Back to Journals » Drug Design, Development and Therapy » Volume 9
Death receptor and mitochondria-mediated hepatocyte apoptosis underlies liver dysfunction in rats exposed to organic pollutants from drinking water
Authors Yang G, Zhou Z, Cen Y, Gui X, Zeng Q, Ao Y, Li Q, Wang S, Li J, Zhang A
Received 18 April 2015
Accepted for publication 28 May 2015
Published 18 August 2015 Volume 2015:9 Pages 4719—4733
DOI https://doi.org/10.2147/DDDT.S86843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Shu-Feng Zhou
Guanghong Yang,1 Zhiwei Zhou,2 Yanli Cen,1 Xiaolin Gui,1 Qibing Zeng,1 Yunxia Ao,1 Qian Li,1 Shiran Wang,1 Jun Li,1 Aihua Zhang1
1Key Laboratory of Environment Pollution Monitoring and Disease Control, Ministry of Education, School of Public Health, Guiyang Medical University, 2Guizhou Provincial Key Laboratory for Regenerative Medicine, Stem Cell and Tissue Engineering Research Center and Sino-US Joint Laboratory for Medical Sciences, Guiyang Medical University, Guiyang, Guizhou, People’s Republic of China
Abstract: Persistent organic pollutants in drinking water impose a substantial risk to the health of human beings, but the evidence for liver toxic effect and the underlying mechanism is scarce. This study aimed to examine the liver toxicity and elucidate the molecular mechanism of organic pollutants in drinking water in normal human liver cell line L02 cells and rats. The data showed that organic extraction from drinking water remarkably impaired rat liver function, evident from the increase in the serum level of alanine aminotransferase, aspartate aminotransferase, and cholinesterase, and decrease in the serum level of total protein and albumin. Organic extraction dose-dependently induced apoptotic cell death in rat liver and L02 cells. Administration of rats with organic extraction promoted death receptor signaling pathway through the increase in gene and protein expression level of Fas and FasL. Treatment of rats with organic extraction also induced mitochondria-mediated apoptosis via increasing the expression level of proapoptotic protein, Bax, but decreasing the expression level of antiapoptotic protein, Bcl-2, resulting in an upregulation of cytochrome c and activation of caspase cascade at both transcriptional and posttranscriptional levels. Moreover, organic extraction enhanced rat liver glutathione S-transferases activity and reactive oxygen species generation, and upregulated aryl hydrocarbon receptor and glutathione S-transferase A1 at both transcriptional and translational levels. Collectively, the results indicate that organic extraction from drinking water impairs liver function, with the involvement of death receptor and mitochondria-mediated apoptosis in rats. The results provide evidence and molecular mechanisms for organic pollutants in drinking water-induced liver dysfunction, which may help prevent and treat organic extraction-induced liver injury.
Keywords: Fas/FasL pathway, mitochondrial pathway, liver injury
Introduction
Due to the rapid growth of the economy, environmental pollution has become a concern that imposes a severe risk to the health of human beings; in particular, water pollution.1,2 Safe drinking water is essential for healthy life; however, there is growing evidence of drinking water pollution worldwide, with worse situations in developing countries in particular.1,3,4 There are a number of water-related diseases that cause a substantial burden to human health. For instance, pollutant drinking water-induced diarrheal disease accounts for approximately 4.1% of the total disability-adjusted life year global burden of disease and is responsible for 1.8 million deaths every year.5 An estimation of 88% of that burden is ascribed to unsafe water supply, sanitation, and hygiene, and the children in developing countries are the most vulnerable subject.5 Thus, it is of great importance to monitor the quality of drinking water, understand the causative factors of water-related diseases, and elucidate the pathogenesis of water-related diseases.
There are many sources of water contamination, including naturally occurring chemicals and minerals, such as arsenic, radon, and uranium, and man-made materials, such as fertilizers and pesticides. Of note, persistent organic pollutants (POPs), organic compounds of anthropogenic origin that resist degradation and accumulate in the food chain, have been proposed to be a dominant artificial type of contaminants posing a threat to the health of human beings owing to their toxicity.4,6 There are 13 groups of POPs, including pentachlorophenol; dichlorodiphenyltrichloroethane; hexachlorocyclohexanes; hexachlorobenzene; heptachlor; polychlorinated dibenzo-p-dioxins and dibenzofurans; polychlorinated biphenyls; polycyclic aromatic hydrocarbons; polychlorinated terphenyls; polybrominated diphenyl ethers; polybrominated dibenzo-p-dioxins and dibenzofuran; short-chain chlorinated paraffins; and Ugilec.6,7 It has been reported that there was a high incidence of liver injury and carcinoma among local population in some regions where the water was severely polluted with POPs in China.8 However, the evidence for the toxic effect and underlying mechanism of POPs in humans are not fully understood. Due to health risk of POPs, it is of great importance to monitor the level of POPs, determine the toxic effect, and unveil the underlying mechanism of POPs.
