Back to Journals » Drug Design, Development and Therapy » Volume 14
Comparison of GnRH-a Prolonged Protocol and Short GnRH-a Long Protocol in Patients with Thin Endometrium for Assisted Reproduction: A Retrospective Cohort Study
Authors Song J , Duan C, Cai W, Wu W, Lv H, Xu J
Received 3 July 2020
Accepted for publication 21 August 2020
Published 11 September 2020 Volume 2020:14 Pages 3673—3682
DOI https://doi.org/10.2147/DDDT.S270519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Jianyuan Song,1 Cuicui Duan,2 Wangyu Cai,1 Wei Wu,1 Houyi Lv,2 Jian Xu1
1The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, Zhejiang 322000, People’s Republic of China; 2Department of Assisted Reproduction, Women’s Hospital School of Medicine Zhejiang University, Hangzhou, Zhejiang 310006, People’s Republic of China
Correspondence: Jian Xu
The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, Zhejiang 322000, People’s Republic of China
Email [email protected]
Purpose: Gonadotrophin releasing hormone agonist (GnRH-a) is widely used for pituitary down-regulation and recruiting more follicles in assisted reproduction. However, no information is available on its value for patients with thin endometrial thickness.
Patients and Methods: This was a retrospective cohort study of 302 patients with endometrium < 8 mm undergoing fresh embryo transfer at a fertility center of a university hospital from January 2016 and December 2018. In 148 cycles of the GnRH-a prolonged protocol, one depot of 3.75 mg GnRH-a was injected on day 2 of the menstrual cycle, while in 154 cycles of the short GnRH-a long protocol, 0.1 mg of GnRH-a was injected daily from the mid-luteal phase. The live birth rate and clinical pregnancy rate were compared between the two groups. Other outcome measures included the implantation rate, miscarriage rate, and characteristics of stimulation procedures.
Results: Live birth rates and clinical pregnancy rates were significantly higher in the GnRH-a prolonged protocol group than in the other group (36.5% vs 20.8%, P=0.002; 43.9% vs 28.2%, P=0.006, respectively). The live birth rate was significantly increased in the prolonged protocol group (crude OR: 2.190, 95% CI: 1.311, 3.660; adjusted OR: 2.458, 95% CI: 1.430, 4.224) compared with that in the reference group. The implantation rate of the former group was also significantly higher than that of the latter group (35.4% vs 15.9%, P=0.000). There was no significant difference in miscarriage rates between the two protocols. In terms of stimulation procedures, the GnRH-a prolonged protocol group required significantly higher Gn time (10.9 vs 9.5 days, P=0.000) and Gn consumption (2625.0 vs 2047.5 IU, P=0.000) than the short GnRH-a long protocol group.
Conclusion: The GnRH-a prolonged protocol in fresh embryo transfer cycles yielded better clinical outcomes of patients with thin endometrium than the short GnRH-a long protocol.
Keywords: ovarian stimulation, GnRH-a prolonged protocol, thin endometrium, IVF, COH
Introduction
Controlled ovarian hyper-stimulation (COH) is necessary in assisted reproductive technology (ART) to recruit more follicles. Routine COH regimens, in most cases using gonadotrophin releasing hormone agonist (GnRH-a), have been generally proven as useful and safe.1–3 Among the various kinds of GnRH-a protocols, the short GnRH-a long protocol, starting GnRH-a in the mid-luteal phase, is recognized as an effective standard in young normogonadotropic women.4 However, some patients who receive conventional COH protocols exhibit poor outcomes, especially when facing thin endometrium.
Endometrial thickness is a key factor for success of ART. Thin endometrial thickness has been known to adversely affect clinical outcomes of infertility in women. A thin endometrium is generally defined as ≤7 or 8 mm at the time of ovulation trigger,5 measured by transvaginal ultrasonography. Alternative treatments have been offered to restore endometrial receptivity, including hormonal manipulation by estrogen and GnRH-a, hysteroscopic adhesiolysis, administration of vitamin E, low dose aspirin, stem cell regeneration, and so on.6–8 However, no exact optimal scheme exists for patients with thin endometrium to date.
