Back to Journals » Drug Design, Development and Therapy » Volume 9
Cromoglycate, not ketotifen, ameliorated the injured effect of warm ischemia/reperfusion in rat liver: role of mast cell degranulation, oxidative stress, proinflammatory cytokine, and inducible nitric oxide synthase
Authors El-Shitany N , El-Desoky K
Received 12 May 2015
Accepted for publication 2 August 2015
Published 16 September 2015 Volume 2015:9 Pages 5237—5246
DOI https://doi.org/10.2147/DDDT.S88337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Nagla A El-Shitany,1,2 Karema El-Desoky3
1Department of Pharmacology and Toxicology, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia; 2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tanta University, Tanta, Egypt; 3Department of Pathology, Faculty of Medicine, Tanta University, Tanta, Egypt
Abstract: Hepatic ischemia/reperfusion (ISCH/REP) is a major clinical problem that is considered to be the most common cause of postoperative liver failure. Recently, mast cells have been proposed to play an important role in the pathophysiology of ISCH/REP in many organs. In contrast, the role played by mast cells during ISCH/REP-induced liver damage has remained an issue of debate. This study aimed to investigate the protective role of mast cells in order to search for an effective therapeutic agent that could protect against fatal ISCH/REP-induced liver damage. A model of warm ISCH/REP was induced in the liver of rats. Four groups of rats were used in this study: Group I: SHAM (normal saline, intravenously [iv]); Group II: ISCH/REP; Group III: sodium cromoglycate + ISCH/REP (CROM + ISCH/REP), and Group IV: ketotifen (KET) + ISCH/REP (KET + ISCH/REP). Liver damage was assessed both histopathologically and biochemically. Mast cell degranulation was assessed histochemically. Lipid peroxidation (malondialdehyde [MDA]) as well as the levels of glutathione (GSH), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), the formation of nitric oxide (NO), and the expression of inducible NO synthase (iNOS) were determined. The results of this study revealed increased mast cell degranulation in the liver during the acute phase of ISCH/REP. Moreover, CROM, but not KET, decreased the activity of alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase and maintained normal liver tissue histology. Both CROM and KET protected against mast cell degranulation in the liver. In addition, both CROM and KET decreased IL-6 and TNF-α. However, CROM, but not KET, decreased MDA formation and increased GSH. Furthermore, KET, but not CROM, increased both NO formation and iNOS expression. In conclusion, this study clearly demonstrated mast cell degranulation in warm ISCH/REP in the liver of rats. More importantly, CROM, but not KET, ameliorated the effect of ISCH/REP-induced injury in rat liver. CROM may protect the liver through mast cell stabilization, inhibition of TNF-α, IL-6, MDA, and iNOS and increased GSH. KET may maintain ISCH/REP-induced liver injury through the NO/iNOS pathway.
Keywords: ischemia/reperfusion, mast cells, liver, sodium cromoglycate, ketotifen, nitric oxide, inducible nitric oxide synthase
Introduction
Hepatic ischemia reperfusion injury (ISCH/REP) is a major clinical problem that arises during liver transplantation and liver partial resection surgery. It is one of the most common causes of postoperative liver dysfunction, graft rejection, and chronic liver diseases.1–4 Until recently, there has been no effective therapy to prevent or treat ISCH/REP-induced liver injury.1
Several hypotheses have explained ISCH/REP-induced tissue damage including, microcirculatory injury, reactive oxygen species (ROS) and reactive nitrogen species generation, leukocyte adhesion, release of proinflammatory cytokines, and mast cell degranulation.5–8 Recently, there has been growing evidence that mast cells play an important role in ISCH/REP-induced damage in many organs, including the intestines, the heart, the lung, and the brain.9,10 However, the exact role of mast cells in ISCH/REP-induced liver damage has not been well clarified. A recent in vitro study concluded that mast cells are not involved in ISCH/REP-induced liver damage.11 On the other hand, Yang et al recently provided evidence that mast cell degranulation was involved in ISCH/REP-induced hepatic injury in rats.12
Ischemia-induced ROS generation causes mast cell degranulation and, hence, the release of histamine and many inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). The release of ROS as well as many inflammatory mediators has been found to contribute to and aggravate REP-induced tissue injury.10,12 This gives rise to the hypothesis that mast cells might play an important role in ISCH/REP-induced liver injury and, consequently, mast cell stabilizing agents could be an effective therapy that might protect against ISCH/REP-induced hepatic damage.
