Back to Journals » Drug Design, Development and Therapy » Volume 13
Comparison of the efficacy and safety of remifentanil versus different pharmacological approaches on prevention of etomidate-induced myoclonus: a meta-analysis of randomized controlled trials
Authors Lang B, Zhang L, Li F, Lin Y, Zhang W, Yang C
Received 3 January 2019
Accepted for publication 10 April 2019
Published 9 May 2019 Volume 2019:13 Pages 1593—1607
DOI https://doi.org/10.2147/DDDT.S200200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Bingchen Lang,1 Lingli Zhang,1–3 Fengshan Li,1 Yunzhu Lin,1 Wensheng Zhang,4 Chunsong Yang1
1Department of Pharmacy, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, People’s Republic of China; 4Department of Anesthesiology, Laboratory of Anesthesia and Critical Care Medicine, Translational Neuroscience Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Objective: Myoclonus was considered as one conundrum in etomidate induction, which led to multiple risks during clinical anesthesia. The present study was conducted to compare the efficacy of pretreatment with remifentanil to different pharmacological approaches on reducing etomidate-induced myoclonus.
Methods: We searched PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure from the inception to October 2018. Randomized controlled trials comparing remifentanil versus other pharmacological approaches in reducing etomidate-induced myoclonus were eligible to be analyzed.
Results: Overall, 13 trials with 1,392 patients met with the inclusion criteria. 1) Pretreatment with remifentanil could reduce the incidence of etomidate-induced myoclonus compared to placebo and fentanyl; few differences were found between the use of remifentanil and the use of midazolam: (incidence of myoclonus: 5.56% with remifentanil vs 71.65% with saline, RR=0.08, with 95% CI [0.05, 0.12], P<0.0001; 3.80% with remifentanil vs 13.33% with fentanyl, RR with 95% 0.31 [0.11, 0.86], P=0.02; 46.00% with remifentanil vs 55.45% with midazolam, RR=0.82, with 95% CI [0.64, 1.06], P=0.13). 2) Compared with placebo, pretreatment with remifentanil could reduce the incidence of mild, moderate, and severe myoclonus; compared with midazolam, patients receiving remifentanil experienced lower occurrence of severe myoclonus; compared with fentanyl, pretreatment with remifentanil associated with significant low occurrence of moderate and severe myoclonus. 3) The outcomes also indicated that pretreatment with remifentanil could prevent excessive hemodynamic changes after endotracheal intubation compared to fentanyl.
Conclusions: Pretreatment with remifentanil could be considered as one operative option to reduce both incidence and severity of etomidate-induced myoclonus. Compared with fentanyl, it also provides efficacy in preventing excessive hemodynamic changes after endotracheal intubation. However, the best treatment and the proper prophylactic dosage calls for more high quality evidence with large sample size.
Keywords: remifentanil, etomidate, myoclonus, meta-analysis, fentanyl, midazolam
Introduction
Etomidate is considered as one of the most suitable hypnotic drugs in elderly patients and hemodynamically unstable patients given that it provides limited effect on circulation and provides minimal breathing suppression.1 However, the side effects caused by etomidate, including injection pain, suppression of adrenal function and myoclonus, cannot be overlooked.2 Especially, myoclonus, which has been reported in 80% of unpremedicated patients during the induction of anesthesia, may result in the high risks of muscle damage, regurgitation, myalgia, and dislodgement of the vascular access.3 According to recent literature, the first two conundrums, injection pain and adrenal suppression, have been solved via lipid formulation3 and synthesis of rapidly metabolized soft analogs,4 however, the reasonable solutions to etomidate-induced myoclonus have not been discovered.
Short acting and negligible effects on hemodynamic parameters should be considered as the ideal characteristics of a prophylactic agent. Although opioids, muscle relaxants, benzodiazepines, and lidocaine have been reported in reducing myoclonic movement,5–8 the drug of choice has yet to be identified, and the question remains attractive and calls for the relevant comparative study.