In our center, a freeze-all policy is used for most patients with thin endometrium who have to wait for a future cycle. But some patients (around 15%) with thin endometrium will choose to proceed with the fresh embryo transfer cycle if they could acquire several good quality embryos. In these patients, the GnRH-a prolonged protocol is the most widely used ovarian stimulation protocol for sufficient down-regulation and a larger number of oocytes. Ideal pregnancy outcomes are often observed in patients receiving the prolonged protocol, in which GnRH-a was administered for at least 40 days before ovarian trigger. We therefore propose that prolonged pituitary down-regulation is effective for improving clinical outcomes of patients with thin endometrium in the fresh embryo transfer cycles.
In order to obtain clear conclusions in this retrospective study, stimulation procedures and clinical outcomes were examined between the two most commonly used stimulation protocols in patients whose endometrium thickness was less than 8 mm.
Materials and Methods
Study Population
All in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles between January 2016 and December 2018 in a fertility center of a university hospital were screened. The inclusion criteria included the following: 1) all women with thin endometrium (<8 mm); 2) fresh embryo transfer cycles; 3) the first ovarian stimulation cycle; and 4) GnRH-a prolonged protocol or short GnRH-a long protocol cycles. The exclusion criteria were: 1) other COH protocol cycles; 2) patients with egg donor; 3) patients with preimplantation genetic diagnosis; and 4) patients without fresh embryo transfer. The flow chart of the study is shown in Figure 1. The study was approved by the Institutional Review Board at Zhejiang University, China (approval number IRB-20,200,164-R) and was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients in accordance with the ethics committee.
 |
Figure 1 Flow chart of the study. |
Stimulation Protocols
Given advice from physicians and informed of detailed information on both protocols, including the days of the pituitary down-regulation, duration of stimulation, and the average previous pregnancy rate, the patients decided to whether to accept the COH regimen or not. However, patients were not given any advice on which protocol was better for women who have thin endometrium or endometriosis. The use of GnRH-a differed in the two protocols: in the GnRH-a prolonged protocol, 3.75 mg of GnRH-a was injected on day 2 of the menstrual cycle. Then, ovarian stimulation with rhFSH ranging from 75 to 300 IU would start 28 days later along with confirmation of pituitary down-regulation. While in the short GnRH-a long protocol, 0.1 mg of GnRH-a was injected daily from the mid-luteal phase. Then, 75–300 IU rhFSH would start 14 days later after confirming down-regulation. Successful down-regulation was confirmed with follicle diameter <8 mm, serum estradiol level <50 pg/mL, luteinizing hormone (LH) <5 IU/L, and endometrium <5 mm. The gonadotropin dosage was adjusted according to age, antral follicle count (AFC) score, and weight. When 2 leading follicles reached a mean diameter of 18 mm, HCG 10,000 IU (HCG; Livzon Pharmaceutical Group Inc., China) was administered to trigger ovulation. Transvaginal oocyte retrieval was performed 34–36 h after HCG administration. All treatment procedures including ovarian stimulation, oocyte retrieval, and fertilization were performed in strict accordance with the standard operating procedure. The two protocols are shown clearly in Figure 2.
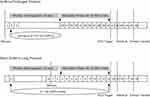 |
Figure 2 Timeline of the two stimulation protocols. |
Embryo transfer was performed on day 3 after fertilization. All embryos were at least good quality (Grade B) with 7–9 cells and less than 10% fragmentation and even symmetry. Embryo quality was analyzed according to the Istanbul consensus workshop on embryo assessment.9
Ultrasound Measurement
Endometrial thickness on the day of ovulation trigger was measured in the midsagittal plane of the uterus as the maximum distance between the two interfaces of endometrial–myometrial junction by transvaginal ultrasound. Thin endometrium is defined as <8 mm.
Sample Size
The sample size was calculated by R according to the difference in clinical pregnancy rate (62% versus 45%) published before.10 To provide a two-tailed significance level of 0.05 and a power of 80%, 134 participants were required in each group.