The liver metabolizes endo- and xenobiotics, detoxifies harmful substances, synthesizes proteins, and performs many other vital functions.9 In particular, given the important role in detoxification of exogenous and endogenous toxins of liver in the human body, it is very important to examine the effect of POPs on liver function in vivo and investigate the molecular mechanism for the liver toxic effect. There are several indicators for liver function.10 When hepatocytes are damaged or destroyed, the proteins in the cells are released into the blood where they can be measured by blood tests such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The increased serum level of AST and ALT indicates the impairment of liver function.10 The impaired liver function is attributed to the perturbation of cellular functions and processes, such as cell survival and death, and redox homeostasis.11,12 Apoptosis and necrosis are two major types of cell death of hepatocytes which are either caspase-dependent or independent.12
Therefore, in order to examine the toxic effect of POPs on the liver and elucidate the underlying mechanism, POPs were detected by gas chromatography–mass spectrometry in our previous research. Fourteen kinds of organic pollutants were found in the tap water, including: undecane, methyl myristate, 2-propenoic acid-pentadecyl crylate, 14-methylpentadecanoic acid methyl ester, methyl palmitate, methyl stearate, methyl cis-9-octadecenoate, 3,3′-iminobispropylamine, methyl benzoate, 1,2-benzenedicarboxylic acid diisooctyl ester, dibutyl phthalate (DBP), di(2-ethyl hexyl)phthalate (DEHP), dichloroacetic acid, and trichloroacetic acid. Among them, DEHP and DBP in tap water were 16.6 and 4.9 μg/L, respectively, exceeding the limit values of National Standard for Drinking Water (GB5749-2006). Other organic compounds were all lower than those of the standards.13 On the basis of our previous results of organic pollutants from drinking water, the present study focused on the effect of organic extraction from drinking water collected from Guiyang City, People’s Republic of China, on liver function, apoptotic cell death, and redox homeostasis in normal human liver cell line L02 cells and rats.
Materials and methods
Chemicals and reagents
Amberlite XAD-2, corn oil, dimethyl sulfoxide, pentobarbital sodium, and sodium chloride were bought from Sigma-Aldrich Co. (St Louis, MO, USA). Methanol, dichloromethane, anhydrous alcohol, and xylene were purchased from East Sichuan Chemical Co., Ltd. (Sichuan, People’s Republic of China). Diagnostic kits used to determine the serum level of ALT, AST, cholinesterase (CHE), total protein (TP), albumin (ALB), cholesterol (CHO), triglyceride (TG), total bilirubin (TBIL), direct bilirubin (DBIL), and glutathione S-transferases (GSTs) were obtained from Nanjing Jiancheng Institute of Biotechnology Co., Ltd. (Nanjing, People’s Republic of China). Reactive oxygen species (ROS) assay kit was purchased from Beyotime Institute of Biotechnology Inc. (Shanghai, People’s Republic of China). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay kit was obtained from Wuhan Boster Biological Technology, Ltd. (Wuhan, People’s Republic of China). Flow cytometry assay kit, Dulbecco’s Modified Eagle’s Medium, and fetal bovine serum were bought from Thermo Fisher Scientific (Waltham, MA, USA). RNAiso Plus reagents; reverse transcription kits; primary antibodies against rat Fas, FasL, Bcl-2, Bax, cytochrome c, cleaved caspase (c-caspase) 3, c-caspase 8, c-caspase 9, c-caspase 12, PARP-1, calpain 1, and GAPDH; and Western blotting kits were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Primers for Fas; FasL; Bcl-2; Bax; cytochrome c; caspase 3, 8, 9, and 12; PARP-1; calpain 1; and GAPDH were obtained from TaKaRa Inc. (Tokyo, Japan).
Preparation of organic extracts from drinking water
A volume of 20,000 L tap water was collected from July to September (abundant water period), 2012 in Guiyang City. Organic pollutants in the drinking water were extracted by solid-phase extraction method as previously described.13,14 The extractant was evaporated with an RE-3000 type rotary evaporator. Then, the resultant extractions were dissolved in corn oil or dimethyl sulfoxide for in vivo or in vitro experiments, respectively. The organic extractions were stored at 4°C.
Animal experiments
A total number of 50 Sprague Dawley rats (80–100 rpm, 25 male and 25 female rats) were purchased from Third Military Medical University of China (Chongqing, People’s Republic of China) (Certificate number SCXK [Chongqing] 2012-0003). All animal experiments were performed according to the protocol approved by the Experimental Animal Ethical Committee of Guiyang Medical University, and the protocol of Guiyang Medical University complied with National Institutes of Health guidelines. Animals were maintained in pathogen-free laboratory under standard conditions of humidity (40%–60%), temperature (22°C–24°C) in a 12-hour light/dark cycle. Animals were fed with standard rodent chow and had free access to pure water. All animals were acclimatized for at least 1 week prior to the experiment. All surgeries were performed under anesthesia and all efforts were made to minimize suffering.