As one commonly used selective μ-receptor agonist, remifentanil features rapid onset time and metabolization.9 Recently, increasing studies have illustrated the efficacy of remifentanil in reducing the incidence of etomidate-induced myoclonus,10–12 however, relevant systematic review about the efficacy of such medication has been not established. Therefore, the present study was performed to evaluate the efficacy of remifentanil and different pharmacological approaches on preventing the incidence and severity of myoclonus incurred by using etomidate.
Methods
This meta-analysis was performed according to the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta Analyses13 statement and the guidelines described in the Cochrane Handbook.
Search strategy
Two independent reviewers (BL and FL) performed the literature search. And we searched the databases included PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI). “Remifentanil,” “Ultiva,” “myoclonus,” “myoclonic movement,” “fasciculation,” “twitching,” “etomidate,” “hypnomidate” were considered as the search terms. Only human studies were involved and there were no restrictions of the publication language. The last literature search was performed on October 26, 2018.
Selection
The studies meeting the following criteria were selected for further analysis: 1) randomized controlled trials (RCTs) in the adult patients with American Society of Anesthesiologists (ASA) physical status IIII who experienced the induction with etomidate; 2) studies comparing remifentanil vs other pharmacological approaches in reducing etomidate-induced myoclonus.
The incidence of myoclonus emergence (the number of patients who had experienced myoclonus) and the severity of myoclonus (the incidence of etomidate-induced myoclonus at different degrees) would be evaluated as the primary outcomes. The duration of myoclonus and the patients’ great hemodynamic changes after endotracheal intubation were considered as the secondary outcomes. Moreover, the adverse effects reported in the enrolled clinical studies were also reviewed.
Literature screening and data extraction
Literature searching and data extraction were performed independently by two reviewers (BL and FL), and then the two reviewers cross-checked with each other. Full texts were obtained when information from titles and abstracts could not be ascertained. A table was designed to collect the general characteristics of these selected studies (Table 1). Disagreements were resolved by consensus through discussion among all authors.
 | Table 1 The general characteristics of the enrolled studies |
Quality assessment
According to Cochrane Collaboration tool for assessing risk of bias in randomized trials,14 two reviewers (BL and FL) independently evaluated the methodological quality which includes seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.15
Statistical analysis
Statistical analyses were done with Review Manager 5.0 software (The Cochrane Collaboration, London, UK). The risk ratio (RR) with 95% CI and the Mantel–Haenszel method (fixed or random models) were used to analyze dichotomous data. The I-squared (I2) test was applied to weigh the impact of heterogeneity on the results. As stated by the Cochrane review guidelines, the random-effects model was chosen when severe heterogeneity was present at I2>50% (or the value of I2 was closed to 50%); otherwise, the fixed-effects model was applied. Additionally, we performed the sensitivity analysis by deleting each study individually to assess the quality and consistency of the results. Publication bias was evaluated by using Begg’s test and Egger’s test when at least ten studies were included in the meta-analysis. A P value less than 0.05 was judged statistically significant.
Results
Literature search results
Of 46 articles identified, 20 were excluded after duplicate removal, and 11 were excluded after title and abstract review. In these 11 excluded items, 9 RCTs were not related to the evaluation of effectiveness in reducing etomidate-induced myoclonus (eg, the evaluation of sedation and the effects on cardiovascular system), the remaining two were an uncorrelated review and a meeting. After full-text review, two items were excluded (one was due to the uncorrelated comparison: rocuronium versus placebo, the full-text of the other one cannot be obtained after contacting the authors). Finally, 13 RCTs were enrolled in the further meta-analysis.10–12,16–25 The identification procedure of these eligible articles is described in Figure 1. The enrolled studies were published from 2006 to 2016.
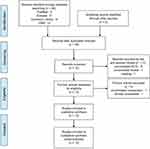 | Figure 1 Flow chart of literature screening and the selection process.Abbreviations: CNKI, China National Knowledge Infrastructure; RCT, randomized controlled trial. |
Basic characteristics of enrolled studies
All enrolled studies reported the incidence of etomidate-induced myoclonus and the severity of myoclonus, and only one study mentioned the duration of myoclonus.16 Normal saline was used as the control in 11 studies,10–12,16,18–24 midazolam was used as the comparison in two studies,21,25 and fentanyl was used as the comparison in four studies.12,22–24 The numbers of patients suffered excessive hemodynamic changes were reported in two studies,12,23 and adverse effects, including apnea, cough, sedation, chest rigidity, bradycardia, hypotension, and postoperative respiratory discomfort, were described in five studies.10,11,17,21,25 In all enrolled studies, a 4-point intensity score was used to grade the severity of etomidate-induced myoclonus.26 The general characteristics of the enrolled studies are shown in Table 1.