This study aimed first to reassess the role of mast cells in warm ISCH/REP-induced liver injury in rats. Second, it investigated and compared the supposed protective effect of two mast cell stabilizers, sodium cromoglycate (CROM) and ketotifen (KET), against ISCH/REP-induced liver injury. Finally, it studied the precise mechanisms of the probable hepatoprotective role of CROM and/or KET, focusing on ROS, nitric oxide (NO), and inflammatory cytokine production.
Materials and methods
Chemicals
Opticrom (4% CROM) (Sanofi-Aventis, Cairo, Egypt) and Zaditen (1 mg KET) (Novartis, Cairo, Egypt) were used in this study. All other chemicals are of analytical grades.
Animals
Twenty-four male albino rats (180–200 g) were used in this study. The rats were maintained under controlled conditions and had free access to food and water. The animals were fasted 12 hours before ISCH/REP induction. The animals were cared for according to institutional guidelines for the care and use of laboratory animals at King Fahd Medical Research Center. All experiments were performed according to the rules and regulations of the Saudi National Committee of Ethics with regard to animal research.
Experimental protocol
Four groups of rats (n=6) were assigned as follows: Group I: SHAM (normal saline, iv); Group II: ISCH/REP; Group III: CROM + ISCH/REP (CROM 50 mg/kg, iv), and Group IV: KET + ISCH/REP (KET 1 mg/kg, iv).9,13
Induction of liver, acute warm ISCH/REP
Total hepatic ISCH was induced by clamping the common hepatic artery and portal vein for 45 minutes, followed by 2 hours REP.7,12 The rats were treated with normal saline, CROM, or KET 0 minute before ISCH induction and immediately after the REP. An additional CROM dose was injected 30 minutes after the REP because of its short half-life.8,9
Sample collection
At the end of the perfusion period, liver samples were collected and kept either frozen (−80°C) or in 10% buffered formalin solution. In addition, blood samples were withdrawn and plasma was separated.
Sample preparation for the biochemical analysis
Frozen livers (−80°C) were used for the analysis of lipid peroxidation (malondialdehyde [MDA]) and to determine the levels of glutathione (GSH) and NO. Homogenization of the frozen liver samples was made in 50 mM potassium phosphate (pH 7.5).14
Assessment of plasma alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase enzyme activity
Concentrations of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were expressed in U/L according to the method recommended by Reitman and Frankel.15 The U/L values were determined using kits from Biodiagnostic (Cairo, Egypt). The absorbance of the samples was read at 546 nm against a reagent blank.
Total plasma lactic dehydrogenase (LDH) activity was measured using kits from Biodiagnostic. Total LDH activity (U/L) was assessed according to the method recommended by Henry.16 The increase in absorbance due to the formation of NADH was measured spectrophotometrically at 340 nm at 1-minute intervals for 3 minutes.
Determination of MDA
A Biodiagnostic kit was used to measure the lipid peroxidation product, MDA (nmol/g tissue), according to the method recommended by Uchiyama and Mihara.17 Briefly, thiobarbituric acid was added to the tissue homogenate and boiled in a water bath, then the color that was formed was extracted with n-butanol and measured at two distinct wavelengths: 535 nm and 525 nm.