Definition of Clinical Outcomes
The primary outcome measures were the live birth rate and the clinical pregnancy rate. The secondary outcomes included the implantation rate, miscarriage rate, and characteristics of stimulation procedures. The live birth rate was classified as delivery of any viable infant after 24 weeks. Clinical pregnancy was identified with the presence of an intrauterine gestational sac with fetal cardiac activity on transvaginal ultrasound 2–3 weeks after a positive pregnancy test. The implantation rate reflects the number of gestational sacs divided by the number of embryos transferred. The early miscarriage rate was defined as pregnancy loss before the 12th gestational week. The late miscarriage rate was defined as pregnancy loss between 13 and 24 weeks of gestation. Ectopic pregnancy was defined as the presence of at least one gestational sac outside the uterine cavity.
Statistical Analysis
Categorical data were expressed as number and percentage and were analyzed using Pearson’s chi-squared test. Continuous data were expressed as mean±standard deviation (SD) and were analyzed using analysis of variance (ANOVA). Endometrial thickness was expressed as median (ranges) for non-normal distribution and evaluated using the Mann–Whitney U-test. Clinical outcomes per cycle were compared between the two protocol groups using Pearson’s chi-squared or Fisher's exact test.
Then, multivariate logistic regression was performed to assess whether ovarian stimulation protocols were correlated with live birth. Firstly, a binary logistic regression model analysis was carried out to determine the confounding factors. Secondly, the significant variables (P<0.1) identified on the univariate analysis or well-known to be associated with the success of IVF cycles, such as female age, were subjected to multivariate logistic regression analysis. Therefore, the live birth rate was adjusted according to female age, years of infertility, AFC, BMI, type of infertility, and concomitant infertility factors. The results are given in terms of crude and adjusted odds ratio (OR) and 95% confidence interval (CI). Finally, the live birth rate and clinical pregnancy rate in the two protocol groups were adjusted as marginal means (95% CIs) according to female age, years of infertility, AFC, BMI, type of infertility, and concomitant infertility factors.
P<0.05 was considered statistically significant. All statistical analyses were carried out using the Statistical Package for Social Science version 22.0 (SPSS, Inc.).
Results
Baseline Characteristics
148 patients who underwent a first GnRH-a prolonged protocol and 154 who underwent a short GnRH-a long protocol between January 2016 and December 2018 were included. Characteristics of included patients are described in Table 1. Endometrial thickness ranged from 4.6 to 7.9 mm and 5.0 to 7.9 mm in the two groups respectively, P=0.053. The average age of the 148 women enrolled in the GnRH-a prolonged protocol group was 31.3±4.0 years (mean±SD), and the 154 women in the other group was 31.1±4.8 years, P=0.832. No statistically significant difference was noted in maternal age, years of infertility, AMH, FSH, AFC, BMI, type of infertility, and concomitant infertility factors between the two groups.
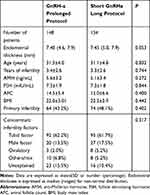 |
Table 1 Baseline Characteristics of the Patients |
Outcomes of Controlled Ovarian Hyper-Stimulation
Table 2 shows ovarian stimulation procedures of the two protocols. Exposure days to GnRH-a before the stimulation phase were enhanced in the GnRH-a prolonged protocol compared with the short GnRH-a long protocol (32.5 vs 14.6 days, P=0.000) as expected, which resulted in prolonged pituitary down-regulation. The GnRH-a prolonged protocol group had significantly higher duration of stimulation (10.9 vs 9.5 days, P=0.000) and total Gn dosage (2625.0 vs 2047.5 IU, P=0.000) than the short GnRH-a long protocol group. While the short GnRH-a long protocol yielded a higher estradiol level (3371.4 vs 2283.4 pg/mL, P=0.000) and LH level (1.7 vs 1.0 mIU/mL, P=0.062) on the hCG day than the prolonged protocol.
 |
Table 2 Outcomes of Controlled Ovarian Hyper-Stimulation |
Each patient was transferred with one or two good quality embryos only and the number of embryos transferred was comparable between the two groups. Other results including the number of oocytes retrieved, two visualized pronuclei (2PN) number, and variable blastocyst frozen were also similar.
