Back to Archived Journals » Journal of Neurorestoratology » Volume 5
Curcumin reduces hippocampal neuron apoptosis and JNK-3 phosphorylation in rats with Aβ-induced Alzheimer's disease: protecting spatial learning and memory
Authors Wang YL, Li JF, Wang YT, Xu CY, Hua LL, Yang XP, Geng S, Wang SS, Wang Z, Yin HL
Received 24 October 2016
Accepted for publication 10 February 2017
Published 21 June 2017 Volume 2017:5 Pages 117—123
DOI https://doi.org/10.2147/JN.S125567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Hongyun Huang
Yun-liang Wang,1,2,* Jin-feng Li,1,* Yu-tong Wang,3 Chun-yang Xu,2 Lin-lin Hua,2 Xiao-peng Yang,2 Shuang Geng,1 Shan-shan Wang,1 Zhen Wang,1 Hong-lei Yin1
1Neurology Department, 148th Hospital, Zibo, Shandong, 2Neurology Department, Second Affiliated Hospital of Zhengzhou University, Zhengzhou, 3Public Health Department, Henan University School of Medicine, Kaifeng, Henan, People’s Republic of China
*These authors contributed equally to this work
Abstract: Amyloid-β peptide (Aβ) toxicity in Alzheimer’s disease (AD) is associated with the c-Jun N-terminal kinase (JNK) signaling pathway. Curcumin may prevent Aβ fiber formation, slowing AD progression. A model of AD was established in 32 Sprague Dawley rats by injection of 10 μg Aβ1–40 into the right hippocampus. Saline was used in sham control (n=16). Sixteen AD model rats received 300 mg/kg curcumin and another 16 received saline daily for 7 days. Spatial learning and memory were assessed using a Morris water maze. Hippocampus neuron apoptosis and hippocampal levels of JNK-3 and p-JNK-3 were assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling, reverse transcription-polymerase chain reaction and Western blotting. Aβ1–40 injection induced slower spatial learning, memory deficit, neuronal apoptosis and increased JNK-3 expression and phosphorylation (all P<0.05). Curcumin relieved spatial learning and memory deficits, hippocampus neuronal apoptosis, and reduced JNK-3 and p-JNK-3 levels (all P<0.05). In conclusion, curcumin may inhibit JNK-3 phosphorylation to protect against hippocampal neuron apoptosis after Aβ injection.
Keywords: Alzheimer’s disease, curcumin, apoptosis, JNK-3, phosphorylation
Introduction
Alzheimer’s disease (AD) is the most common type of dementia seen in patients worldwide; in China, in those aged over 60, AD has a prevalence of 1.9%.1 AD has an even higher prevalence in western countries, with >5 million people affected in the USA, and this is expected to rise in future.2 AD is a neurodegenerative disease characterized by the deposition of senile plaques in the brain, a pathogenesis that probably starts years before the symptoms of AD develop.3 Senile plaques are mainly composed of diverse amyloid-β peptides (Aβ).4 The pathologic process of AD is complicated. Aβ neurotoxicity is thought to be an important reason for the pathologic development of AD, leading to neuronal degeneration and ultimately dementia.5 Wide deposition of Aβ in the brain could cause neurotoxicity through the induction of oxygen free radicals, cell apoptosis, inflammation and calcium overload.6 It has been suggested that the toxicity of Aβ is associated with the c-Jun N-terminal kinase (JNK) pathway.7,8 Cell apoptosis needs extracellular signals to be transmitted to the nucleus, for which the JNK pathway is suggested to be critical.9 The JNK signaling pathway is one of the important pathways of the mitogen-activated protein kinase (MAPK) family. The activation of JNK pathway is closely related to cell apoptosis; it can mediate cytokines, protein synthesis inhibitors and osmotic stress.10 The JNK pathway could be activated by various factors such as reactive oxygen species, ischemia/reperfusion and inflammation. The JNK subfamily are members of MAPK, they are constitutive serine/threonine protein kinases. There are three genes encoding JNK in vertebrates, JNK1, JNK2 and JNK3.11 JNK1 and JNK2 are widely expressed and JNK3 is specifically expressed in the brain.