Rats were randomly divided into five groups and each group consists of ten animals. According to the design principle of subchronic animal experiment, high exposure dose may cause obvious toxic effect in animals, but the dose can’t induce animal death or it can only induce one animal death (the death rate is less than 10%). In our pre-experiment, we found that under the experimental condition, there was obvious liver toxicity (liver function and histopathology) in the exposure group at a dose of 80 L/kg (organic extraction from 80 L of drinking water). Therefore, 80 L/kg was set as the high-dose exposure group. Then, under four-times spacing, the 20 L/kg exposure group and 5 L/kg exposure group were set as middle- and low-dose groups, respectively. Blank control group without any treatment and vehicle control group with corn oil treatment were also set. The rats were administered through intragastric gavage once a day (1 mL/100 g body weight), 5 days a week, for 12 weeks. The animals were sacrificed under deep anesthesia 12 hours after the last dosage. Blood samples were collected by cardiac puncture and allowed to clot for 45 minutes at room temperature. The liver tissue was carefully excised, washed twice with saline, blotted dry on a filter paper, weighed, and cut into two pieces. One part was used for TUNEL analysis and the other was used for hepatic homogenate preparation.
Cell line and cell culture
The normal human hepatic cell line L02 cells were provided by the Cell Bank of Kunming Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Kunming, People’s Republic of China). L02 cells were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum and 1% penicillin/streptomycin, and the cells were maintained at 37°C in a 5% CO2/95% air humidified incubator.
According to the design principle of experiment in vitro, cell viability in the high-dose group is usually 20%–30%, and three to five exposure groups should be included in the experiment in vitro. In our pre-experiment, we found that under the experimental condition, cell viability was 30% at a dose of 5.0 L/mL (organic extraction from 5 L drinking water). So, high dose was 5.0 L/mL. Then, the next four low-dose groups were set up under double spacing (they were 2.5, 1.25, 0.625, and 0.3125 L/mL, respectively).
Examination of liver function
The blood samples were centrifuged at 600× g for 15 minutes to separate the serum. Serum levels of ALT, AST, CHE, TP, ALB, CHO, TG, TBIL, and DBIL were measured using respective kits according to the manufacturer’s instruction. Liver tissues of the rats were rinsed in ice-cold saline and homogenized in Tris-HCl buffer (0.01 M, pH =7.4) to get a 10% homogenate. Homogenates were centrifuged at 4°C, 3,000 rpm for 10 minutes and supernatants were collected for further analysis.
TUNEL assay
The TUNEL assay was performed to examine the hepatocyte apoptosis in rat liver according to the manufacturer’s instruction. Briefly, the paraffin sections of rat liver tissue were dewaxed, hydrated, and rinsed with phosphate-buffered saline, followed by the addition of H2O2 in methanol solution (3 mL/L) to block endogenous peroxidase activity. Then, liver sections were penetrated using penetrating solution (1 g/L Triton X-100 dissolved in 0.1% sodium citrate). The TUNEL reaction buffer and converter-POD were added, and liver sections were incubated with diaminobenzidine for chromogenic reaction. After the incubation, the sections were counter-stained with hematoxylin to identify TUNEL-negative cells in the background. The hepatic apoptosis was observed under a microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan). Apoptotic liver cells were characterized with brown particles confined within the nucleus. Five fields were randomly selected from each section at high magnification (400×). The percentage of apoptotic cells was calculated with the ratio of TUNEL-positive cell number to the total liver cell number per field. The results were counted by a blind observer.
Examination of apoptosis of L02 cells
In order to verify the effect of organic extraction on apoptosis of hepatocytes, the flow cytometry assay was performed to detect the apoptosis of liver cells in vitro. L02 cells were seeded into six-well plates (2.0×105 cells per well) and treated with organic extraction which were extracted from 0.3125, 0.6250, 1.2500, 2.5000, and 5.0000 L water for 72 hours. The cells were collected after the treatment and resuspended with phosphate-buffered saline. Following that, the cells were incubated with 195 μL apoptosis assay buffer containing 5 μL annexin V-fluorescein isothiocyanate (annexin V-FITC) for 10 minutes at room temperature in the dark. Then, the cells were pelleted via centrifugation at 1,000 rpm for 5 minutes and incubated with 10 μL staining solution for 10 minutes on ice in the dark. Subsequently, the samples were subject to flow cytometric analysis. The excitation wavelength and emission wavelength were 488 nm and 530 nm, respectively. Annexin V-FITC green fluorescence was detected through FL1 channel, and red fluorescence of propidium iodide was detected through FL2 channel. The results were counted by a blind observer. All experiments were performed in triplicate.