Quality assessment
Seven items mentioned above were evaluated in accordance with the Cochrane Collaboration tool for assessing risk of bias. A total of 23% (3/13) of the studies performed an adequate method of random sequence generation,12,21,22 and two studies reported allocation concealment with detailed descriptions (using opaque, sealed envelopes).11,12 Five studies mentioned the blinding procedure of participants and personnel,10,16,21,22,25 and seven studies mentioned the blinding procedure of outcome assessment.10–12,16,21,22,25 The details of risk of bias assessment are shown in Figure 2.
 | Figure 2 Risk of bias assessment of included studies. Green + dot, low risk of bias; yellow ? dot, unclear risk of bias; red - dot, high risk of bias. |
Incidence of etomidate-induced myoclonus
Remifentanil vs placebo
Eleven studies involving 778 patients were included in the study, and 450 of them were given remifentanil to alleviate the etomidate-induced myoclonus. Considering no statistical heterogeneity exists among the study results (I2=38%), the fixed-effects model was chosen. According to the result, pretreatment of remifentanil unquestionably reduced the incidence of etomidate-induced myoclonus (incidence of myoclonus: 5.56% with remifentanil vs 71.65% with saline, RR=0.08, with 95% CI [0.05, 0.12], P<0.0001, I2=38%).The results indicated that pretreatment with remifentanil exhibited a significantly lower incidence of etomidate-induced myoclonus (Figure 3A). A publication bias was detected in this analysis by use of both Begg’s (P=0.029) and Egger’s (P=0.001) tests.
Remifentanil vs midazolam
Two RCTs involving a total of 201 patients were enrolled in the study, and 100 of them were given the remifentanil. The I2 of 0% indicated that substantial heterogeneity did not exist, thus the fixed effect model was used. The result indicated that there were no significant differences between the using of remifentanil and midazolam (incidence of myoclonus: 46.00% with remifentanil vs 55.45% with midazolam, RR=0.82, with 95% CI [0.64, 1.06], P=0.13, I2=0%). The result is shown in Figure 3B.
Remifentanil vs fentanyl
Four RCTs involving 210 patients were included in the study, and 105 of them were pretreated with remifentanil to reduce the incidence of myoclonus. The I2 of 0% indicated that substantial heterogeneity did not exist, thus the fixed effect model was used. Compared with the fentanyl, the pretreatment of remifentanil presented a definite advantage in reducing the incidence of myoclonus caused by etomidate (incidence of myoclonus: 3.80% with remifentanil vs 13.33% with fentanyl, RR with 95% 0.31 [0.11, 0.86], P=0.02, I2=0%). The result is shown in Figure 3C.
Remifentanil 2 ng/ml vs remifentanil 4 ng/ml (TCI)
The effect-site concentration of remifentanil was set by the target controlled infusion (TCI) system. This system was designed for the administration of various opioids and other anesthetics by TCI. By using computer simulation of known infusion protocol, the pharmacokinetic parameters were selected. And the system with the computer-compatible infusion pump provided appropriate target concentrations of anesthetic drugs in many clinical trials.27 Three RCTs involving 186 patients were included, and half of them were pretreatment with 2 ng/ml of remifentanil. The I2 of 0% revealed that substantial heterogeneity did not exist; therefore, the fixed effect model was chosen. The results indicated that pretreatment of remifentanil with a concentration of 4 ng/ml was associated with significantly decreased incidence of myoclonus compared to remifentanil with a concentration of 2 ng/ml (incidence of myoclonus: 16.13% with remifentanil 2 ng/ml vs 0% with remifentanil 4 ng/ml, RR with 95% 16.00 [2.19, 117.07], P=0.006, I2=0%). The result is shown in Figure 3D.