Clinical Outcomes
The live birth rate was 36.5% (54/148) in the prolonged protocol group and 20.8% (32/154) in the short GnRH-a long protocol group, P=0.002 (Table 2). The clinical pregnancy rate and implantation rate of the former group were also significantly higher than that of the latter group (43.9% vs 28.2%, P=0.006; 35.4% vs 15.9%, P=0.000, respectively). There were no significant differences in the miscarriage rate and ectopic pregnancy between the two protocols (P>0.05).
Multivariable Logistic Regression Models
We further conducted logistic regression analysis to evaluate whether the ovarian stimulation protocol was an independent factor associated with the live birth rate (Table 3). The logistic regression model showed that in patients with thin endometrium, the independent variables predictive of live birth were the ovarian stimulation protocol (P=0.001) and AFC (P=0.016). Compared with that in the reference group, the live birth rate was significantly increased in the prolonged protocol group (crude OR: 2.190, 95% CI: 1.311, 3.660; adjusted OR: 2.458, 95% CI: 1.430, 4.224). However, female age, years of infertility, BMI, type of infertility, and concomitant infertility factors were not associated with live birth.
 |
Table 3 Logistic Regression Model of Live Birth |
Adjusted Live Birth Rate and Clinical Pregnancy Rate by Protocols
Table 4 shows the marginal means (95% CIs) of the live birth rate and clinical pregnancy rate by the two protocols. In women with thin endometrium, after adjustment for female age, years of infertility, AFC, BMI, type of infertility, and concomitant infertility factors, the live birth rate and clinical pregnancy rate were significantly higher in the prolonged group than that in the other group (39% vs 20%, P=0.001; 45% vs 28%, P=0.004, respectively).
 |
Table 4 Live Birth Rates and Clinical Pregnancy Rates by Protocols: Unadjusted and Adjusted |
Discussion
This study was the first research with the largest sample size (n=302) to estimate the novel use of GnRH-a for improving the live birth rates of patients with thin endometrium. The results of this retrospective cohort study showed that the GnRH-a prolonged protocol is an effective treatment for improving clinical outcomes of patients with thin endometrium.
Treatment of thin endometrium remains a challenge and unresolved problem in ART. The pregnancy outcomes are significantly declined with an endometrial thickness below 6–8 mm on the day of ovulation trigger.11,12 Various treatments have been proposed to improve endometrial receptivity and increase pregnancy rates, such as adding additional estrogen and GnRH-a, hysteroscopic adhesiolysis, administration of vitamin E, low dose aspirin, stem cell regenerative therapy, and so on. Many patients, however, could get limited benefits from these methods.13
Several studies revealed that the thickness of endometrium on the day of ovarian trigger of at least 8 mm is a prognostic factor for successful implantation.14,15 Also, the endometrium of 8 mm has been widely used as the threshold of endometrial receptivity in FET cycles.16–18 When doctors and infertile women are faced with extremely low endometrium (≤4–5 mm), freezing all embryos may be the best choice for them. However, with low endometrium (≤6–8 mm) and other good conditions, patients may hesitate about the next step considering time and cost.
In our center, the GnRH-a prolonged protocol and the short GnRH-a long protocol are the most widely used ovarian stimulation protocols for patients with thin endometrium. Usually, ideal pregnancy outcomes are obtained. In our results, with comparable baseline characteristics and similar stimulation procedure, patients of the prolonged protocol group had significantly higher live birth rate, clinical pregnancy rate, and implantation rate than those of the short GnRH-a long protocol group. Several retrospective studies also demonstrated that the GnRH-a prolonged protocol consisting of one depot of long-acting GnRH-a analogues before ovarian stimulation offered relatively high pregnancy rates in normal responders and especially in patients with endometriosis.19–23 No information, however, is available regarding their value in improving endometrium.