Determination of reduced GSH
The level of GSH (nmol/g tissue) was determined according to the method described earlier by Ellman18 using a Biodiagnostic kit. This assay is based on the reduction of bis-(3-carboxy-4-nitrophenyl) disulfide reagent by the thiol group to form 2-nitro-5-mercaptobenzoic acid. The absorbance of the formed yellow color was measured spectrophotometrically at 412 nm.19
Determination of NO
A Biodiagnostic kit was used to measure NO (μmol/g tissue). NO was assayed spectrophotometrically (550 nm) by measuring total nitrate plus nitrite (NO3− + NO2−) using Griess reagent.20
Determination of IL-6 and TNF-α
Liver tissues were homogenized in ice-cold phosphate-buffered saline containing protease inhibitor cocktail and 0.05% Tween-20. The samples were centrifuged at 12,074× g for 10 minutes. The resulting supernatant was used to analyze the IL-6 and TNF-α levels in an ELISA as a part of the Assaymax IL-6 and TNF-α kits (Gentaur, Dublin, Ireland) using monoclonal antibodies specific for IL-6 and TNF-α, respectively. Cytokine concentrations were calculated using standard purified recombinant cytokines.
Histopathologic examination of the liver sections
The liver tissues were fixed in buffered formalin and embedded in paraffin wax. Then, 3–5 μm sections were stained with hematoxylin and eosin (H&E). Assessment of the histopathologic changes was done microscopically.
Liver mast cell degranulation analysis (toluidine blue staining)
The liver sections were stained with 0.5% toluidine blue in 0.5 M of hydrochloric acid. Degranulated mast cells were defined as the cells with reduced granule density.
Immunohistochemical determination of inducible nitric oxide synthase expression
Inducible nitric oxide synthase (iNOS) expression was detected by the immunostaining of the tissue sections prepared from formalin-fixed, paraffin-embedded liver using a kit obtained from Lab Vision (Fremont, CA, USA). An immunoperoxidase (PAP, peroxidase/anti-peroxidase) technique was adopted. In this way, the cytoplasm of each iNOS (+) cell was stained brown. The brown staining was graded as follows: no brown color (−) (negative staining), mild brown staining (+) (mild positive), moderate brown staining (++) (moderate positive), and severe brown staining (+++) (severe positive).
Semiquantitative analysis of antibody immunoreactivity was assessed using Image-Pro Plus from Media Cybernetics-USA software version 6.0. The software used in the analysis is labeling intensity (mean intensity) and extension of the reaction (area percentage) of iNOS in 30 field ×40 objective lens and ×10 ocular lens.
Statistical analysis
One-way analysis of variance test was used for comparison between different groups followed by Dunnett’s t-test (two-sided) multiple comparisons to detect significant differences among individual mean values of all groups. Results are expressed as the mean ± standard deviation. The level of significance was set at P≤0.05. Statistical analysis was generated using SPSS software for windows, version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
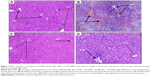
Effect of CROM and KET on ISCH/REP-induced injury changes in the microscopic appearance of liver sections after H&E staining
As shown in Figure 1, induction of ISCH/REP resulted in severe congestion and dilation of the portal tract vessels and central veins, with a mild inflammatory reaction in the portal tract (Figure 1B). Pretreatment of the ISCH/REP rats with CROM protected the liver against ISCH/REP-induced pathological changes (Figure 1C). On the other hand, the liver sections of the KET-pretreated ISCH/REP rats showed a mild inflammatory reaction of the portal tract together with a mild dilation and congestion of the portal tract and central veins (Figure 1D).
Effect of CROM and KET on plasma ALT, AST, and LDH levels in ISCH/REP-induced liver injury
As shown in Table 1, induction of ISCH/REP significantly increased the plasma ALT, AST, and LDH levels (approximately 16-, six-, and twofold, respectively) as compared with the rats in the SHAM group (P=0.000, 0.000, and 0.030, respectively). Pretreatment of the ISCH/REP rats with CROM significantly decreased the plasma ALT, AST, and LDH levels (62%, 25%, and 39%, respectively) compared with the ISCH/REP rats (P=0.001, 0.006, and 0.046, respectively). In contrast, pretreatment of the ISCH/REP rats with KET did not change the plasma ALT, AST, and LDH levels compared with the ISCH/REP rats (P=0.789, 0.855, and 0.54, respectively).