Severity of etomidate-induced myoclonus
Visual judgement was used to determine the degree of myoclonus. A transient movement of a body segment (eg, a finger or shoulder) was defined as mild myoclonus. Moderate myoclonus was described as slight movement of two different muscle groups (eg, face and leg). Intense clonic movement of two or more muscle groups was defined as severe myoclonus (eg, fast abduction of a limb).2 The severity of myoclonus was graded according to the 4-point intensity score: 0, no myoclonus; 1, mild myoclonus; 2, moderate myoclonus; or 3, severe myoclonus.
Remifentanil vs placebo
Eleven RCTs involving 778 patients were included, and 450 of them were pretreated with remifentanil.
Owing to statistical heterogeneity (I2=46%), the random-effects model was used. The results indicated that the intervention of remifentanil reduced the incidence of etomidate-induced mild myoclonus (the incidence of etomidate-induced mild myoclonus: 4.00% with remifentanil vs 27.13% with saline, RR=0.19 with 95% CI [0.09, 0.40], P<0.0001, I2=46%). The I2 of 46% indicated existing heterogeneity, attributable to the Lee study (Lee et al, 2009). The heterogeneity was resolved after removing this study (I2=16%), and the summary estimate was unchanged in essence (the incidence of etomidate-induced mild myoclonus: 2.56% with remifentanil vs 28.19% with saline, RR=0.16, 95% CI [0.08, 0.32], P<0.00001). The result is shown in Figure 4A. By using both Begg’s (P=0.062) and Egger’s (P=0.002) tests, publication bias was found in the analysis.
The I2 of 0% demonstrated that substantial heterogeneity does not exist; thus, a fixed-effects model was chosen to perform the analysis, The results (Figure 4B) indicated that pretreatment with remifentanil produced a significantly lower incidence of moderate myoclonus (incidence of etomidate-induced moderate myoclonus: 1.11% with remifentanil vs 25.00% with saline, RR=0.08 with 95% CI [0.04, 0.16], P<0.0001, I2=0%). The results of Begg’s (P=0.484) and Egger’s (P=0.022) tests indicated that a publication bias existed in this analysis.
No statistical heterogeneity was detected among the study results (I2=0%) and the fixed-effects model was used. The results revealed that pretreatment with remifentanil reduced the incidence of etomidate-induced severe myoclonus (the incidence of etomidate-induced severe myoclonus: 0.02% with remifentanil vs 19.51% with saline, RR=0.07 with 95% CI [0.03, 0.14], P<0.0001, I2=0%). The result is shown in Figure 4C. Publication bias was not significant (P=0.312 for Begg’s test and P=0.941 for Egger’s test).
Remifentanil vs midazolam
Two RCTs involving a total of 201 patients were enrolled in the study, and 100 of them were given the remifentanil.
The random-effects model was used for the existing statistical heterogeneity. The data from those studies implied that there was no significant difference between the remifentanil group and the midazolam group in the incidence of etomidate-induced mild myoclonus (incidence of etomidate-induced mild myoclonus: 15% with remifentanil vs 7.92% with midazolam, RR=1.61 with 95% CI [0.26, 10.09], P=0.13, I2=76%). The I2 of 76% again indicated substantial heterogeneity but the source could not be clearly attributed to a single study (Figure 5A).
The fixed-effects model was selected due to no statistical heterogeneity (I2=0%). And the results revealed pretreatment with remifentanil did not significantly decrease the incidence of moderate myoclonus compared to midazolam (incidence of etomidate-induced moderate myoclonus: 16% with remifentanil vs 17.82% with midazolam, RR=0.89 with 95% CI [0.50, 1.61], P=0.71, I2=0%). The result is shown in Figure 5B.
The fixed-effects model was chosen because statistical heterogeneity did not exist (I2=26%). Compared with the midazolam, the intervention of remifentanil reduced the incidence of etomidate-induced severe myoclonus, which was described as the occurrence of myoclonus (incidence of etomidate-induced severe myoclonus: 15.00% with remifentanil vs 29.70% with midazolam, RR=0.51, with 95% CI [0.30, 0.86], P=0.01, I2=26%). The result is shown in Figure 5C.