RNA isolation and quantitative real-time polymerase chain reaction
The expression level of genes, including Fas; FasL; Bcl-2; Bax; cytochrome c; caspase 3, 8, 9, and 12; PARP-1; calpain 1; AhR; GSTA1; and GAPDH, which were involved in Fas/FasL signaling pathway, mitochondrial pathway, and endoplasmic reticulum pathway were examined using quantitative real-time polymerase chain reaction (PCR). Total cellular RNA was extracted from rat liver homogenate using RNAiso Plus kit according to the manufacturer’s instructions. The specific primer of each gene was designed using Primer 3.0 application software and the primer sequences were synthesized by TaKaRa. The primers sequences are shown in Table 1. The extracted RNA was reversely transcribed into cDNA using reverse transcription kit and the resultant cDNA was used for quantitative real-time PCR assay in a reaction volume of 20 μL according to the manufacturer’s instructions. Briefly, SYBRR Premix Ex Taq II (10 μL), forward primer (0.8 μL), reverse primer (0.8 μL), template cDNA (2 μL), ROX Reference Dye (0.4 μL), and distilled water (6 μL) were added to each well. The PCR plate was subject to 40 cycles of the following conditions: 1) activation at 95°C for 30 seconds; 2) denaturation at 95°C for 5 seconds; and 3) annealing/extension at 60°C for 30 seconds. All samples and controls were run in triplicate using CFX-96 Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA). The relative expression level of each gene was calculated and analyzed by Bio-Rad CFX Manager software. GAPDH was used as an internal reference gene to normalize the expression of targeted genes.
  | Table 1 Sequences of primers for targeted genes |
Western blotting assay
A volume of 600 μL radioimmunoprecipitation assay buffer containing protease inhibitor cocktail was used to lysate 20 mg liver tissue, and the protein concentration was detected using bicinchoninic acid assay. Then, equal amounts of protein were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane. Following that, the membrane was blocked with 5% skimmed milk, probed with primary antibodies at room temperature for 2 hours, and incubated with respective secondary antibody conjugated with horseradish peroxidase at room temperature for 2 hours. Then, visualization was performed using Bio-Rad ChemiDocTM XRS system (Bio-Rad Laboratories Inc.) with enhanced chemiluminescence substrate and the blots were analyzed using Image Lab 3.0 (Bio-Rad Laboratories Inc.). Protein level was normalized to the matching densitometric value of internal control.
Measurement of the activity of GSTs and level of ROS in liver homogenate
To further examine the liver toxicity of organic extraction, the effect of organic extraction on the liver GSTs activity and ROS generation was measured using liver homogenate. The GST activity and ROS level assays were performed according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation. Multiple comparisons were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison. A value of P<0.05 was considered statistically significant. All experiments were performed at least three times independently.
Results
Organic extraction from drinking water affects rat liver function
We first examined the effect of organic extraction on rat liver function through the evaluation of serum levels of ALT, AST, CHE, TP, ALB, CHO, TG, TBIL, and DBIL. As shown in Figure 1, administration of organic extraction at different concentrations to the rat dramatically increased the serum level of ALT, AST, and CHE, while decreasing serum level of TP and ALB. In comparison to the vehicle group, administration to rat of 80 L/kg organic extraction markedly increased the serum level of ALT and AST by 19.5% and 17.0% respectively (P<0.01 or 0.001 by one-way ANOVA; Figure 1). The serum level of CHE was elevated by 22.0% and 25.5% when rats were administered organic extraction at 20 and 80 L/kg, compared to vehicle control, respectively (P<0.001 by one-way ANOVA; Figure 1). However, in comparison to the vehicle control, there was a 11.1% and 11.9% reduction in the serum level of TP and ALB, respectively, when rats were treated with 80 L/kg organic extraction (P<0.05 or 0.01 by one-way ANOVA; Figure 1). There was no statistical significance observed in the serum level of CHO, TG, TBIL, and DBIL when rats received the organic extraction treatment (P>0.05 by one-way ANOVA; Figure 1). Taken together, the results show that administration of organic extraction impairs the rat liver function.
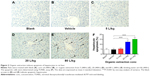
Organic extraction from drinking water induces apoptosis of hepatocytes in rat liver
Following the examination of the effect of organic extraction on liver function, we evaluated the apoptotic effect of organic extraction in rat liver using TUNEL assay. Treatment of rats with organic extraction dose-dependently increased the apoptosis of hepatocytes in rat liver (Figure 2A–E). There was no significant difference between blank group and vehicle group (Figure 2A and F). In comparison to the vehicle control group (Figure 2B), there was evident increase in the apoptosis of hepatocytes in rat liver when rats were dosed with 20 and 80 L/kg organic extraction (Figure 2D and E), with a 2.7- and 3.3-fold elevation, respectively (P<0.001 by one-way ANOVA, Figure 2F). There was a 1.2-fold increase in the apoptosis of hepatocytes in rat liver without statistical significance (Figure 2C and F).
