Back to Journals » Drug Design, Development and Therapy » Volume 12
Comparison of the efficacy and survival analysis of neoadjuvant chemotherapy for Her-2-positive breast cancer
Authors Li S, Wei W, Jiang Y, Li Q, Huang Q, Yang H, Liu J
Received 19 April 2018
Accepted for publication 14 August 2018
Published 21 September 2018 Volume 2018:12 Pages 3085—3093
DOI https://doi.org/10.2147/DDDT.S171534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Si Li,1,* Wei Wei,1,* Yi Jiang,1 Qiuyun Li,1 Qinghua Huang,1 Huawei Yang,1 Jianlun Liu1,2
1Department of Breast Surgery, The Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, China; 2Department of General Surgery, Longdong Hospital of Guangxi Medical University, Nanning, Guangxi, China
*These authors contributed equally to this work
Purpose: The objective of this research was to compare the short- and long-term efficacy of the following four neoadjuvant chemotherapy (NAC) regimens: docetaxel/carboplatin/trastuzumab (TCH), docetaxel/epirubicin/cyclophosphamide (TEC), Xeloda/epirubicin/cyclophosphamide followed by Xeloda/docetaxel (XEC-XT), and 5-fluorouracil/epirubicin/cyclophosphamide followed by docetaxel (FEC-T) in human epidermal growth factor receptor-2-positive (Her-2-positive) breast cancer.
Patients and methods: According to treatment preferences, 139 patients with Her-2-positive breast cancer were divided into the following four groups: 39 patients in the TCH group, 35 patients in the TEC group, 33 patients in the XEC-XT group, and 32 patients in the FEC-T group. The primary end points were disease-free survival (DFS) and 5-year overall survival (5-year OS). The secondary end points were the efficacy and toxicity of NAC.
Results: The TCH, TEC, XEC-XT, and FEC-T groups demonstrated overall response rates of 87.1%, 74.3%, 75.8%, and 62.5% (P=0.031), respectively, and pathological complete response rates of 25.6%, 18.2%, 20.0%, and 18.2% (P=0.041), respectively. The DFS rates for the TCH, TEC, XEC-XT, and FEC-T groups were 84.6%, 62.9%, 65.7%, and 46.9% (P=0.01), respectively. The 5-year OS rates for the TCH, TEC, XEC-XT, and FEC-T groups were 87.2%, 69.7%, 71.4%, and 59.4% (P=0.069), respectively. The mean survival time was 59.3 months (TCH group), 53.5 months (TEC group), 55.3 months (XEC-XT group), and 52.4 months (FEC-T group). The difference in survival among the four groups was statistically significant (P=0.04).
Conclusion: In four NAC regimens for the treatment of Her-2-positive breast cancer, the TCH group exhibited better DFS and 5-year OS. The TCH regimen significantly enhanced the pathological complete remission rate of NAC with similar side effects compared to the TEC, XEC-XT, and FEC-T regimens. In terms of long-term efficacy, the XEC-XT treatment was superior to the FEC-T and TEC treatment, and there was no significant difference between the FEC-T and TEC groups.
Keywords: Her-2-positive breast cancer, neoadjuvant chemotherapy, trastuzumab, efficacy, survival analysis, toxic side effects
Introduction
Breast cancer is the most common cancer affecting women worldwide. It is estimated that in 2018, there will be ~260,000 women diagnosed with breast cancer. This means that breast cancer alone accounts for 30% of all new cancer diagnoses in women.1 Human epidermal growth factor receptor-2 (Her-2) is a transmembrane tyrosine kinase receptor implicated in cell growth, differentiation, and survival,2 and ~18%–20% of breast cancer patients have Her-2-positive disease.3 Extensive clinical trials have shown that Her-2-positive disease predicts poor prognosis compared to Her-2-negative disease.4
Neoadjuvant chemotherapy (NAC), which was originally proposed by Frei et al in the 1980s,5,6 has been extensively explored in recent years. Many studies have since explored the clinical significance of NAC in breast cancer treatment, including two well-known large-scale clinical trials, namely, the NSAPB (National Surgical Adjuvant Breast and Bowel Project) B18 and B27.7 NAC has attained widespread promotion and continuous application in clinics and is now an imperative part of the preoperative systematic treatment for breast cancer.8,9 In the strategy of NAC, the combination of chemotherapeutic drugs, anti-vascular-targeting drugs, and anti-Her-2 dual-targeting drugs is often used to further increase pathological complete remission (pCR), leading to greater survival benefits. This strategy has several advantages. First, with new adjuvant chemotherapy reducing the clinical stage, even patients who previously had difficulty in surgery have the opportunity for radical operation. Second, this strategy improves the success rate of conservative surgery and increases the satisfaction level of breast esthetics. Third, preoperative systemic chemotherapy is used to control tumor and subclinical metastasis, which can lead to improvements in the cure rate in early breast cancer or the enhancement of overall survival (OS) for patients with locally advanced cancers. Moreover, unlike adjuvant chemotherapy, indicated in the absence of any measurable disease, this strategy provides in vivo chemosensitivity testing and offers a reference for the future selection of adjuvant chemotherapy regimens.