Remifentanil vs fentanyl
Four RCTs involving 210 patients were selected in the study, and 105 of them were pretreated with remifentanil to reduce the incidence of myoclonus.
No statistical heterogeneity was found among the study results (I2=0%) and the fixed-effects model was chosen. There was no significant difference between the remifentanil group and the fentanyl group in the incidence of etomidate-induced mild myoclonus (incidence of etomidate-induced mild myoclonus: 3.81% with remifentanil vs 8.57% with fentanyl, RR=0.47, with 95% CI [0.16, 1.41], P=0.18, I2=0%). The result is shown in Figure 6A.
The fixed-effects model was used due to the absence of statistical heterogeneity (I2=0%). No significant differences were observed between the two groups in the incidence of etomidate-induced moderate myoclonus; And no patients experienced moderate myoclonus in remifentanil group (incidence of etomidate-induced moderate myoclonus: 0% with remifentanil vs 4.76% with midazolam, RR=0.23, with 95% CI [0.04, 1.32], P=0.10, I2=0%). The result is shown in Figure 6B.
No patients experienced etomidate-induced severe myoclonus in either the remifentanil or the fentanyl group, therefore, the relevant analysis was not performed.
Remifentanil 2 ng/ml vs remifentanil 4 ng/ml (TCI)
Three RCTs involving 186 patients were included, and half of them were pretreatment with 2 ng/ml of remifentanil.
No statistical heterogeneity was found among the study results (I2=0%) and the fixed-effects model was chosen. The results indicated that pretreatment with 4 ng/ml of remifentanil produced a significantly lower incidence of mild myoclonus (incidence of etomidate-induced mild myoclonus: 11.83% with a concentration of 2 ng/ml remifentanil vs 0% with a concentration of 4 ng/ml remifentanil, RR=12.00, with 95% CI [1.61, 89,58], P=0.02, I2=0%). No patients experienced mild myoclonus in 4 ng/ml of remifentanil group. The result is shown in Figure 7A.
The fixed-effects model was chosen due to the abscence of statistical heterogeneity (I2=0%). No significant differences were observed between the two groups in the incidence of etomidate-induced moderate myoclonus; And no patients experienced moderate myoclonus in 4 ng/ml of remifentanil group (incidence of etomidate-induced moderate myoclonus: 3.17% with a concentration of 2 ng/ml remifentanil vs 0% with a concentration of 4 ng/ml remifentanil, RR=3.00, with 95% CI [0.32, 28.09], P=0.34, I2=0%). The result is shown in Figure 7B.
Two patients in the 2 ng/ml of remifentanil group in only one study experienced etomidate-induced severe myoclonus,17 thus, the relevant analysis was not performed.
Duration of etomidate-induced myoclonus
Only one study,16 involving 90 patients, described the duration of etomidate-induced myoclonus. Therefore, the relevant analysis was not performed. However, based on the existing data from this study, pretreatment with remifentanil significantly decreased the duration of myoclonus compared to placebo (duration of myoclonus: 36.0±27.0 sec with remifentanil 1 μg/kg vs 93.8±59.5 sec with saline).
The numbers of patients experienced great hemodynamic changes
Due to etomidate’s insufficient suppression on laryngopharyngeal reflexes during endotracheal intubation, hypertension and tachycardia occurred frequently after intubation. Thus, the occurrence of great hemodynamic changes was evaluated and it was defined as an increase or decrease in systolic blood pressure (SBP) or heart rate (HR) of more than 30% of the baseline level.12
Remifentanil vs fentanyl
Two RCTs involving 120 patients were evaluated in the study, and 60 of them were givenremifentanil.
Due to the absence of statistical heterogeneity (I2=0%), the fixed-effects model was applied. The results indicated that pretreatment with remifentanil was associated with significantly low incidence of great changes in SBP compared to fentanyl (incidence of experiencing great changes in SBP: 0% with remifentanil vs 61.67% with fentanyl, RR=0.03, with 95% CI [0.00, 0.19], P=0.0003, I2=0%). The result is shown in Figure 8A.