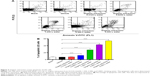
To further examine the apoptosis-inducing effect of organic extraction in hepatocytes, we employed human normal hepatocyte L02 cells. L02 cells were treated with organic extraction from 0.3125, 0.6250, 1.250, 2.500, and 5.000 L of water for 72 hours and then the apoptosis was examined by flow cytometry (Figure 3A). Organic extraction concentration-dependently increased the percentage of apoptotic L02 cells (Figure 3A and B). The percentage of apoptotic L02 cells was 7.6%, 7.1%, 7.8%, 12.1%, 28.1%, 43.0%, and 54.2% when cells were treated with blank, vehicle, and organic extraction from 0.3125, 0.6250, 1.250, 2.500, and 5.000 L water for 72 hours, respectively. There was a 1.7-, 3.9-, 6.0-, and 7.6-fold increase in the percentage of apoptotic L02 cells when treated with organic extraction from 0.6250, 1.250, 2.500, and 5.000 L water for 72 hours, respectively (P<0.001 by one-way ANOVA; Figure 3B). In aggregate, organic extraction from water remarkably induces apoptosis of liver cells, which may explain the impaired liver function.
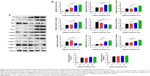
Organic extraction modulates the gene and protein expression level of key regulators involved in Fas/FasL and mitochondrial signaling pathways in rat liver
Given the observation of the potent apoptosis-inducing effect of organic extraction on hepatocytes, we further investigated the mechanisms underlying this proapoptotic effect with a focus on key regulators involved in the Fas/FasL, mitochondrial, and endoplasmic reticulum signaling pathways. We examined the effect of organic extraction on the expression of these key regulators at transcriptional and translational levels. First, we examined the expression level of Fas and FasL which play an important role in the initiation of cell death signaling pathway.15 As shown in Figure 4, there was a remarkable elevation in the gene expression level of Fas and FasL when rats received organic extraction treatment. Organic extraction induced a dose-dependent increase in the gene expression level of Fas (Figure 4). In comparison to the vehicle control group, there was a 2.0-, 3.3-, and 3.7-fold increase when treated with organic extraction at 5, 20, and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 4). There was a similar effect of organic extraction on the gene expression of FasL, and there was a 1.7- and 1.8-fold increase when treated with organic extraction at 20 and 80 L/kg, compared to the vehicle control group, respectively (P<0.001 by one-way ANOVA; Figure 4).
Next, we also examined the effect of organic extraction on mitochondria-mediated apoptosis pathway. Mitochondria-mediated apoptosis is a critical cellular process that has been implicated in the initiation and development of a variety of diseases.16–18 Mitochondria-mediated apoptosis requires the interplay of a number of pro- and antiapoptotic Bcl-2 family proteins and caspase cascade.16–18 As shown in Figure 4, there was a dramatic alteration in the gene expression of key regulators involved in mitochondria-mediated apoptosis, including Bax; Bcl-2; cytochrome c; caspase 3, 8, and 9; and PARP-1. Organic extraction dose-dependently decreased the gene expression of the antiapoptotic protein Bcl-2 with a 12.9%, 37.3%, and 41.4% reduction when treated with 5, 20, and 80 L/kg, compared to vehicle control, respectively (P<0.05 or 0.001 by one-way ANOVA; Figure 4); whereas organic extraction dose-dependently increased the gene expression of the proapoptotic protein Bax with a 32.0% and 77.7% reduction when treated with 20 and 80 L/kg, compared to vehicle control, respectively (P<0.01 or 0.001 by one-way ANOVA; Figure 4). Alteration in the balance between Bcl-2 and Bax leads to change in cytochrome c through promoting the release of cytochrome c from mitochondria to cytoso.18 Administration of organic extraction promoted the gene expression of cytochrome c in a dose-dependent manner (Figure 4). There was a 1.2-, 2.4-, and 2.9-fold increase in the expression of cytochrome c when dosed with 5, 20, and 80 L/kg organic extraction, respectively (Figure 4). Further, the gene expression of caspase 3, 8, and 9 was remarkably increased in rats dosed with organic extraction in a dose-dependent manner (Figure 4). In comparison to the vehicle control, administration of rats with organic extraction at 5, 20, and 80 L/kg led to a 1.1-, 1.8-, and 2.2-fold increase, respectively, in the gene expression of caspase 3 compared to vehicle control (Figure 4), and there was a 1.4- and 2.0-fold elevation in gene expression of caspase 8, and 2.2- and 2.7-fold rise in the gene expression of caspase 9, when rats received organic extraction at 20 and 80 L/kg, respectively (P<0.01 or 0.001 by one-way ANOVA; Figure 4), whereas the gene expression level of PARP-1 was decreased by 42.3% and 49.3% when rats were treated with organic extraction at 20 and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 4). Additionally, there was no significant alteration in the expression level of caspase 12 and calpain 1 when rats were administered with organic extraction.