Curcumin is a major ingredient of Curcuma longa, commonly called turmeric and a member of the ginger family, which is a safe compound with low toxicity with diverse bioactivities including anti-inflammation, antitumor, antioxidation and antiapoptosis.12 As no side effect has been reported of this compound from clinic and laboratory studies, curcumin is implicated to be a safe and effective treatment strategy of AD.13,14 It is now a promising multitarget drug for treating AD.15,16 Curcumin has been demonstrated to inhibit the deposition and improve the disaggregation of Aβ. It inhibited the aggregation of Aβ in old amyloid precursor protein transgenic mice.17
The hippocampus plays an important role in learning and memory, especially short-term memory. It is one of the most vulnerable parts of brain; it suffers damage earlier than the cortex. Early in AD development, the neurons significantly decrease in the Cornu Ammonis region of the hippocampus. Therefore, the hippocampus is thought to be an important target region for studying AD.18 In this study, the AD rat model was established by injection of Aβ1–40 into rat right hippocampus. We investigated the effects of curcumin on neuronal apoptosis and JNK-3 phosphorylation in the hippocampus of Aβ-induced AD rat to provide an experimental and theoretical basis for the clinical treatment of AD with curcumin.
Materials and methods
Study design
Male Sprague Dawley rats aged 3 months old (N=48; body weight [BW]: 230–250 g) were obtained from the Laboratory Animal Center of Zhengzhou University (Henan, People’s Republic of China). Animals were maintained in a light–dark (12–12 h) cycle at constant temperature (25°C) and humidity (60%–70%) with free access to regular chow diet and drinking water. The rats were fasted without water deprivation 12 h before surgery. The rats were randomly divided into three groups (n=16 in each group) including Aβ-induced AD rat model group, AD model rats treated with curcumin (curcumin-treated group) and sham-operation group. Animal care and experimental protocols were conducted according to the guidelines of the Institutional Animal Care and Use Committee of Zhengzhou University and the 148th Hospital. All experiments were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Zhengzhou University and the 148th Hospital.
Aβ-induced AD rat model
Aβ1–40 (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in normal saline (NS) to a concentration of 10 μg/μL and allowed to aggregate at 37°C for 7 days before use. The Aβ-induced AD rat model was established according to previous reports.19–22 Briefly, the rats were anesthetized with an intraperitoneal injection of 3.5% chloral hydrate (1 mL/100 mg BW), and the right hippocampus of each rat was injected with 10 μL of Aβ1–40 according to the following coordinates: anteroposterior –2.8 mm, mediolateral ±2.2 mm and dorsoventral –3.0 mm from bregma. The injection lasted for 10 min, and the needle, along with the syringe, was left on the injection site for another 5 min to ensure the completion of Aβ infusion. NS was injected in the sham-operation group. After surgery, the animals were kept in individual cages until they recovered from anesthesia. The successful establishment of the model was based on the results of the rat’s behavior in the Morris water maze test.23 Curcumin (Sigma-Aldrich)-treated rats were given curcumin (10 μL dimethyl sulfoxide [DMSO] was added to 990 μL volume of NS, and the final concentration of DMSO was 1% v/v; 75 mg curcumin was added to the above mixture of DMSO and NS, and curcumin and the mixture were blended in a t-branch pipe in order to ensure the curcumin is completely dissolved). The solution is the required volume for a rat. The rats were intraperitoneally injected once each day (n=16, 300 mg/kg BW) for 7 days.24 Rats in the sham-operation group and Aβ-induced AD model group were given 0.1% DMSO (n=16 in each group).
Morris water maze test
One month after the animal model was established, the spatial learning ability and memory capability of the rats were evaluated by Morris water maze test.23
To test place navigation, the rat was placed in a large circular pool and had to find a platform that allowed it to escape the water. This allowed the learning ability of rats to be quantitatively measured. The experiment lasted for 5 days, and the rat was tested twice a day. The rat was placed into the pool facing the wall from two different locations, and the time taken to find the platform and the swimming pattern within 2 min were recorded. The escape latency was 120 s if the rat could not find the platform within 2 min; the rat was guided by the observer with a wooden rod to the platform, and placed back in the cage after 10 s on the platform.