The fixed-effects model was used due to the absence of statistical heterogeneity (I2=0%). Compared with the fentanyl, the using of remifentanil associated with the minor effects on the changes in HR (incidence of experienced great changes in HR: 0.00% with remifentanil vs 73.33% with fentanyl, RR=0.14, with 95%CI [0.06, 0.30], P<0.00001, I2=0%). The result is shown in Figure 8B.
The side effects
A total of five studies10,11,17,21,25 mentioned the side effects related to the drugs used as premedication. According to the records, side effects appeared frequently in the patients who received remifentanil 4 ng/ml by the TCI system, the details were as follows: 13 of them experienced cough, 15 of them suffered chest wall rigidity, four of them experienced bradycardia, and apnea developed in seven patients. Additionally, two patients who received remifentanil 1 μg/kg pretreatment experienced bradycardia. Except for the above-mentioned cases, none of the patients experienced any side effects after injection of either drugs.
Publication bias
Publication bias was explored by using Begg’s test and Egger’s test to indicate the possible presence of bias. The result (Figure 9) showed that publication bias existed in the analysis of the efficacy of remifentanil in alleviating etomidate-induced myoclonus compared to saline. For evaluating severe myoclonus between the remifentanil and placebo groups, publication bias was not significant.
Discussion
Myoclonus was considered as one serious problem in patients who experienced etomidate induction. As previously stated, it may raise the risk to muscles and may disturb the assessment of the depth of anesthesia. Therefore, such adverse effects during anesthesia induction should be prevented by any means necessary. The present study was a meta-analysis of the published RCTs to compare the efficacy of remifentanil vs different pharmacological approaches on reducing the etomidate-induced myoclonus. In our study, lower incidence of myoclonus at different grades was exhibited in the remifentanil group compared to the saline group based on the analysis of existing data from 13 RCTs. It demonstrated that pretreatment of remifentanil could reduce both the incidence and the severity of etomidate-induced myoclonus.
The results from searching in several databases showed that the existing comparative studies involved remifentanil vs midazolam, remifentanil vs fentanyl, and remifentanil 2 ng/ml vs remifentanil 4 ng/ml administered by the TCI system.
As one of the short acting benzodiazepines, midazolam acts selectively through the GABA A receptor. And it has been investigated as one approach to preventing etomidate-induced myoclonus. According to our analysis, there was no significant difference between the use of remifentanil and midazolam in reducing etomidate-induced myoclonus, but the patients who received remifentanil experienced lower incidence of etomidate-induced severe myoclonus. However, we found that the conclusions from two included studies were opposite. The difference of effects on reducing myoclonus between midazolam 0.5 mg/kg with remifentanil 1 μg/kg were compared by Hwang et al21 and the report indicated that no significant difference was found in the incidence of myoclonus between the two groups. Sedighinejad et al25 reported the superiority of remifentanil to midazolam. Based on the current evidences, the difference might result from the large gap of the dosage of midazolam (0.05 mg/kg vs 0.015 mg/kg). Particularly, there are currently too few data (only two relevant studies) to draw a satisfactory conclusion that remifentanil was a preferable option compared to midazolam.
Fentanyl, another opioid, also acting as one intervention to reduce myoclonus, was evaluated in the present study. And the results from four RCTs comparing remifentanil with fentanyl indicated that the former one was associated with significantly lower occurrences of myoclonus and low incidence of moderate and severe myoclonus. It is common knowledge that etomidate provides the stable hemodynamic parameters during anesthesia induction for its negligible effects on myocardial contractility. However, it does not reduce the sympathetic response to endotracheal intubation due to its lack of analgesic efficacy,28,29 which was reflected in the tendency for an increase in HR and SBP in patients after the endotracheal intubation. We also analyzed the occurrence of excessive hemodynamic changes (an increase in SBP) or HR of more than 30% of the baseline level) in patients who receiving remifentanil and fentanyl. Compared with fentanyl, excessive hemodynamic changes after endotracheal intubation could be prevented effectively by using remifentanil, which showed low numbers of patients who experienced great systolic blood pressure changes and HR changes.