Following the examination of the expression of key regulators involved in cell death pathways at transcriptional level, the cellular level of corresponding protein was also tested. As shown in Figure 5A and B, administration of rats with organic extraction markedly altered the expression level of pro- and antiapoptotic proteins involved in death receptor signaling, mitochondria-mediated apoptosis, and endoplasmic reticulum signaling pathways. Organic extraction dose-dependently increased the expression of Fas and FasL (Figure 5A and B). In comparison to the vehicle control, there was a 2.0-, 3.3-, and 3.7-fold rise in the expression level of Fas when dosed with organic extraction at 5, 20, and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 5A and B), and there was a 1.7- and 1.8-fold elevation in the expression level of FasL when administered with organic extraction at 20 and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 5A and B). Further, in comparison to the vehicle control, there was 1.2- and 1.8-fold increase in the expression level of Bax when rats were administered organic extraction at 20 and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 5A and B), whereas there was a 13.1%, 36.7%, and 41.8% reduction in the expression level of Bcl-2 when rats were treated with organic extraction at 5, 20, and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 5A and B). Treatment of organic extraction promoted the release from mitochondria to cytosol with a 1.2-, 3.1-, and 4.0-fold increase when rats were dosed with 5, 20, and 80 L/kg organic extraction, compared to the vehicle control, respectively (Figure 5A and B). Also, treatment of organic extraction activated the caspase cascade leading to dramatic increase in the level of c-caspases in a dose-dependent manner (Figure 5A and B). In comparison to the vehicle control, there was a 1.4- and 2.0-fold increase in the level of c-caspase 8; 1.7- and 1.9-fold rise in the level of c-caspase 3; and 1.6- and 2.0-fold elevation in the level of c-caspase 9, when rats were treated with organic extraction at 20 and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 5A and B). On the other hand, the level of PARP-1 was remarkably decreased by 42.7% and 49.3% when rats were dosed with 20 and 80 L/kg organic extraction, compared to the vehicle control, respectively (P<0.001 by one-way ANOVA; Figure 5A and B). In addition, there was a significant increase in the expression level of PARP-1 when treated with 5 L/kg organic extraction, and there was no significant alteration in the expression level of c-caspase 12 and calpain 1. Taken together, organic extraction from drinking water remarkably induces apoptosis of hepatocytes in rat liver through the increase in the level of proapoptotic factors and decrease in the level of antiapoptotic factors at both transcriptional and translational levels, with the involvement of death receptor and mitochondria-mediated signaling pathways.
Organic extraction promotes the GSTs activity and ROS generation in rat liver
The effect of organic extraction on liver GST activity and ROS generation was also examined. Figure 6 shows that treatment of rats remarkably enhances the GST activity and increases the ROS generation in rat liver. In comparison to the vehicle control group, there was a 7.7- and 6.0-fold increase in the activity of GST in rat liver when rats were treated with organic extraction at 20 and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 6), and there was a 1.3- and 1.5-fold elevation in ROS generation in rat liver when rats were administered organic extraction at 20 and 80 L/kg, respectively (P<0.01 or 0.001 by one-way ANOVA; Figure 6). Collectively, the data shows a promoting effect of organic extraction on GST activity, and ROS generation in rat liver that may contribute to the proapoptotic effect of organic extraction on hepatocytes.
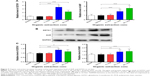
Organic extraction increases the gene and protein expression of AhR and glutathione S-transferase A1
Additionally, the effect of organic extraction on the expression of AhR and glutathione S-transferase A1 was examined at transcriptional and translational levels. Administration of organic extraction markedly increased the gene expression level of AhR and GSTA1. In comparison to the vehicle control group, there was a 3.9- and 2.9-fold elevation in the gene expression of GSTA1, and a 1.9- and 2.7-fold rise in the gene expression level of AhR, when rats were dosed with organic extraction at 20 and 80 L/kg, respectively (P<0.001 by one-way ANOVA; Figure 7A). Moreover, there was a remarkable increase in the protein expression level of glutathione S-transferase A1 and AhR. Treatment of rats with organic extraction at 20 and 80 L/kg led to a 1.4- and 1.2-fold increase in the expression level of glutathione S-transferase A1, and a 1.3- and 1.4-fold rise in the expression level of AhR, compared to the vehicle control, respectively (P<0.05 or 0.001 for GSTA1, P<0.01 or 0.001 for AhR by one-way ANOVA; Figure 7B and C). Taken together, the data indicate that organic extraction enhances the expression of glutathione S-transferase A1 and AhR at transcriptional and translational levels, providing an explanation for the enhanced GST activity and ROS generation.
POPs in drinking water impose a threat to the health of human beings which has gained increasing attention. The toxic effect of organic extraction from drinking water urgently needs to be determined, and understanding of the potential toxic targets and the underlying mechanism is helpful to prevent and treat POP-induced toxic effects. Our previous research suggests that POPs exist in the drinking water and the main active organic pollutants are DEHP and DBP. In the present study, we observed that liver toxicity is induced by organic extraction from the drinking water. The impaired liver function is accompanied by an increase in the serum level of AST, ALT, and CHE and a decrease in the serum level of TP and ALB. Organic extraction exerts an apoptosis-inducing effect in human liver cell line L02 cells and rat liver through regulating the key modulators involved in the death receptor signaling and mitochondria-medicated signaling pathways at both transcriptional and translational levels. Of note, the perturbed redox homeostasis is also a contributor to the organic extraction-induced liver toxicity in rats.