For memory probe trials, the escape platform was removed on day 6 of the experiment, and the rat was placed into the pool from a random location. The swimming pattern and time were recorded within 2 min, and the time spent in each quadrant was recorded.
Tissue processing
On the day of sacrifice, after completion of the Morris water maze test, eight rats from each group were given 3.5% sodium chloride by intraperitoneal injection; the brains were then collected in a dish on ice. The bilateral hippocampus was collected and stored in sterile vials that were pretreated with diethylpyrocarbonate-treated water overnight. After being stored for 2–3 h in liquid nitrogen, the samples were then stored at −80°C. The other eight rats from each group were perfused intracardially with 4% paraformaldehyde, fixed and the hippocampus was collected. Sections as thin as 4 μm were sliced from paraffin-embedded hippocampus for subsequent terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay.
Apoptosis of hippocampal neuron was determined by TUNEL assay
We used the TUNEL assay as a sensitive method of measuring apoptosis.25 After paraffin dewaxing was completed, sections were incubated with protease K (20 mg/L, pH 7.4–8.0) followed by TUNEL reaction mixture (Sigma-Aldrich) and converter-peroxidase for 1 h at 37°C, respectively. 3,3′-Diaminobenzidine substrate was then applied onto the sections at room temperature. The slides were counterstained with hematoxylin. TUNEL reactions without terminal deoxynucleotidyl transferase enzyme were performed as negative controls.
JNK-3 messenger RNA (mRNA) in hippocampus was evaluated by reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the hippocampus tissues by one-step Trizol (TakaRa, Dalian, People’s Republic of China) method. RNA purity was determined using absorbance at 260 and 280 nm (A260/280), and the integrity of the RNA was verified by electrophoresis on formaldehyde gels. The parameters for the reverse transcription step were 30°C, 10 min; 50°C, 30 min; 99°C, 5 min; 5°C, 5 min. The cDNA synthesized in the reverse transcription reaction was used as a template for the polymerase chain reaction. A total of 10 μL of cDNA, 0.5 μL of sense and antisense primer, respectively, were used in the reaction mixture (Table 1). The amplification conditions were 94°C for 3 min; 35 cycles of 94°C for 30 s, 57°C for 30 s and 72°C for 40 s; 3 min final extension at 72°C and 4°C hold. A RT-PCR Kit was purchased from TakaRa. The results were run on an agarose gel and analyzed by Quantity One-4.6.2 software (Bio-Rad Laboratories Inc., Hercules, CA, USA). The gray-scale ratios of target gene bands to β-actin bands were calculated as the relative levels of the target genes.
  | Table 1 Primer sequences for RT-PCR Abbreviations: JNK, c-Jun N-terminal kinase; RT-PCR, reverse transcription-polymerase chain reaction. |
Western blot analysis of JNK-3 and p-JNK-3 protein expression levels
Total proteins were extracted from the hippocampus tissues using the protein lysate kit (KeyGEN, Nanjing, People’s Republic of China). Then, equal amounts of proteins (30 µg) were separated by 5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene fluoride membranes (KeyGEN). The transferred membranes were blocked overnight in 5% non-fat milk diluted in Tris-buffered saline with 0.05% Tween 20. The membranes were then exposed to anti-JNK-3 monoclonal antibody (rat; Sigma-Aldrich) and rabbit anti-rat against p-JNK-3 polyclonal antibody and β-actin antibody (KeyGEN) at a dilution of 1:1000 for 2 h at 4°C. After washing, the membranes were incubated in horseradish peroxidase-conjugated secondary antibody (1:5000, goat anti-rabbit IgG and rabbit anti-rat IgG) for 1 h at 4°C. The resulting immunoblots were visualized using Super enhanced chemiluminescence substrate (Tiangen Biotechnology Co., Ltd., Beijing, People’s Republic of China). The results were analyzed by Quantity One-4.6.2 analysis software. The gray-scale ratios of target protein bands to β-actin bands were calculated as the relative expression levels of the proteins.