Effect of CROM and KET on liver MDA, reduced GSH, and NO concentrations in ISCH/REP-induced liver injury
Induction of ISCH/REP significantly increased both liver MDA and NO (81% and 18%, respectively) as compared with the SHAM rats (P=0.003 and P=0.012, respectively) (Figures 2 and 3). On the other hand, ISCH/REP significantly decreased the level of GSH (21%) as compared with the SHAM rats (P=0.01) (Figure 4). Pretreatment of the ISCH/REP rats with CROM significantly decreased liver MDA (29%) and increased liver GSH (22%) as compared with the ISCH/REP rats (P=0.025 and 0.009, respectively) (Figures 2 and 4). On the other hand, CROM did not affect NO formation in comparison with the ISCH/REP rats (P=0.119) (Figure 3). Pretreatment of the ISCH/REP rats with KET did not change either the hepatic MDA or GSH content in comparison with the ISCH/REP rats (P=0.48 and 0.341, respectively) (Figures 2 and 4). On the other hand, pretreatment of the ISCH/REP rats with KET significantly increased the liver NO (27%) content as compared with the ISCH/REP rats (P=0.003) (Figure 3).
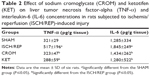
Effect of CROM and KET on liver TNF-α and IL-6 concentrations in ISCH/REP-induced liver injury
As shown in Table 2, induction of ISCH/REP significantly increased liver TNF-α and IL-6 (61% and 44%, respectively) (P=0.044 and 0.008, respectively) as compared with the SHAM rats. Pretreatment of the ISCH/REP rats with both CROM and KET significantly decreased liver TNF-α (38% and 44%, respectively) (P=0.050 and 0.026, respectively) and IL-6 (87% and 44%, respectively) (P=0.019 and 0.038, respectively) as compared with the ISCH/REP rats.
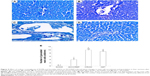
Effect of CROM and KET on ISCH/REP-induced injury changes in mast cell recruitment and granulation in liver sections after toluidine blue staining
Figure 5A showed normal mast cells around the portal tract. Induction of ISCH/REP resulted in increased drainage and degranulation of the mast cells around the portal tract (Figure 5B and E). Pretreatment of the ISCH/REP rats with both CROM and KET stabilized the mast cell membranes and protected them from degranulation (Figure 5C–E).
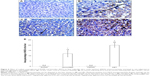
Effect of CROM and KET on ISCH/REP-induced injury changes in liver iNOS immunoreactivity
Figure 6A showed normal liver with no iNOS immunoreactivity. The liver sections of the ISCH/REP rats showed moderately positive immunoreactivity toward iNOS (P=0.0001) (Figure 6B and E). Pretreatment of the ISCH/REP rats with CROM resulted in negative iNOS immunoreactivity (Figure 6C and E). However, pretreatment of the ISCH/REP rats with KET resulted in a severe increase in iNOS immunoreactivity (P=0.0003) (Figure 6D and E).