In the GnRH-a prolonged protocol, 3.75 mg of GnRH-a is injected once on day 2 of the menstrual cycle. Most researchers hold the view that the beneficial effects might be associated with increased endometrial receptivity during embryo implantation. In our study, patients with thin endometrial thickness could also benefit a lot from the GnRH-a prolonged stimulation protocol. Our results showed that this protocol may increase pregnancy outcomes by improving endometrial receptivity rather than just endometrial thickness. Another study supported that the increasing concentrations of GnRH-a could improve endometrial receptivity by regulating the expression of the related enzymes and cytokines directly.24 A recent molecular mechanism study proved that the administration of higher doses of long-acting GnRH-a may have a beneficial effect on endometrial receptivity by protecting the expression of endometrial receptivity markers such as HOXA10, MEIS1, and LIF.25 However, a longer period of GnRH-a administration is not recommended. Previous studies showed that the ultralong administration of GnRH-a before IVF did not improve the clinical pregnancy rate in women with endometrial factors.26,27 In the ultralong GnRH-a protocol, 3.75 mg of GnRH-a was injected three times on days 1, 28, and 56 after the menstrual cycle. Thus, the duration of the down-regulation of this protocol is 90 days. While in the GnRH-a prolonged protocol of our study, the average duration of the down-regulation is 40 days. But the reason why a prolonged ovarian suppression resulted in better clinical outcomes needs to be explored in future studies.
This study was a retrospective design from a single medical center, but baseline characteristics of included patients were matched (Table 1). 302 patients whose endometrium was less than 8 mm were finally enrolled from 21,750 cycles. All 148 patients who underwent the first GnRH-a prolonged protocol and 154 who underwent the short GnRH-a long protocol had acquired good quality fresh embryos for transfer. “Special” patients like egg donors who might introduce confounding factors were routinely excluded according to the criteria as showed in the flow chart of the study in Figure 1. As expected, maternal age, years of infertility, AMH, FSH, AFC, BMI, type of infertility, and concomitant infertility factors were comparable between the two groups. In addition, multivariate logistic regression analysis and adjusted marginal means (95% CIs) of the live birth rate and clinical pregnancy rate also contributed to eliminating the confounding factors. The average age, known as the best predictor of embryo quality, was both 31 years in the two groups. Most patients were young and have good quality, which might be the reason why the female age was not found to be associated with the live birth rate in our regression analysis result.
The purpose of the present study was to determine whether prolonged pituitary down-regulation is an effective treatment for improving clinical outcomes of patients with endometrium <8 mm. Though with similar stimulation procedures including the number of oocytes retrieved, 2PN, and variable blastocyst frozen, the live birth rate, clinical pregnancy rate, and implantation rate of the prolonged protocol group were significantly higher than those of the short GnRH-a long protocol group. The explanation for better outcomes may lie in increased exposure to GnRH-a in the prolonged protocol group. Our results were supported by some studies, which justified the effectiveness of adding GnRH-a before oocyte retrieval for better IVF outcomes.28,29 Another reason for better outcomes may be the decreased LH levels in the prolonged protocol group (1.0 vs 1.7), even though the difference was not significant (P=0.062). As we know, relatively high LH serum level has a dramatic impact on pregnancy achievement. The underlying mechanism may be the role of LH receptor expression in determining the fate of embryos, which is influenced by different ovarian stimulation protocols.30 Our data suggested a stronger suppression on the pituitary and ovary in the GnRH-a prolonged protocol group. Some publications proved that more successful pregnancies can be obtained if an endogenous premature LH surge could be inhibited.31–33 Additionally, another significantly different hormone value between two protocols was the estradiol level on the HCG day. The short GnRH-a long protocol yielded a higher estradiol serum level (3371.4 vs 2283.4 pg/mL) (Table 2), but the two protocols had a similar number of oocytes retrieved. Thus, an increase in estradiol levels may induce a premature LH surge with follicles growing in the short GnRH-a long protocol – an important limiting factor in achieving pregnancy.