Statistical analysis
All statistical analyses were conducted using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as mean ± standard error of the mean. Statistical analysis was performed using one-way analysis of variance. P<0.05 was considered statistically significant.
Results
Effects of curcumin on spatial learning and memory deficits in Aβ-induced AD rats
The place navigation of the rats in the Morris water maze test is shown in Table 2. It shows that the escape latency was gradually shortened, indicating that the spatial learning ability in finding the escape platform was increased with a series of training. The escape latency of rats from the AD rat model group was significantly longer, compared with that of rats from the sham-operation group (P<0.05). The time of curcumin-treated group was similar to that of sham-operation group and significantly shorter than that of AD rat model group (P<0.05). The swimming pattern was along the pool wall or random, suggesting successful establishment of the animal model. Once the escape platform was removed as shown in Table 3, rats in the AD model group spent significantly less time in the original platform quadrant (the second quadrant) compared with the sham-operation group and the curcumin treatment group (P<0.05, Table 2), indicating significant retardation of memory. But there was no significant difference between curcumin treatment group and sham-operation group (P>0.05), suggesting that curcumin has a positive effect on cognitive impairment of rats and leads to improved learning and memory capability.
Effect of curcumin on neuronal apoptosis in the hippocampus of Aβ-induced AD rats
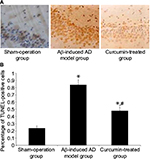
In the sham-operation group, blue products were found in cell nuclei. Sporadic TUNEL-positive cells in the hippocampus were found with an optical density value of 0.24±0.03. More TUNEL-positive cells were seen in the Aβ-induced AD model group as brown granular shapes in the nuclei, the optical density value of which was 0.84±0.07. However, granular cells were reduced in the curcumin-treated group and the optical density value was reduced to 0.48±0.05 (P<0.05, Figure 1).
Effects of curcumin on JNK-3 mRNA and protein expression levels in the hippocampus of AD model rats
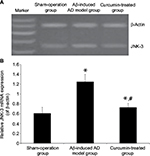
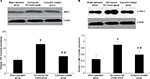
JNK-3 mRNA was significantly increased in the rat hippocampus of the Aβ-induced AD model group compared to the sham-operation group (1.25±0.15 versus 0.61±0.12, P<0.05; Figure 2). Results from Western blotting showed that the protein expression level of JNK-3 was also significantly induced in the Aβ-induced AD model group compared to the sham-operation group (1.75±0.17 versus 0.81±0.14, P<0.05; Figure 3A). However, after curcumin treatment, both mRNA and protein expression levels of JNK-3 were significantly decreased, compared to the Aβ-induced AD model group (0.73±0.08 versus 1.25±0.15 and 1.03±0.07 versus 1.75±0.17, respectively, all P<0.05), although they were still higher than those in the sham-operation rats (Figures 2 and 3A).
Effect of curcumin on the protein expression of p-JNK-3 in the hippocampus of AD
p-JNK-3 protein expression level followed a similar pattern to the expression of total JNK-3 (Figure 3B). The sham-operation group had a level of 0.45±0.13, and this was significantly increased in the Aβ-induced AD model group at 0.90±0.12 (P<0.05). The curcumin-treated group had a decreased p-JNK-3 protein expression level, compared to the Aβ-induced AD model group (0.59±0.13 versus 0.90±0.12, P<0.05), although it was still higher than that in the sham-operation rats (Figure 3B). Optical density of neuron apoptosis in the hippocampus was positively correlated with p-JNK expression in each group (r=0.46, P<0.05).