Discussion
This study’s results revealed increased mast cell degranulation in the liver during the acute phase of warm ISCH/REP. Moreover, CROM protected the liver against ISCH/REP-induced acute injury, which was confirmed by the decrease in the activity of ALT, AST, and LDH and the normal liver tissue histology revealed by H&E staining. On the other hand, KET did not protect against acute phase ISCH/REP-induced liver injury. Both CROM and KET protected against mast cell degranulation in the liver that was induced during the acute phase of ISCH/REP. In addition, both CROM and KET decreased the levels of IL-6 and TNF-α that were induced in the liver during the acute phase of ISCH/REP. Taken together, CROM-induced inhibition of mast cell degranulation and proinflammatory cytokine release are not the key mechanisms accounting for its protective role against the acute phase of warm ISCH/REP in the liver. CROM, but not KET, decreased MDA formation and increased the GSH content in the liver during the acute phase of ISCH/REP, suggesting that CROM protected the liver against the toxic action of ISCH/REP through an antioxidant mechanism and that the same protective effect did not occur with KET. Furthermore, KET, but not CROM, increased both NO formation and iNOS expression in the liver during the acute phase of ISCH/REP. From our results, we could suggest that KET increased the reactive nitrogen species and NO through iNOS; hence, it promoted liver injury during the acute phase of ISCH/REP.
Our results appear to be consistent with Yang et al12 who found increased mast cell degranulation in the liver and a protective effect of CROM against ISCH/REP-induced liver injury. In addition, Huang et al21 recently reported on the protective role of CROM against liver injury triggered by small intestinal ISCH/REP. Conversely, Shibamoto et al11 found ISCH/REP-induced injury in the isolated perfused liver of genetically mast cell deficient (Ws/Ws) rats. The Shibamoto group analyzed ISCH/REP-induced injury 1 hour after REP, whereas we analyzed ISCH/REP-induced injury 2 hours after REP.11 Most of the research studies used the 2 hours or greater time frame to investigate the role of mast cells during ISCH/REP.12,22,23 To the best of our knowledge, no study has reported on the effect that KET has on ISCH/REP-induced liver injury; nonetheless, our results appear to be contrary to the findings from a previous study conducted by Huang et al21 who reported a protective role of KET against liver injury triggered by small intestinal ISCH/REP.
On the one hand, CROM produced a hepatoprotective effect while, on the other hand, KET did not have any effect on ISCH/REP-induced liver injury. Interestingly, this type of paradoxical effect could be explained, depending on the nature and mechanism of action of each agent. CROM is a mast cell membrane stabilizer, so it could inhibit mast cell degranulation and the release of histamine, TNF-α, and other inflammatory mediators.24 In contrast, KET is a second-generation histamine H1-receptor antagonist.25,26 In various experimental and clinical conditions, KET has been noted to reduce mast cell degranulation and to decrease the release of histamine, mast cell proteases, myeloperoxidase, leukotrienes, platelet-activating factor, and various prostaglandins.25,27–29 Depending on these facts and our results, we could conclude that both CROM and KET stabilized the mast cell membrane during ISCH/REP; hence, we expected that both would decrease histamine release. As KET, but not CROM, blocked the H1 receptor, our results suggested that the small amount of histamine released after mast cell stabilization played a protective role via the H1 receptor. A preliminary in vitro study showed that histamine, at slightly elevated concentrations, aggravated hypoxia/reoxygenation-induced rat liver BRL-3A cell injury.4 Therefore, it was reasonable to speculate that ISCH/REP might mediate liver injury primarily via mast cell activation and the subsequent elevated release of histamine.21