Our research has some limitations. First, the data of endometrial thickness were not collected before a cycle was initiated. But endometrial thickness on the day of ovarian trigger is the key determinant for clinicians and infertile couples to choose to proceed with the fresh embryo transfer or cryopreserve all embryos for a future cycle. In our study, patients had similar endometrial thickness on the day of ovarian trigger, ranging from 4.6 to 7.9 mm and 5.0 to 7.9 mm in the two groups respectively. Despite this, they could still benefit a lot from the GnRH-a prolonged stimulation protocol. Second, this study was a retrospective design from a single medical center. These conclusions warrant further confirmation by other multicenter, larger, prospective studies. However, the large sample size, use of a multivariate regression model for a wide array of possible confounding factors, and marginal means of live birth rate adjusted by protocols rendered the conclusion relatively reliable.
Conclusion
Our large, retrospective cohort study found that the GnRH-a prolonged protocol in fresh embryo transfer cycles offered better clinical outcomes of patients with thin endometrium than the short GnRH-a long protocol.
Acknowledgment
This study was funded by National Key Research and Development Program of China (grant no. 2019YFC0121000).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Lambalk CB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–579. doi:10.1093/humupd/dmx017
2. Haahr T, Roque M, Esteves SC, Humaidan P. GnRH agonist trigger and LH activity luteal phase support versus hCG trigger and conventional luteal phase support in fresh embryo transfer IVF/ICSI cycles-a systematic PRISMA review and meta-analysis. Front Endocrinol (Lausanne). 2017;8:116. doi:10.3389/fendo.2017.00116
3. Gallos ID, Eapen A, Price MJ, et al. Controlled ovarian stimulation protocols for assisted reproduction: a network meta-analysis. Cochrane Database Syst Rev. 2017;2017(3).
4. Depalo R, Jayakrishan K, Garruti G, et al. GnRH agonist versus GnRH antagonist in in vitro fertilization and embryo transfer (IVF/ET). Reprod Biol Endocrinol. 2012;10:26. doi:10.1186/1477-7827-10-26
5. Kasius A, Smit JG, Torrance HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530–541. doi:10.1093/humupd/dmu011
6. Ranisavljevic N, Raad J, Anahory T, Grynberg M, Sonigo C. Embryo transfer strategy and therapeutic options in infertile patients with thin endometrium: a systematic review. J Assist Reprod Genet. 2019;36(11):2217–2231. doi:10.1007/s10815-019-01576-w
7. Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in asherman syndrome and thin endometrium: stem cell- based therapy. Biomed Pharmacother. 2018;102:333–343. doi:10.1016/j.biopha.2018.03.091
8. Eid ME. Sildenafil improves implantation rate in women with a thin endometrium secondary to improvement of uterine blood flow; “pilot study”. Fertil Steril. 2015;104(3):e342. doi:10.1016/j.fertnstert.2015.07.1066
9. Balaban B, Brison D, Calderon G. Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. The istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–1283. doi:10.1093/humrep/der037
10. Song J, Sun X, Qian K. Endometrial but not ovarian response is associated with clinical outcomes and can be improved by prolonged pituitary downregulation in patients with thin and medium endometrium. Reprod Sci. 2019;26(11):1409–1416. doi:10.1177/1933719118816835
11. Lebovitz O, Orvieto R. Treating patients with “thin” endometrium - an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–414. doi:10.3109/09513590.2014.906571
12. Gao G, Cui X, Li S, Ding P, Zhang S, Zhang Y. Endometrial thickness and IVF cycle outcomes: a meta-analysis. Reprod Biomed Online. 2020;40(1):124–133. doi:10.1016/j.rbmo.2019.09.005