Discussion
Curcumin has diverse bioactivities including antioxidation, anti-inflammation and antiamyloidosis, making it potentially effective for AD treatment. The aim of this study was to investigate whether curcumin could reduce spatial learning and memory deficits in a rat model of AD and if the potential benefits were related to apoptosis and the activity of JNK-3. The study by Begum et al reported that the activation of JNK in the cortex of Tg2576 transgenic mouse was inhibited by curcumin,15 which was similar to our results. As JNK-3 is critical for apoptosis and is specifically expressed in the brain, we hypothesized that JNK-3 might have a role to play in inhibition of apoptosis by curcumin. The JNK pathway may be active in neurons around the region of Aβ deposits in AD patients, and this is associated with neuronal apoptosis.26
The results showed that curcumin reduced the effects of Aβ-induced neuronal injury in terms of spatial learning and memory, and this was accompanied by a reduction in the level of apoptosis and reduced levels of JNK-3 mRNA and protein. Curcumin also reduced the levels of phosphorylated JNK-3 that had been increased by Aβ injury.
Aβ-induced neuronal injury rat model is an important method for studying the pathologic development of AD, with advantages over other methods such as it is less expensive and can be undertaken during a short experimental period.19–22 We have also had experience of the method and found it easy to use in previous studies.27 The rats in this study clearly demonstrated significant spatial learning and memory deficits when the AD model was established. These deficits were then reduced to levels similar to those of the sham-operation group when the rats received curcumin treatment. Cell death by apoptosis has been a hot topic of research into AD pathologic development in recent studies, and it is suggested that excessive apoptosis might be the main reason for AD.28–33
In this study, we found in TUNEL assay that neuronal apoptosis was increased by the injection of Aβ in the hippocampus, which was inhibited by curcumin treatment. Other studies investigating the role of curcumin in AD have found similar results using flow cytometry.34–36 This led us to investigate the mechanism by which curcumin might inhibit apoptosis. A study of this kind has some limitations; any animal model, however close to the disease state, may have some differences from the clinical situation. Also, we did not perform a full evaluation of all the other factors related to JNK signaling or measures of oxidative stress and inflammation.
Conclusion
Our work demonstrated a significant increase in the mRNA and protein expression levels of JNK-3 and p-JNK-3 in the hippocampus of Aβ-induced AD model rats. However, both mRNA and protein expression levels of JNK-3 and p-JNK-3 were inhibited by curcumin administration. The results indicated that curcumin treatment may ameliorate spatial learning and memory deficits as well as neuronal apoptosis in the hippocampus after Aβ injection, which might be associated with inhibition of JNK-3 phosphorylation. This suggests a preliminary mechanism for the protective effect of curcumin on Aβ-induced neuronal injury in rats.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under the grant number 30770762. Our sincere thanks to Dr Ming-yao Du and Jing Zhao, who made great contributions to the behavioral tests.
Disclosure
The authors report no conflicts of interest in this work.
References
Zhang Y, Xu Y, Nie H, et al. Prevalence of dementia and major dementia subtypes in the Chinese populations: a meta-analysis of dementia prevalence surveys, 1980–2010. J Clin Neurosci. 2012;19(10):1333–1337. | ||
Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014; 6:37–48. | ||
Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging. 2014; 35(Suppl 2):S29–S34. | ||
Qiao L, Huang H, Muresanu DF. Clinical neurorestorative progress in Alzheimer’s disease. Journal of Neurorestoratology. 2015; 3:1–9. | ||
Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J Neurochem. 2000;75(1):117–124. | ||
McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126(4):479–497. | ||
Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13(18):1875–1886. | ||
Yoon SO, Park DJ, Ryu JC, et al. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron. 2012;75(5):824–837. | ||
Kuan CY, Whitmarsh AJ, Yang DD, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100(25):15184–15189. | ||