Trastuzumab, namely, herceptin, which has a high affinity for the Her-2 receptor, was the first Her-2 molecular-targeted drug. The principal mechanism of action of trastuzumab is as follows: binding to the extracellular domain of the Her-2 receptor causes cell growth to be terminated at the G stage, so the replication ability is weakened. By reducing the expression of Her-2, the dimerization of the receptor is disrupted. In addition, trastuzumab can inhibit the activity of CDK2 through the signal transduction of the downstream P13K pathway and terminate the cell life cycle.10 Trastuzumab can also inhibit angiogenesis by inducing antiangiogenic factors and inhibiting angiogenesis factors.
Capecitabine, namely, Xeloda® (Shanghai Roche Pharmaceuticals Ltd., Shanghai, China), is a new 5-fluorouracil (5-FU) anti-metabolite drug. After oral ingestion, Xeloda is quickly absorbed by the intestinal mucosa and is then converted into the inactive intermediate 5′-DNA-5′ fluorine cytidine by carboxylic esterase in the liver. After the function of cytidine deaminase in the liver, tumor tissue converts the intermediate into 5′-deoxy-5′ fluorouridine, and finally the enzymes in the tumor tissue are catalyzed by thymidine phosphorylation of 5-FU, inhibiting cell division and interfering with RNA and protein synthesis.11,12 Since the concentration of thymidine phosphorylase in cancer cells is higher than that in the healthy tissues, capecitabine has selective and targeted anti-tumor effects. Due to the low concentration of 5FU in the healthy tissue, the toxic side effects are relatively small. In conclusion, capecitabine has advantages of convenient administration, few side effects, and definite curative effect in the treatment of breast and gastrointestinal cancer.13
Patients and methods
General clinical data
Between October 2010 and December 2014, the Affiliated Cancer Hospital of Guangxi Medical University (Guangxi, China) enrolled 139 female patients whose age ranged from 27 to 70 years, with a median age of 47 years. All patients had no previous history of malignancy. All patients underwent a preoperative biopsy, breast ultrasound, and mammography. All patients were diagnosed with invasive breast cancer for the first time by core needle biopsy. If prompted, the patient underwent a hollow needle biopsy of the axillary lymph nodes under ultrasound to determine lymph node metastasis. Prior to starting the NAC, all 139 famale patients had undergone CT scan, including the head, chest, abdomen, pelvis, and bone, and single-photon emission computed tomography (SPECT) to ensure that there was no distant metastasis. The patients had not received any special treatment, including traditional Chinese medicine, hormonal treatment, radiotherapy, surgery, or other chemotherapy regimens. The individual patient characteristics are shown in Table 1. The staging of the tumor was performed according to the international tumor TNM staging system. Written informed and chemotherapy consent were obtained prior to the study. This study was conducted with the approval of the tumor hospital affiliated with Guangxi Medical University, which conforms to the ethical standards of Helsinki’s 1964 declaration and its later revision.