Toxic substances released after ISCH/REP, including MDA, ROS, neutrophils, pro-inflammatory cytokines, and adhesion molecules, contribute to liver injury through portal circulation and induced oxidative stress and inflammatory responses in the liver.21 Excessive degranulation of mast cells elicited by a severe ischemic insult has been shown to cause the release of certain biochemical mediators to an extent that was cytotoxic; thus, it mediated ischemic cell death and the accompanying secondary inflammatory processes.30 Mast cells were abundant in the microcirculatory beds in which the inflammatory effects of ISCH were demonstrated and they are known to degranulate and release various inflammatory mediators, including leukotriene B4 and platelet activating factor. Mast cells have been shown to play a role in leukocyte recruitment into tissue after ISCH/REP.31,32 The present study showed significantly increased levels of TNF-α and IL-6 in the liver of the ISCH/REP rats as compared with the SHAM rats. These results are consistent with the findings of many previous studies.33–35 Similar to our results, previous studies have found that treatment with either CROM or KET could significantly decrease the levels of TNF-α and IL-6 in the intestine in comparison with an intestinal ISCH/REP model.10,36
The small amount of NO, which was generated from endothelial NOS, was essential to maintain microvascular circulation. Consequently, it could protect the liver cells against early ISCH/REP-induced damage.37 The large amount of NO, which was formed by iNOS, has been shown to have a significant role in numerous immune and inflammatory reactions.38 However, the impact of iNOS in liver warm ISCH/REP injury is still an issue of debate.39 Previously, it was reported that aminoguanidine, a selective inhibitor of iNOS, reduced liver warm ISCH/REP injury in rats.40 Another study reported that a selective inhibitor of iNOS exacerbated liver damage during warm ISCH/REP.41 As a product of iNOS, NO might act as an antioxidant and/or a prooxidant molecule.42 NO reacted with a superoxide radical to form peroxynitrite anion, a potent nitrogen-free radical that damages cells.43 However, as an antioxidant, NO reacted with the superoxide anion and removed it.44,45 The results of the present study showed that ISCH/REP induced an increase in iNOS immunoreactivity, which was further increased upon the administration of KET. CROM inhibited ISCH/REP-induced iNOS immunoreactivity. In addition, both ISCH/REP and KET increased the NO content in the liver. Similar to our results, Trocha et al46 reported a significant increase in iNOS protein concentration caused by ISCH/REP in the liver of rats. In addition, Jiang et al47 reported activated iNOS gene transcripts in warm ISCH/REP rats. It has also been previously reported that KET enhanced iNOS activity in the renal cortex of rats and in human colonic tissue homogenates.48 Taken together, CROM might protect against warm ISCH/REP-induced liver through the inhibition of iNOS immune expression; moreover, KET might maintain warm ISCH/REP-induced liver injury by increasing the NO content in the liver and by increasing iNOS immune expression.
The present study results showed that CROM, but not KET, decreased MDA and increased GSH in the liver after ISCH/REP. The lipid peroxidation product, MDA, was used as a quantitative measure of liver oxidative stress. In the present study, ISCH/REP increased liver MDA, which was accompanied by increased NO and iNOS, indicating that iNOS plays a role in liver damage during warm ISCH/REP via ROS. Non-enzymatic (GSH) and enzymatic (superoxide dismutase and glutathione peroxidase) antioxidants have been found to be involved in minimizing the damaging effects of ROS on the liver.49 Our results showed that CROM increased GSH in the liver after ISCH/REP. This finding might suggest that GSH plays a protective role in ISCH/REP-induced liver injury through improved antioxidant defense.
In conclusion, this study clearly demonstrated mast cell degranulation in warm ISCH/REP in the liver of rats. More importantly, CROM, but not KET, was found to ameliorate the injury effect of ISCH/REP in the liver of rats. CROM may protect the liver through mast cell stabilization, inhibition of TNF-α, IL-6, MDA, and iNOS, and increased GSH. Moreover, KET may maintain ISCH/REP-induced liver injury through the NO/iNOS pathway.
Recommendation
More studies are needed to resolve the actual role that the mast cell stabilizers, CROM and KET, play in warm ISCH/REP-induced liver injury.