Liver is the major organ responsible for the biotransformation, metabolism, protein synthesis, and detoxification of endogenous and exogenous materials, which also renders liver as very vulnerable to malicious stimuli, leading to liver damage and failure with clinical presentations of hepatic dysfunction, abnormal liver biochemical values, coagulopathy, encephalopathy, or even multiorgan failure and death.19 ALT and AST, responsible for transamination reaction, have been considered as two biochemical markers of liver dysfunction.10 Any type of liver cell injury can increase ALT and AST levels, such as liver tissue degeneration and necrosis. In the present study, our data clearly showed that organic extraction from drinking water elevated serum ALT and AST levels in rats, indicating an impairment of liver function. Other liver function indicators have also been monitored when rats were dosed with organic extraction. The decrease in the serum level of TP and ALB suggested that organic extraction compromised the protein synthesis function of liver. In addition, our data showed that organic extraction did not affect the lipid and bilirubin metabolism of liver, evident from the unaffected serum level of CHO, TG, TBIL, and DBIL. Taken together, the findings show that organic extraction from the drinking water in Guiyang City impairs liver function. Measurements should be taken to control pollution due to POPs (especially for DEHP and DBP).
Given the dominant type and critical role in metabolizing and detoxifying the endo- and xenobiotics, manufacturing critical circulating proteins, and generating bile acid-dependent bile flow, hepatocytes are vulnerable to injury, and the perturbation of cellular processes and functions of hepatocytes results in hepatocyte cell death, which, as a consequence, leads to liver dysfunction and failure.9,12 There are several types of cell death, and apoptosis and necrosis are considered as the most widely recognized forms of hepatocyte cell death in liver injury.12 As noted in our findings, treatment with organic extraction from drinking water caused considerable apoptotic cell death in both human normal hepatic cell line L02 and rat liver, which occurred in a dose-dependent manner. As a consequence, hepatocyte apoptosis contributes to the development of a number of liver diseases.
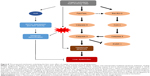
Apoptosis of hepatocytes exhibits unique morphological features, including membrane blebbing, shrinkage of the cell, chromatin condensation, and nuclear fragmentation,20 and hepatocyte apoptosis is susceptible to death receptor-mediated injury with the involvement of different death receptors, such as Fas, TNF receptor 1, and TRAIL receptor 1/2. These death receptors are major mediators of the apoptotic pathway in the liver.21–23 The death receptor signaling pathways is also orchestrated with extrinsic and intrinsic apoptosis pathways for efficient cell killing.20,21 Activation of caspase cascade leads to the cleavage of critical functional proteins for cell structure and function, and activation of the intrinsic pathway also results in mitochondrial release of several proapoptotic proteins and mitochondrial dysfunction, which eventually cause cell death through both caspase-dependent and caspase-independent mechanisms (Figure 8).
As observed in the present study, organic extraction significantly promoted the expression of Fas and FasL at both transcriptional and translational levels in rat liver. Meanwhile, the findings showed an activation of caspase cascade with a remarkable increase in gene and protein expression levels of caspase 8, 3, and 9 in rat liver. Upon stimulation by death receptor ligand, FasL, the death receptor oligomerizes and recruits different adaptor proteins which activate the initiator caspase 8 and likely caspase 10, which in turn activate the downstream target caspase 3, leading to apoptosis. Moreover, organic extraction increased the expression of cytochrome c and Bax, but deceased the expression of Bcl-2 at both transcriptional and translational levels in rat liver. The disturbance of antiapoptotic Bcl-2 and proapoptotic Bax promoted the release of cytochrome c from mitochondria to cytosol, which activates caspase 9. Activated caspase 9 in turn stimulates the downstream target caspase 3, leading to mitochondria-mediated apoptosis.20,21 Moreover, the remarkable alteration in the gene and protein expression levels of PARP-1 indicated hepatocyte apoptosis. Taken together, organic extraction impairs liver function through death receptor signaling and mitochondria-mediated pathways.
Of note, it has been reported that endoplasmic reticulum stress or the unfolded protein response contributes to hepatocyte cell death during alterations of lipid and fatty acid metabolism.12 However, our findings showed that organic extraction did not significantly affect the lipid and bilirubin metabolism in rat liver. Moreover, organic extraction did not significantly alter the gene and protein expression level of calpain 1 in rat liver. Calpains, a family of cytosolic cysteine proteinases with Ca2+-dependent enzymatic activities, are involved in a variety of biological processes, including integrin-mediated cell migration, cytoskeletal remodeling, cell differentiation, and apoptosis.24,25 Calpains are activated under endoplasmic reticulum stress, which in turn activates caspase 12, leading to apoptosis. However, our data showed that there was no significant activation of caspase 12 in rat liver when treated with organic extraction from drinking water. The findings indicate that organic extract does not induce endoplasmic reticulum stress in rat liver.