Chemotherapy regimen
According to the treatment preference, 139 cases of Her-2-positive breast cancer were divided into the following four groups: 39 cases in the docetaxel/carboplatin/trastuzumab (TCH) group, 35 cases in the docetaxel/epirubicin/cyclophosphamide (TEC) group, 33 cases in the Xeloda/epirubicin/cyclophosphamide followed by Xeloda/docetaxel (XEC-XT) group, and 32 cases in the 5-FU/epirubicin/cyclophosphamide followed by docetaxel (FEC-T) group. The TCH regimen was administered as follows: on day 1, patients received a 75 mg/m2 intravenous injection of docetaxel and a carboplatin dose to achieve an area under the curve of 6. One chemotherapy cycle lasted 3 weeks, for a total of six courses. On day 2, trastuzumab was administered and then was administered every fourth week; the first dose was 8 mg/kg, followed by a course of 3 weeks with a dose of 6 mg/kg. Trastuzumab must be used for up to 1 year.
The XEC-XT regimen was composed of 1,000 mg/m2 capecitabine orally ingested twice daily on days 1 through 14, plus an infusion of 100 mg/m2 epirubicin and 500 mg/m2 cyclophosphamide on day 1. One chemotherapy cycle lasted 3 weeks, for a total of four courses. Additionally, patients received another four cycles of docetaxel (100 mg/m2 on day 1, via intravenous drip, every 3 weeks) and capecitabine (1,000 mg/m2 orally, twice daily on days 1 through 14) after surgery.
The FEC-T regimen was composed of an infusion of 500 mg/m2 5-FU on day 1 plus an infusion of 100 mg/m2 epirubicin and 500 mg/m2 cyclophosphamide on day 1 before surgery. One chemotherapy cycle lasted 3 weeks for a total of six courses. Patients received another four cycles of docetaxel (100 mg/m2 day 1, every 3 weeks) after surgery.
The TEC regimen included docetaxel (175 mg/m2), epirubicin (70 mg/m2), and cyclophosphamide (600 mg/m2), repeated every 3 weeks for one cycle, for a total of six cycles.
All patients with four degrees of leukocytopenia were admitted to the hospital immediately, followed by isolation, granulocyte-colony stimulating factor therapy, and injection of antibiotics to prevent infection. Upon completion of the adjuvant chemotherapy, all patients received radiotherapy and were concurrently treated with tamoxifen or letrozole when the hormone receptor (HR) was positive.
Evaluation of treatment efficacy
The maximal diameters of tumor masses and involved axillary lymph nodes were measured before NAC and before the operation. The responses to NAC were graded as pCR, clinical partial remission (cPR), progressive disease (PD), and stable disease (SD) according to the WHO criteria.14 In this study, pCR was defined as the absence of invasive carcinoma in either the primary site or the axillary node; however, pCR was still considered if the ductal carcinoma remained in situ in the primary site.15
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3.16
Histopathology
After NAC, the surgically resected tumor specimens were histologically graded and classified following H&E staining. All pathological examinations of the biopsies revealed invasive carcinoma, including 116 cases of invasive ductal carcinoma, 17 cases of invasive lobular carcinoma, and six cases of invasive ductal lobular carcinoma. The expression of estrogen receptor and progesterone receptor of all the pathological sections were examined by immunohistochemistry.17 According to the criteria established by Allred et al, semiquantitative tissue chemical assessment was used to analyze the results of ER and PR staining.18 When ≥10% of the cancer cells were positive by immunohistochemistry, the tumor’s molecular typing was classified into HR-positive.19 Her-2 state was assessed on a 0 (negative), 1 (weak positive), 2 (intermediate), and 3 (strongly positive) scale according to the standard set by Dako et al; staining scored as 3 was considered as Her-2 positive. When the score was 2 (intermediate), fluorescence in situ hybridization was necessary to estimate the Her-2 status.20
Follow-up
Follow-up started from the first day of treatment. All patients were followed up for 36–70 months until February 2018, with the median follow-up time of 49 months and the mean follow-up time of 48 months. Disease-free survival was defined as the period from the first day after NAC to the date of recurrence or metastasis. Total survival was defined as the period from the first day after NAC to the date of death or last visit.