The comparison between remifentanil 2 ng/ml and remifentanil 4 ng/ml administered by the TCI system was reported in three studies. The results of our analysis demonstrated that pretreatment with remifentanil with a concentration of 4 ng/ml leads to a lower occurrence rate of myoclonus at different grades. However, the vast majority of side effects in the present study such as apnea, cough, sedation, chest rigidity, and bradycardia was described in the patients receiving remifentanil with a concentration of 4 ng/ml. Therefore, given the paucity of existing evidence, the pros and cons of each approach should be discussed in future studies.
As far as we know, the present study is the first meta-analysis to review the efficacy and safety of pretreatment with remifentanil to alleviate etomidate-induced myoclonus. However, there are some limitations which should be taken into account. First, the results of Begg’s test and Egger’s test suggests that publication bias is present in the analysis of remifentanil vs saline in reducing incidence of etomidate-induced myoclonus. This phenomenon might be incurred by the absence of trials with negative results, because the comparison was performed between the pharmacological intervention group and the saline control group. Even so, we cannot rule out that publication bias might lead to an overestimation of the true treatment effect. Second, sample sizes, quality, and design of the enrolled trials in the present study incurred limitations. Although we performed a thorough search including not only several international but also a Chinese database to make the existing evidence as comprehensive as possible, the evidence about pretreatment with remifentanil to prevent etomidate-induced myoclonus was limited. Unfortunately, the full-text of one relevant study30 could not be obtained after contacting the authors.
Conclusion
Pretreatment with remifentanil could be considered as one effective way to reduce both incidence and severity of etomidate-induced myoclonus. Compared with using fentanyl and midazolam, remifentanil might be considered as the preferable option for its efficacy in preventing both the myoclonus and excessive hemodynamic changes after endotracheal intubation. Nevertheless, more high-quality evidence with a large sample size is required. The best treatment and most suitable dosage to resolve such clinical practical problems remains to be established.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114(3):695–707. doi: 10.1097/ALN.0b013e3181ff72b5.
2. Holdcroft A, Morgan M, Whitwam JG, Lumley J. Effect of dose and premedication on induction complications with etomidate. Br J Anaesth. 1976;48:199–205. doi:10.1093/bja/48.3.199
3. Nyman Y, Von Hofsten K, Palm C, Eksborg S, Lönnqvist PA. Etomidate-lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. Br J Anaesth. 2006;97(4):536–539. doi:10.1093/bja/ael187
4. Cotten JF, Husain SS, Forman SA, et al. Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111(2):240–249. doi:10.1097/ALN.0b013e3181ae63d1
5. Hueter L, Schwarzkopf K, Simon M, Bredle D, Fritz H. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand. 2003;47(4):482–484. doi:10.1034/j.1399-6576.2003.00081.x
6. Hüter L, Schreiber T, Gugel M, Schwarzkopf K. Low-dose intravenous midazolam reduces etomidate-induced myoclonus: a prospective, randomized study in patients undergoing elective cardioversion. Anesth Analg. 2007;105(5):1298–1302. doi:10.1213/01.ane.0000287248.25610.c0
7. Choi JM, Choi IC, Jeong YB, Kim TH, Hahm KD. Pretreatment of rocuronium reduces the frequency and severity of etomidate-induced myoclonus. J Clin Anesth. 2008;20(8):601–604. doi:10.1016/j.jclinane.2008.06.010
8. Gultop F, Akkaya T, Bedirli N, Gumus H. Lidocaine pretreatment reduces the frequency and severity of myoclonus induced by etomidate. J Anesth. 2010;24(2):300–302. doi:10.1007/s00540-010-0869-6
9. Glass PS, Hardman D, Kamiyama Y, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. 1993;77:1031–1040. doi:10.1213/00000539-199311000-00028
10. Kelsaka E, Karakaya D, Sarihasan B, Baris S. Remifentanil pretreatment reduces myoclonus after etomidate. J Clin Anesth. 2006;18(2):83–86. doi:10.1016/j.jclinane.2005.05.004
11. Ri HS, Shin SW, Kim TK, Baik SW, Yoon JU, Byeon GJ. The proper effect site concentration of remifentanil for prevention of myoclonus after etomidate injection. Korean J Anesthesiol. 2011;61(2):127–132. doi:10.4097/kjae.2011.61.2.127
12. Ko BJ, Oh JN, Lee JH, Choi SR, Lee SC, Chung CJ. Comparison of effects of fentanyl and remifentanil on hemodynamic response to endotracheal intubation and myoclonus in elderly patients with etomidate induction. Korean J Anesthesiol. 2013;64(1):12–18.