Acknowledgments
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No 166-529-D1435. The authors therefore, gratefully acknowledge the DSR technical and financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
Ren F, Duan Z, Cheng Q, et al. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54(2):687–696. | ||
Dai Y, Cui J, Cun Y, Shi A. Tetrahydrobiopterin ameliorates hepatic ischemia-reperfusion Injury by coupling with eNOS in mice. J Surg Res. 2012;176(2):e65–e71. | ||
Feng M, Wang H, Wang Q, Guan W. Matrix metalloprotease 9 promotes liver recovery from ischemia and reperfusion injury. J Surg Res. 2013;180(1):156–161. | ||
Wu T, Gan X, Zhou S, Ge M, Zhang Z, Hei Z. Histamine at low concentrations aggravates rat liver BRL-3A cell injury induced by hypoxia/reoxygenation through histamine H2 receptor in vitro. Toxicol In Vitro. 2013;27(1):378–386. | ||
Jessup JM, Battle P, Waller H, et al. Reactive nitrogen and oxygen radicals formed during hepatic ischemia-reperfusion kill weakly metastatic colorectal cancer cells. Cancer Res. 1999;59(8):1825–1829. | ||
Han JY, Fan JY, Horie Y, et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117(2):280–295. | ||
Ozkan E, Yardimci S, Dulundu E, et al. Protective potential of montelukast against hepatic ischemia/reperfusion injury in rats. J Surg Res. 2010;159(1):588–594. | ||
Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013;19(11):1683–1698. | ||
Cordeiro PG, Lee JJ, Mastorakos D, Hu QY, Pinto JT, Santamaria E. Prevention of ischemia-reperfusion injury in a rat skin flap model: the role of mast cells, cromolyn sodium, and histamine receptor blockade. Plast Reconstr Surg. 2000;105:654–659. | ||
Hei Z, Gan X, Luo G, Li S, Cai J. Pretreatment of cromolyn sodium prior to reperfusion attenuates early reperfusion injury after the small intestine ischemia in rats. World J Gastroenterol. 2007;13(38):5139–5146. | ||
Shibamoto T, Tsutsumi M, Kuda Y, Ohmukai C, Zhang W, Kurata Y. Mast cells are not involved in the ischemiareperfusion injury in perfused rat liver. J Surg Res. 2012;174:114. | ||
Yang MQ, Ma YY, Tao SF, et al. Mast cell degranulation promotes ischemia-reperfusion injury in rat liver. J Surg Res. 2014;186(1):170–178. | ||
Vural KM, Liao H, Oz MC, Pinsky DJ. Effects of mast cell membrane stabilizing agents in a rat lung ischemia-reperfusion model. Ann Thorac Surg. 2000;69(1):228–232. | ||
Uraz S, Tahan V, Aygun C, et al. Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci. 2008;53(4):1071–1077. | ||
Reitman S, Frankel S. Colorimetric method for aspartate and alanine amino transferases. Am J Clin Pathol. 1957;28:56–63. | ||
Henry RJ. Colorimetric determination of lactic dehydrogenase. In: Henery RJ, editor. Clinical Chemistry: Principles and Techniques. 2nd ed. Hagerstown, NJ: Harper and Row; 1974:819–831. | ||
Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1979;86:271–278. | ||
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;74:214–226. | ||
Theodorus P, Akerboom M, Sies H. Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Meth Enzymol. 1981;77:373–383. | ||
Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. | ||
Huang P, Gan X, Liu J, Liu D, Wang Y, Hei Z. Effects of anti-histamine treatment on liver injury triggered by small intestinal ischemia reperfusion in rats. Chin J Physiol. 2014;57(5):271–278. | ||
Hei ZQ, Gan XL, Huang PJ, Wei J, Shen N, Gao WL. Influence of ketotifen, cromolyn sodium, and compound 48/80 on the survival rates after intestinal ischemia reperfusion injury in rats. BMC Gastroenterol. 2008;8:42. | ||
Santen S, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Mast-cell-dependent secretion of CXC chemokines regulates ischemia-reperfusion-induced leukocyte recruitment in the colon. Int J Colorectal Dis. 2008;23:527. | ||