Statistical methods
The hypothesis of this study was that disease-free survival (DFS) would be significantly improved in the trial group compared to the control group. In our preliminary experiment, the incidence of DFS was 58%, while the DFS in the trial groups was 67%, 78%, and 74%. The sample size was calculated with the minimum meaningful effect size. Using a two-sided alpha level of 5% and a beta error of 20%, it was determined that each group needed to recruit 28 participants. In addition, it was predicted that 10% of the participants in each group could be lost or withdrawn from this study; therefore, each group needed to include 32 participants.
This study was a retrospective clinical Phase III study and the data were analyzed by SPSS 24.0 statistical software (version 24.0; SPSS Inc., Chicago, IL, USA). The ANOVA test was used for the comparison of clinical and pathological response rates and toxicities in the two treatment groups. A P-value <0.05 was statistically significant. The survival data were described and compared with the Kaplan–Meier estimator.
Results
Adverse reactions
Tolerance of all regimens was good (Table 2). Four groups of patients exhibited varying degrees of gastrointestinal reactions, which could be eased with antiemetic treatment. A few patients had mild liver dysfunction, oral ulcers, rashes, and neurotoxicity, but these symptoms were well tolerated after symptomatic treatment. In the TCH group, echocardiography was used to monitor cardiac function at 1, 3, 6, and 9 months, and the cardiac systolic function was maintained in good condition with an ejection fraction greater than 60%. No chemotherapy-related death or cardiac dysfunction occurred in this study, and no episodes of congestive heart failure were observed. There were no statistically significant differences in the adverse reactions of alopecia, gastrointestinal reaction, or muscle pain. The incidence of grade 4 leukopenia was more prevalent in the TEC group compared with the TCH, XEC-XT, and FEC-T groups (54.3% vs 33.3%, 36.7%, and 25.0%, respectively, P=0.311). Moreover, in the XEC-XT group, hand-foot syndrome occurred more frequently compared with the TCH, TEC, and FEC-T groups (54.5% vs 12.5%, 17.1%, and 18.8%, respectively), demonstrating statistical significance (P=0.001).
Efficacy
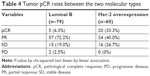
The efficacy of the four NAC regimens was not the same, and the patients experienced the following responses: in the TCH cohort (n=39), ten (25%) patients obtained pCR, whereas 24 (61.5%) patients obtained a pathologic partial response (pPR) and five (13.5%) patients had stable disease, considered as no response; in the XEC-XT group (n=33), six patients (18.2%) obtained pCR and 20 patients (60.6%) obtained pPR; in the FEC-T group (n=32), two patients (6.3%) obtained pCR and 18 (56.2%) patients obtained pPR; in the TEC group (n=35), seven (20%) patients obtained pCR and 19 patients (54.3%) obtained pPR. In the FEC-T group, two (6.2%) patients progressed. The TCH, XEC-XT, FEC-T, and TEC groups demonstrated overall response rates of 87.1%, 78.7%, 62.5% and 74.3%, respectively (Table 3). These results indicate that TCH chemotherapy was more efficacious compared with other chemotherapy regimens. In the luminal B arm, six patients (6.3%) obtained pCR; in the Her-2 overexpression arm , 20 patients (33.3%) obtained pCR. Tumor pCR rates were significantly different among the two molecular types of breast cancer between the two arms (P=0.002), as shown in Table 4. These findings demonstrate that Her-2 overexpression breast cancer is more sensitive than luminal B to neochemotherapy, and the possibility of obtaining CR is higher in Her-2 overexpression breast cancer compared with luminal B.
DFS and OS
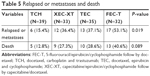
The recurrence and survival results of the patients during treatment are indicated in Table 5. In this study, six (15.4%) patients in the TCH group, 13 (37.1%) patients in the TEC group, 12 (36.4%) patients in the XEC-XT group, and 17 (53.1%) patients in the FEC-T group (P=0.01) relapsed or experienced metastasis during treatment. The Kaplan–Meier analysis was used to analyze the DFS and the 5-year OS. During the 5 years of follow-up, the survival rate of each group was as follows: 87.2% (TCH group), 71.4% (TEC group), 72.7% (XEC-XT group), and 59.4% (FEC-T group). The mean OS was 59.3 months (TCH group), 53.5 months (TEC group), 55.3 months (XEC-XT group), and 52.4 months (FEC-T group). The analysis of the disease-free and OS curves for the four groups are shown in Figures 1 and 2. The disease-free and 5-years overall survival curve of the TCH group was clearly superior to the other groups, while the XEC-XT 5-years overall survival curve was better than the TEC and FEC-T groups (P=0.021, Figure 1; P=0.040, Figure 2).