13. Moher D, Liberati A, Tetzlaff J, Altman DG;
14. Higgins JP, Altman DG, Gøtzsche PC, et al.;
15. Higgins JP, Altman DG, Sterne JA, editors. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2008:187–241.
16. Lee SW, Gill HJ, Park SC, et al. The effect of remifentanil for reducing myoclonus during induction of anesthesia with etomidate. Korean J Anesthesiol. 2009;57(4):438–443. doi:10.4097/kjae.2009.57.4.438
17. Zou L, Yuan H, Wang HY, Geng ZY, Xu L, Sun L. [Role of target controlled infusion of remifentanil for the prevention of etomidate induced myoclonus during general anesthesia]. Acta Acad Med Sin. 2013;35(1):112–115. Chinese.
18. Zhao XH, Li JB, Deng XM. [Remifentanil pretreatment reduces myoclonus after etomidate]. J Clin Anesthesiol. 2008;24:482–483. Chinese.
19. Sun ZC, Huang XL, Zhang RJ, Qi C. Gao L. [Reducing etomidate injection induced myoclonus symptom by remifentanil target control infusion]. J Trop Med. 2012;12(11):1367–1369. Chinese.
20. Yuan LL, Wen XB, Zhou W, Xu DP. [Effects of remifentanil on myoclonus induced by etomidate]. Clin J Med Offic. 2012;40(2):298–299. Chinese.
21. Hwang JY, Kim JH, Oh AY, Do SH, Jeon YT, Han SH. A comparison of midazolam with remifentanil for the prevention of myoclonic movements following etomidate injection. J Int Med Res. 2008;36(1):17–22. doi:10.1177/147323000803600103
22. Chen Y, Wang GL, Yu YH. [Effects of different opioids on myoclonus and bispectral index during etomidate induction]. Tianjin Med J. 2009;37(5):368–370. Chinese.
23. Song ZG, Zhang ZP, Fang NN, Li X, Sun GH. [Comparison of effects of fentanyl,remifentanil and sufentanil on hemodynamic response to endotracheal intubation and myoclonus in elderly patients with etomidate induction]. Pract Pharm Clin Remedies. 2014;17(10):1262–1265. Chinese.
24. Xie YF, Song DD, Zhou J, Liu GL. [Preventive effect of fentanyl and remifentanil on myoclonus induced by etomidate in elderly patients]. Pract Pharm Clin Remedies. 2016;19(2):187–189. Chinese.
25. Sedighinejad A, Naderi Nabi B, Haghighi M, et al. Comparison of the effects of low-dose midazolam, magnesium sulfate, remifentanil and low-dose etomidate on prevention of etomidate-induced myoclonus in orthopedic surgeries. Anesth Pain Med. 2016;6(2):e35333. doi:10.5812/aapm.35333
26. Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. Reducing myoclonus after etomidate. Anesthesiology. 1999;90(1):113–119. doi:10.1097/00000542-199901000-00017
27. Guarracino F, Lapolla F, Cariello C, et al. Target controlled infusion: TCI. Minerva Anestesiol. 2005;71(6):335–337.
28. Nauta J, Stanley TH, de Lange S, Koopman D, Spierdijk J, van Kleef J. Anaesthetic induction with alfentanil: comparison with thiopental, midazolam, and etomidate. Can Anaesth Soc J. 1983;30:53–60. doi:10.1007/BF03007717
29. Giese JL, Stockham RJ, Stanley TH, Pace NL, Nelissen RH. Etomidate versus thiopental for induction of anesthesia. Anesth Analg. 1985;64:871–876. doi:10.1213/00000539-198509000-00006
30. Safavi M, Honarmand A, Zolfaghari S. Comparing the effect of sodium thiopental, magnesium sulfate, midazolam, prime etomidate, and remifentanil on myoclonus incidence and pain induced by anesthesia with etomidate. J Isfahan Med Sch. 2018;35(453):1515–1520.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