Hemmati AA, Nazari Z, Motlagh ME, Goldasteh S. The role of sodium cromolyn in treatment of paraquat-induced pulmonary fibrosis in rat. Pharmacol Res. 2002;46(3):229–234. | ||
Craps LP, Ney UM. Ketotifen: current views on its mechanism of action and their therapeutic implications. Respiration. 1984;45(4):411–421. | ||
Grant SM, Goa KL, Fitton A, Sorkin EM. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40(3):412–448. Review. Erratum in: Drugs. 1991;41(2):192. | ||
Karmeli F, Eliakim R, Okon E, Rachmilewitz D. Gastric mucosal damage by ethanol is mediated by substance P and prevented by ketotifen, a mast cell stabilizer. Gastroenterology. 1991;100(5 Pt 1):1206–1216. | ||
Pothoulakis C1, Karmeli F, Kelly CP, et al. Ketotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileum. Gastroenterology. 1993;105(3):701–707. | ||
Grönbech JE, Lacy ER. Substance P attenuates gastric mucosal hyperemia after stimulation of sensory neurons in the rat stomach. Gastroenterology. 1994;106(2):440–449. | ||
Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. | ||
Kubes P, Granger DN. Leukocyte-endothelial cell interactions evoked by mast cells. Cardiovasc Res. 1996;32:699–708. | ||
Kurose I, Argenbright LW, Wolf R, Lianxi L, Granger DN. Ischemia/reperfusion-induced microvascular dysfunction: role of oxidants and lipid mediators. Am J Physiol Heart Circ Physiol. 1997;272:H2976–H2982. | ||
Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85(6):1936–1943. | ||
Scales WE, Campbell DA Jr, Green ME, Remick DG. Hepatic ischemia/reperfusion injury: importance of oxidant/tumor necrosis factor interactions. Am J Physiol. 1994;267(6 Pt 1):G1122–G1127. | ||
El-Mahdy NA, El-Sisi AE, Dewidar BI, El-Desouky KI. Histamine protects against the acute phase of experimentally-induced hepatic ischemia/re-perfusion. J Immunotoxicol. 2013;10(1):9–16. | ||
Zi-qing H, Xiao-liang G, Pin-jie H, Jing W, Ning S, Wan-ling G. Influence of Ketotifen, Cromolyn Sodium, and Compound 48/80 on the survival rates after intestinal ischemia reperfusion injury in rats. BMC Gastroenterol. 2008;8:42. | ||
Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–565. | ||
Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005;19:1155–1157. | ||
Shah V, Kamath PS. Nitric oxide in liver transplantation: pathobiology and clinical implications. Liver Transpl. 2003;9:1–11. | ||
Yaylak F, Canbaz H, Caglikulekci M, et al. Liver tissue iNOS expression and lipid peroxidation in experimental hepatic ischemia reperfusion injury stimulated with lipopolysaccharide: the role of aminoguanidine. J Surg Res. 2008;148:214–223. | ||
Hsu CM, Wang JS, Liu CH, Chen LW. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock. 2002;17:280–285. | ||
Casini A, Galli G, Salzano R, Rotella CM, Surrenti C. Acetaldehyde-protein adducts, but not lactate and pyruvate, stimulate gene transcription of collagen and fibronectin in hepatic fat-storing cells. J Hepatol. 1993;19:385–392. | ||
Crow JP, Spruell C, Chen J, et al. On the pH-dependent yield of hydroxyl radical products from peroxynitrite. Free Radic Biol Med. 1994;16:331–338. | ||
Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. | ||
Rubbo H, Radi R, Trujillo M, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. | ||
Trocha M, Merwid-Lad A, Szuba A, et al. Effect of simvastatin on nitric oxide synthases (eNOS, iNOS) and arginine and its derivatives (ADMA, SDMA) in ischemia/reperfusion injury in rat liver. Pharmacol Rep. 2010;62(2):343–351. | ||
Jiang W, Kong L, Li G, Wang X. Expression of iNOS in early injury in a rat model of small-for-size liver transplantation. Hepatobiliary Pancreat Dis Int. 2009;8(2):146–151. | ||
Heyman SN, Karmeli F, Brezis M, Rachmilewitz D. The effect of ketotifen on nitric oxide synthase activity. Br J Pharmacol. 1997;120:1545–1551. | ||
Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577–596. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.