Discussion
The current mainstream idea is that breast cancer is a multifaceted disease composed of different biological subtypes. These subtypes are increasingly considered to have different clinical and molecular characteristics and different prognostic and therapeutic significance. In this study, five (6.3%) cases of NAC reached pCR in the luminal B group, compared to 20 (33.3%) cases in the Her-2 overexpression group (P=0.002) (Table 4). Our retrospective study also showed that Her-2 overexpression can increase pCR in NAC compared with luminal B. It has been reported that hormone-positive breast cancer is less sensitive to primary chemotherapy compared with hormone-negative breast cancer. Hugh et al reported a simple immunopanel that divides breast cancers into biological subtypes. The strong prognostic effects seen with this molecular subdivisioning is primarily due to the ability to predict the sensitivity of each subtype to each treatment regimen, not the degree of malignancy between subtypes or the potential for metastasis.21 Triple-negative and Her-2 overexpression breast cancer exhibit a higher pCR rate compared with luminal-type breast cancer. Carey et al used the AC (doxorubicin/cyclophosphamide) regimen for NAC in patients with locally advanced breast cancer.22 Basal-like clinical efficacy reached 85%, Her2+/ER-type clinical effectiveness reached 70%, and luminal type was only 47% (P<0.0001); however, the OS rate of the former two (P=0.02) was still lower than the latter. Because luminal-type breast cancer is not sensitive to chemotherapy, neoadjuvant endocrine therapy is also advocated for those who need systematic preoperative treatment.23
Depending on the patient’s age, tumor size, condition of axillary lymph node metastasis, estrogen and progesterone receptor status, and Her-2 expression, doctors formulate individualized treatment strategies and achieve remarkable results. Her-2 status is also a predictor of certain systemic treatments. Slamon et al first proposed that Her-2 state is an independent prognostic factor of breast cancer, independent of tumor size, lymph node, and HR status; moreover, HER-2 is an independent prognostic factor for tumor recurrence and survival.3 Her-2-positive disease predicts the benefits of anthracycline treatment. The recurrence-free survival (RFS) of the FEC-T regimen containing anthracycline has an advantage over conventional CMF (cyclophosphamide/methotrexate/5-FU) regimen, whereas in HER-2-negative patients the RFS for these two regimens is not significantly different.24
Previous studies have shown that Her-2 overexpression breast cancer is resistant to chemotherapeutic agents and is not sensitive to endocrine therapy or radiotherapy. Therefore, Her-2 overexpression often predicts a poor prognosis in breast cancer patients. However, the appearance of trastuzumab changed this situation. Trastuzumab was originally used in breast cancer patients with metastases and has now been applied to conventional chemotherapy. Many studies have shown that trastuzumab can extend the survival of HER-2-positive breast cancer patients. Trastuzumab enhances the anti-tumor activity of paclitaxel and anthracycline on Her-2-positive breast cancer.25 Both the NSABP (National Surgical Adjuvant Breast and Bowel Project) B31 test and the NCCTG (North Central Cancer Treatment Group) N9831 test found that adding trastuzumab in AC sequential T can significantly reduce the risk of recurrence and mortality.26,27 In the Breast Cancer International Research Group (BCIRG) 006 trial, a combination program of trastuzumab, docetaxel, and carboplatin was designed to reduce the risk of recurrence and death of breast cancer and avoid the cardiotoxicity caused by the combination of trastuzumab and anthracycline. Furthermore, a study on the Noah test results showed that for patients with neoadjuvant trastuzumab treatment, the efficacy of pCR was almost twice that of the group that did not receive trastuzumab (35% vs 18%). In our study, the pCR rate of the TCH group was superior to that of the other three groups, and the difference was statistically significant (P=0.041). The results of this study are in agreement with the results of other neoadjuvant therapy involving trastuzumab.28