Additionally, organic extraction exhibited a substantial regulating effect on redox homeostasis, evident from the alteration in the GST activity and ROS generation in rat liver with a remarkable increase in the gene and protein expression level of GSTA1 and AhR. AhR has a critical role in xenobiotic-induced toxicity and carcinogenesis through the transcriptional regulation of target genes, such as GST and CYPs.26 Upregulation of the expression level of AhR contributes to the enhancement of GST activity, which may help accelerate the detoxification process in the liver. On the other hand, organic extraction-promoted ROS generation contributes to the hepatocyte apoptosis and liver dysfunction, through the deterioration of the hepatocyte function and cellular process.
In summary, the present study shows that organic extraction from the drinking water induces liver dysfunction and causes hepatocyte apoptosis in normal human liver cell line L02 cells and rats. The molecular mechanisms underlying the liver toxic effects are the death receptor signaling and mitochondria-mediated apoptosis signaling pathways. The perturbation of redox homeostasis contributes to organic extraction-induced hepatocyte apoptosis and liver toxicity. The findings provide further evidence and molecular mechanisms for organic extraction from drinking water-induced liver dysfunction, which may be helpful to prevent and treat organic extraction-induced liver injury.
Acknowledgment
This research was supported by the Science and Technique Research Projects of Guizhou, No [2014]7102; and the Science and Technique Research Projects of Guiyang, No [2013103]19.
Disclosure
The authors report no conflicts of interest in this work.
References
Schwarzenbach RP, Egli T, Hofstetter TB, von Gunten U, Wehrli B. Global Water Pollution and Human Health. Annu Rev Environ Resour. 2010;35:109–136. | ||
Hogan CM. Water pollution [webpage on the Internet]. The Encyclopedia of the Earth; 2014 [cited April 6, 2015]. Available from: http://www.eoearth.org/view/article/156920. Accessed January 10, 2015. | ||
Lu Y, Song S, Wang R, et al. Impacts of soil and water pollution on food safety and health risks in China. Environ Int. 2015;77:5–15. | ||
UNECE. Health Risks of Persistent Organic Pollutants from Long-Range Transboundary Air Pollution. Geneva: World Health Organization; 2003. | ||
Hutton G, Haller L. Evaluation of the Costs and Benefits of Water and Sanitation Improvements at the Global Level. Geneva: World Health Organization; 2004. | ||
Lohmann R, Breivik K, Dachs J, Muir D. Global fate of POPs: current and future research directions. Environ Pollut. 2007;150(1):150–165. | ||
Mosharraf Hossain M, Nazmul Islam KM, Rahman IMM. An Overview of the Persistent Organic Pollutants in the Freshwater System. In: Voudouris K, editor. Ecological Water Quality – Water Treatment and Reuse. InTech; 2012:455–470. | ||
Bao LJ, Maruya KA, Snyder SA, Zeng EY. China’s water pollution by persistent organic pollutants. Environ Pollut. 2012;163:100–108. | ||
Weber C. Liver: Reprogramming a hepatocyte’s memory of liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(5):250. | ||
Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 2009;3:17. | ||
Marí M, Colell A, Morales A, von Montfort C, Garcia-Ruiz C, Fernández-Checa JC. Redox control of liver function in health and disease. Antioxid Redox Signal. 2010;12(11):1295–1331. | ||
Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90(3):1165–1194. | ||
Wang S, Yang G, Zhang A, et al. The quality of source water and tap water from a distict of G city. Jouranl of Guiyang Medical College. 2014;39(2):187–189,197. Chinese. | ||
Sánchez-Avila J, Fernandez-Sanjuan M, Vicente J, Lacorte S. Development of a multi-residue method for the determination of organic micropollutants in water, sediment and mussels using gas chromatography-tandem mass spectrometry. J Chromatogr A. 2011;1218(38): 6799–6811. | ||
Waring P, Müllbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77(4): 312–317. | ||
Brenner C, Kroemer G. Apoptosis. Mitochondria – the death signal integrators. Science. 2000;289(5482):1150–1151. | ||
Soriano ME, Scorrano L. Traveling Bax and forth from mitochondria to control apoptosis. Cell. 2011;145(1):15–17. | ||
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281(5381):1309–1312. | ||
Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013; 369(26):2525–2534. | ||
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. | ||
Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. | ||
Yin XM, Ding WX. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med. 2003;3(6):491–508. | ||
Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology. 1999;29(1):1–4. | ||
Shi M, Zhang T, Sun L, et al. Calpain, Atg5 and Bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium. Apoptosis. 2013;18(4):435–451. | ||
Khorchid A, Ikura M. How calpain is activated by calcium. Nat Struct Biol. 2002;9(4):239–241. | ||
Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581(19):3608–3615. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.