Xu Y, Hou XY, Liu Y, Zong YY. Different protection of K252a and N-acetyl-L-cysteine against amyloid-beta peptide-induced cortical neuron apoptosis involving inhibition of MLK3-MKK7-JNK3 signal cascades. J Neurosci Res. 2009;87(4):918–927. | ||
Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28(9):923–934. | ||
Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44(2):97–111. | ||
Zhou Y, Su Y, Li B, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003; 302(5648):1215–1217. | ||
Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA. NSAID and antioxidant prevention of Alzheimer’s disease: lessons from in vitro and animal models. Ann N Y Acad Sci. 2004;1035:68–84. | ||
Begum AN, Jones MR, Lim GP, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326(1):196–208. | ||
Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. | ||
Ma QL, Yang F, Rosario ER, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29(28):9078–9089. | ||
Rissman RA, Mishizen-Eberz AJ, Carter TL, et al. Biochemical analysis of GABA(A) receptor subunits alpha 1, alpha 5, beta 1, beta 2 in the hippocampus of patients with Alzheimer’s disease neuropathology. Neuroscience. 2003;120(3):695–704. | ||
Qiu G, Wu XQ, Luo XG. [Effect of polygonum multiflorum thunb on BDNF expression in rat hippocampus induced by amyloid beta-protein (Abeta) 1–40]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006; 31(2):194–199. | ||
Khazipov R, Zaynutdinova D, Ogievetsky E, et al. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front Neuroanat. 2015;9:161. | ||
Fan H, Guo Y, Liang X, et al. Hydrogen sulfide protects against amyloid beta-peptide induced neuronal injury via attenuating inflammatory responses in a rat model. J Biomed Res. 2013;27(4):296–304. | ||
Rahimian R, Fakhfouri G, Ejtemaei Mehr S, et al. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur J Clin Invest. 2013;43(10):1039–1051. | ||
Wang Y, Yin H, Li J, et al. Amelioration of beta-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associated with suppression of CRMP-2 hyperphosphorylation. Neurosci Lett. 2013;557 Pt B:112–117. | ||
Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74(8):969–985. | ||
Deng X, Wang Y, Chou J, et al. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93(1):64–69. | ||
Sclip A, Arnaboldi A, Colombo I, et al. Soluble Aβ oligomer-induced synaptopathy: c-Jun N-terminal kinase’s role. J Mol Cell Biol. 2013; 5(4):277–279. | ||
Yin HL, Wang YL, Li JF, et al. Effects of curcumin on hippocampal expression of NgR and axonal regeneration in Aβ-induced cognitive disorder rats. Genet Mol Res. 2014;13(1):2039–2047. | ||
Modi KK, Jana A, Ghosh S, et al. A physically-modified saline suppresses neuronal apoptosis, attenuates tau phosphorylation and protects memory in an animal model of Alzheimer’s disease. PLoS One. 2014;9(8):e103606. | ||
Cervia D, Perrotta C, Moscheni C, et al. Nitric oxide and sphingolipids control apoptosis and autophagy with a significant impact on Alzheimer’s disease. J Biol Regul Homeost Agents. 2013;27(2 Suppl):11–22. | ||
Song J, Park KA, Lee WT, Lee JE. Apoptosis signal regulating kinase 1 (ASK1): potential as a therapeutic target for Alzheimer’s disease. Int J Mol Sci. 2014;15(2):2119–2129. | ||
Thinnes FP. Apoptogenic interactions of plasmalemmal type-1 VDAC and Abeta peptides via GxxxG motifs induce Alzheimer’s disease – a basic model of apoptosis? Wien Med Wochenschr. 2011;161(9–10):274–276. | ||
Bertoni-Freddari C, Fattoretti P, Casoli T, et al. Neuronal apoptosis in Alzheimer’s disease: the role of age-related mitochondrial metabolic competence. Ann N Y Acad Sci. 2009;1171:18–24. | ||
Genc S, Egrilmez MY, Yaka E, et al. TNF-related apoptosis-inducing ligand level in Alzheimer’s disease. Neurol Sci. 2009;30(3):263–267. | ||
Ahmed T, Gilani AH. A comparative study of curcuminoids to measure their effect on inflammatory and apoptotic gene expression in an Abeta plus ibotenic acid-infused rat model of Alzheimer’s disease. Brain Res. 2011;1400:1–18. | ||
Mulik RS, Monkkonen J, Juvonen RO, et al. ApoE3 mediated poly (butyl) cyanoacrylate nanoparticles containing curcumin: study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol Pharm. 2010;7(3):815–825. | ||
Pan R, Qiu S, Lu DX, et al. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin Med J (Engl). 2008;121(9):832–839. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.