In the present study, six cases (18.2%) in the XEC-XT group achieved pCR, and the objective remission rate (ORR) was 78.7%, while two patients (6.3%) in the FEC-T group achieved pCR and the ORR was 62.5%. In terms of short-term efficacy, the efficacy of XEC-XT was superior to that of FEC-T. A number of clinical studies have shown that the use of capecitabine can achieve 20%–40% efficiency for breast cancer patients who fail treatment with anthracyclines or taxanes.29–31 In the study by Joensuu et al,33 1,500 patients with axillary node-positive or high-risk node-negative breast cancer were randomly assigned to receive either three cycles of docetaxel and capecitabine (TX) followed by three cycles of cyclophosphamide, epirubicin, and capecitabine (XEC-XT; n=753) or three cycles of docetaxel (T) followed by three cycles of cyclophosphamide, epirubicin, and 5-FU (FEC-T; n=747). TX/CEX improved breast cancer–specific survival (HR, 0.64; 95% CI, 0.44–0.95; P=0.027) and RFS in women with triple-negative disease and in women who had more than three metastatic axillary lymph nodes at the time of diagnosis. In this study, the fact that 92% of patients with triple-negative breast cancer achieved ORR in the XEC-XT group compared with only 73% in the FEC-T group implies that treatment with XEC-XT has a better curative effect on triple-negative breast cancer compared to FEC-T. This study also compared the long-term efficacy of XEC-XT and FEC-T. In the 5-year follow-up, the survival rate of the XEC-XT group (72.7%) was significantly better than that of the FEC-T group (59.4%).
In our study, comparing FEC-T and XEC-XT groups, it was seen that the TEC arm had a quicker onset and a better degree of pathological relief. Nevertheless, the incidence of fourth-degree bone marrow suppression in the TEC group was much higher compared with the FEC-T and XEC-XT groups. Compared with the XEC-XT group, the TEC group had a relatively short-term effect. The reason for this finding may be that anthracycline combined with docetaxel and cyclophosphamide greatly improves the effect of chemotherapy. The BCIRG 001 adjuvant trial demonstrated that docetaxel, doxorubicin, and cyclophosphamide (TAC) resulted in improved DFS and OS compared to 5-FU, doxorubicin, and cyclophosphamide (FAC) in the triple-negative breast cancer node-positive subset.32 From the perspective of long-term effects, such as DFS and 5-year OS, the results of the TEC group were relatively poor compared with the XEC-XT group. According to the relevant reports in HR-negative or histological grade 3 breast cancer, the addition of capecitabine to NAC results in greater pCR rates, which may be due to the interaction of capecitabine with other chemotherapy drugs; the greater pCR rates may also be related to the long-term use of capecitabine.33
Adverse reactions caused by the administration of chemotherapeutic agents include diverse degrees of bone marrow suppression, gastrointestinal reactions, alopecia, hand-foot syndrome, and peripheral nerve reaction.34 However, in the present study, none of the 139 famale patients experienced severe toxic side effects or cardiac insufficiency. From the comparison of adverse events, the incidence rate of hand-foot syndrome was most prevalent in the XEC-XT arm (12.5% [TCH] vs 54.5% [XEC-XT] vs 17.1% [FEC-T] vs 18.8% [TEC], P<0.001). Several studies have shown that, due to the pharmacological properties of capecitabine, the occurrence rate of hand-foot syndrome is high.35 In the present study, large doses of vitamin B6 were given orally when severe HFS was encountered, which reduced the incidence and symptoms of HFS.
