;2023(3–Second Mustan Intern Dental Confer–art. no. 7): 1–7).doi:10.5339/jemtac.2023.midc.7
17. Shizukuishi S, Hayashi N, Tamagawa H, et al. Lifestyle and periodontal health status of Japanese factory workers. Ann Periodontol. 1998;3(1):303–311. doi:10.1902/annals.1998.3.1.303
18. Atri M, Srivastava D, Kharbanda J, Bugalia A, Yousuf A, Occupational Stress AN. Salivary cortisol, and periodontal disease: a clinical and laboratory study. J Intern Oral Health. 2015;7(9):65–69.
19. Islam MM, Ekuni D, Yoneda T, Yokoi A, Morita M. Influence of occupational stress and coping style on periodontitis among Japanese workers: a cross-sectional study. Int J Environ Res Public Health. 2019;16(19):3540. doi:10.3390/ijerph16193540
20. Ones DS, Sinangil HK, Viswesvaran C. The SAGE Handbook of Industrial. Work Organ Psy. 2015;2015:1–672.
21. Papadopoulos G, Georgiadou P, Papazoglou C, KJSs M. Occupational and public health and safety in a changing work environment: an integrated approach for risk assessment and prevention. Safety science. 2010;48(8):943–949.
22. Li Y, Sun X, Ge H, Liu J, LJIjoer C, Health P. The status of occupational stress and its influence the quality of life of copper-nickel miners in Xinjiang. China. 2019;16(3):353.
23. Leka S, Griffiths A, Cox T, Organization WH. Work organisation and stress: systematic problem approaches for employers, managers and trade union representatives. World Health Organization. 2003;2003:1.
24. Marcenes WS, Sheiham A. The relationship between work stress and oral health status. Soc sci med. 1992;35(12):1511–1520. doi:10.1016/0277-9536(92)90054-T
25. Linden GJ, Mullally BH, Freeman R. Stress and the progression of periodontal disease. Journal of Clinical Periodontology. 1996;23(7):675–680. doi:10.1111/j.1600-051X.1996.tb00593.x
26. Yoshino K, Suzuki S, Ishizuka Y, Takayanagi A, Sugihara N, Kamijyo H. Relationship between job stress and subjective oral health symptoms in male financial workers in Japan. Industrial Health. 2017;55(2):119–126. doi:10.2486/indhealth.2016-0120
27. Swanson C, Lorentzon M, Conaway HH, Lerner UH. Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bones. Endocrinology. 2006;147(7):3613–3622. doi:10.1210/en.2005-0717
28. Ng JS, Chin KY. Potential mechanisms linking psychological stress to bone health. Int J Med Sci. 2021;18(3):604–614. doi:10.7150/ijms.50680
29. Genco RJ, Ho AW, Grossi SG, Dunford RG, Tedesco LA. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. Journal of Periodon. 1999;70(7):711–723. doi:10.1902/jop.1999.70.7.711
30. Akhter R, Hannan MA, Okhubo R, Morita M. Relationship between stress factor and periodontal disease in a rural area population in Japan. Eur J Med Res. 2005;10(8):352–357.
31. Lee W, Lim SS, Kim B, Won JU, Roh J, Yoon JH. Relationship between long working hours and periodontitis among the Korean workers. Sci Rep. 2017;7(1):7967. doi:10.1038/s41598-017-08034-6
32. Green LW, Tryon WW, Marks B, Huryn J. Periodontal disease as a function of life events stress. J Human Stress Spring. 1986;12(1):32–36. doi:10.1080/0097840X.1986.9936764
33. da Silva AM M, Oakley DA, Newman HN, Nohl FS, Lloyd HM. Psychosocial factors and adult onset rapidly progressive periodontitis. Journal of Clinical Periodontology. 1996;23(8):789–794. doi:10.1111/j.1600-051X.1996.tb00611.x
34. Moss ME, Beck JD, Kaplan BH, et al. Exploratory case-control analysis of psychosocial factors and adult periodontitis. Journal of Periodon. 1996;67(10 Suppl):1.
35. Vettore MV, Leão AT, Da Silva AM M, Quintanilha RS, Lamarca GA. The relationship of stress and anxiety with chronic periodontitis. Journal of Clinical Periodontology. 2003;30(5):394–402. doi:10.1034/j.1600-051X.2003.10271.x
36. Wiebe DJ, McCallum DM. Health practices and hardiness as mediators in the stress-illness relationship. Health Psy. 1986;5(5):425–438. doi:10.1037/0278-6133.5.5.425
37. Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontology. 2010;53(1):138–153. doi:10.1111/j.1600-0757.2010.00340.x
38. Shankardass K. Place-based stress and chronic disease: a systems view of environmental determinants. In: Rethinking Social Epidemiology: Towards a Science of Change. Springer; 2011:113–136.
39. Wimmer G, Janda M, Wieselmann-Penkner K, Jakse N, Polansky R, Pertl C. Coping with stress: its influence on periodontal disease. Journal of Periodon. 2002;73(11):1343–1351. doi:10.1902/jop.2002.73.11.1343
40. Trombelli L, Scapoli C, Tatakis DN, Grassi L. Modulation of clinical expression of plaque-induced gingivitis: effects of personality traits, social support and stress. Journal of Clinical Periodontology. 2005;32(11):1143–1150. doi:10.1111/j.1600-051X.2005.00835.x
41. Kawakami N, Haratani T. Epidemiology of job stress and health in Japan: review of current evidence and future direction. Industrial Health. 1999;37(2):174–186. doi:10.2486/indhealth.37.174
42. Chu L. Impact of long working hours on health based on observations in China. BMC Public Health. 2021;21(1):1347. doi:10.1186/s12889-021-11190-0
43. Ali Daily Z, Al-Ghurabi BH, Al-Qarakhli AMA, Hussein HM. Association between AIM2 and pycard genes polymorphisms and susceptibility to periodontitis with coronary heart disease. Clin Cosmetic Invest Dent. 2023;15:307–320. doi:10.2147/CCIDE.S440577
44. Freeman R, Goss S. Stress measures as predictors of periodontal disease--a preliminary communication. Comm Dentist Oral Epidemiol. 1993;21(3):176–177. doi:10.1111/j.1600-0528.1993.tb00748.x
45. Bandar KJjoku T. The association between periodontal disease and job stress in Baghdad City. J kerbala univer. 2009;5(4):47–57.
46. Al-Ghurabi BH, BCoD MS. Salivary level of RANKL and OPG in chronic periodontitis. Int J Mod Sci. 2015;27(1):189–194.
47. Hathaway-Schrader JD, Novince CM. Maintaining homeostatic control of periodontal bone tissue. Periodontology. 2021;86(1):157–187. doi:10.1111/prd.12368
48. Mengel R, Bacher M, Flores-de-jacoby L. Interactions between stress, interleukin-1beta, interleukin-6 and cortisol in periodontally diseased patients. Journal of Clinical Periodontology. 2002;29(11):1012–1022. doi:10.1034/j.1600-051X.2002.291106.x
 © 2024 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2024 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.