Conclusion
The advantages of TCH were demonstrated by comparing four chemotherapy regimens. For the remaining three groups, which did not receive trastuzumab, we concluded that the short-term efficacy of the XEC-XT group and the TEC group were not significantly different, and that both were superior to the FEC-T group. However, in terms of long-term efficacy, the XEC-XT group was superior to both the FEC-T group and the TEC group. There was no difference in the long-term prognosis between the TEC and FEC-T groups (Figures 1 and 2). No optimal sequence is currently known, and the choice of individual regimens and drugs depends on patient preferences regarding the schedule and side-effect pattern, as well as the aforementioned therapies and residual toxic effects. Compared with the AC-TH regimen (pirarubicin/cyclophosphamide follow by docetaxel/trastuzumab), the advantage of the TCH regimen is that the early use of trastuzumab can rapidly cause tumor reduction and prevent tumor progression. In conclusion, with the promotion and evolution of NAC, the NAC scheme is often varied; however, in Her-2-positive breast cancer, the TCH regimen would occupy the dominant position.
In recent years, therapy has progressed adequately with a decline in therapy intensity, both for locoregional and systemic therapy; avoiding overtreatment, but also undertreatment, has become a major focus. Therapy has a curative intent and should be decided in a multidisciplinary setting, taking into account the molecular subtype and locoregional tumor load. Primary conventional surgery is no longer the optimal choice for all patients. Neoadjuvant therapy has become a common choice in Her-2-positive primary early breast cancer. In non-advanced breast cancer, the treatment goal should be a cure. Advances in neoadjuvant therapy, endocrine therapy, and combinations thereof, as well as the prospect of new targeted therapies for Her-2-positive breast cancer, make the desire for curing breast cancer in the early or middle stages a reality.
Disclosure
The authors report no conflicts of interest in this study.
References
Siegel RL, Miller KD, Jemal A, Statistics C. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Ullrich A, Coussens L, Hayflick JS, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309(5967):418–425. | ||
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. | ||
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. | ||
Frei E, Miller D, Clark JR, Fallon BG, Ervin TJ. Clinical and scientific considerations in preoperative (neoadjuvant) chemotherapy. Recent Results Cancer Res. 1986;103(1):1–5. | ||
Frei E, Miller D, Clark JR. Clinical and Scientific Considerations in Preoperative (Neoadjuvant). Chemotherapy: Springer Berlin Heidelberg; 1986. | ||
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. | ||
van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. | ||
Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–1934. | ||
Molina MA, Codony-Servat J, Albanell J, et al. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744. | ||
Judson IR, Beale PJ, Trigo JM, et al. A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14C-labelled drug. Invest New Drugs. 1999;17(1):49–56. | ||
Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85–104. | ||
Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759–1768. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
García-Martínez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. | ||
Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67(5):1025–1039. | ||
Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. | ||
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. | ||
Almasri NM, Al Hamad M, Hm A. Immunohistochemical evaluation of human epidermal growth factor receptor 2 and estrogen and progesterone receptors in breast carcinoma in Jordan. Breast Cancer Res. 2005;7(5):1–7. | ||
Pauletti G, Godolphin W, Press MF, Slamon DJ. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene. 1996;13(1):63–72. | ||
Hugh J, Hanson J, Cheang MCU, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2009;27(8):112–113. | ||
Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. | ||
Alba E, Calvo L, Albanell J, et al; GEICAM. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23(12):3069–3074. | ||
Pritchard KI, Shepherd LE, O’Malley FP, et al; National Cancer Institute of Canada Clinical Trials Group. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354(20):2103–2111. | ||
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58(13):2825–2831. | ||
Slamon D, Eiermann W, Robert N, et al; Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. | ||
Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–3373. | ||
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. | ||
Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17(2):485–493. | ||
Talbot DC, Moiseyenko V, van Belle S, van BS, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86(9):1367–1372. | ||
Lueck H, Minckwitz GV, Du BA, et al. Epirubicin/paclitaxel (EP) vs. capecitabine/paclitaxel (XP) in first-line metastatic breast cancer (MBC): A prospective, randomized multicentre phase III study of the AGO breast cancer study group.[C]// Meeting of the American-Society-Of-Clinical-Oncology. 2006:7S–7S. | ||
Noronha V. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;353(9):954. | ||
Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: final analysis of the randomized FinXX trial. J Clin Oncol. 2012;30(1):11–18. | ||
Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 2001;2001(30):135–142. | ||
Huang H, Jiang Z, Wang T, et al. Single-agent capecitabine maintenance therapy after response to capecitabine-based combination chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2012;23(7):718–723. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.