13. Liu KE, Hartman M, Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian fertility and andrology society. Reprod Biomed Online. 2019;39(1):49–62. doi:10.1016/j.rbmo.2019.02.013
14. Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221–228. doi:10.1097/GCO.0b013e328302143c
15. Mouhayar Y, Franasiak JM, Sharara FI. Obstetrical complications of thin endometrium in assisted reproductive technologies: a systematic review. J Assist Reprod Genet. 2019;36(4):607–611. doi:10.1007/s10815-019-01407-y
16. Jimenez PT, Schon SB, Odem RR, Ratts VS, Jungheim ES. A retrospective cross-sectional study: fresh cycle endometrial thickness is a sensitive predictor of inadequate endometrial thickness in frozen embryo transfer cycles. Reprod Biol Endocrinol. 2013;11:35. doi:10.1186/1477-7827-11-35
17. Gingold JA, Lee JA, Rodriguez-Purata J, et al. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104:
18. Bu Z, Wang K, Dai W, et al. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol Endocrinol. 2016;32(7):524–528. doi:10.3109/09513590.2015.1136616
19. Ren J, Sha A, Han D, Li P, Geng J, Ma C. Does prolonged pituitary down-regulation with gonadotropin-releasing hormone agonist improve the live-birth rate in in vitro fertilization treatment? Fertil Steril. 2014;102(1):75–81. doi:10.1016/j.fertnstert.2014.03.030
20. Surrey MW, Surrey ES, Silverberg KM, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril. 2002;78(4):699–704. doi:10.1016/S0015-0282(02)03373-3
21. Zikopoulos K, Kolibianakis EM, Devroey P. Ovarian stimulation for in vitro fertilization in patients with endometriosis. Acta Obstet Gynecol Scand. 2004;83(7):651–655. doi:10.1111/j.0001-6349.2004.00543.x
22. Mao GH, Feng Z, He Y, Huang YR. Comparisons of the effects of long-acting and short-acting GnRHas on embryo quality, endometrial thickness and pregnancy rate in human in vitro fertilization. Arch Med Sci. 2014;10:161–166. doi:10.5114/aoms.2014.40743
23. Geng Y, Xun Y, Hu S, Lai Q, Jin L. GnRH antagonist versus follicular-phase single-dose GnRHa protocol in patients of normal ovarian response during controlled ovarian stimulation. Gynecol Endocrinol. 2019;35:309–313. doi:10.1080/09513590.2018.1528221
24. Ruan HC, Zhu XM, Luo Q, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod. 2006;21(10):2521–2529. doi:10.1093/humrep/del215
25. Xu B, Geerts D, Hu S, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod. 2020;35(6):1306–1318. doi:10.1093/humrep/deaa086
26. Kaponis A, Chatzopoulos G, Paschopoulos M, et al. Ultralong administration of gonadotropin-releasing hormone agonists before in vitro fertilization improves fertilization rate but not clinical pregnancy rate in women with mild endometriosis: a prospective, randomized, controlled trial. Fertil Steril. 2020;113(4):
27. Georgiou EX, Melo P, Baker PE, et al. Long-term GnRH agonist therapy before in vitro fertilisation (IVF) for improving fertility outcomes in women with endometriosis. Cochrane Database Syst Rev. 2019;11:CD013240.
28. Tiras B, Leylek OA, Halicigil C, Saltik A, Kavci N. Does single dose GnRH agonist administration after oocyte pick-up in antagonist cycles improve endometrial receptivity and pregnancy rates? Fertil Steril. 2013;100(3):S59–S60. doi:10.1016/j.fertnstert.2013.07.1864
29. Schachter M, Friedler S, Ron-El R, et al. Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril. 2008;90(4):1087–1093. doi:10.1016/j.fertnstert.2007.07.1316
30. Maman E, Yung Y, Kedem A, et al. High expression of luteinizing hormone receptors messenger RNA by human cumulus granulosa cells is in correlation with decreased fertilization. Fertil Steril. 2012;97(3):592–598. doi:10.1016/j.fertnstert.2011.12.027
31. Venetis CA, Kolibianakis EM, Tarlatzi TB, Tarlatzis BC. Benefits of luteinizing hormone activity in ovarian stimulation for IVF. Reprod Biomed Online. 2009;18(Suppl 2):31–36. doi:10.1016/S1472-6483(10)60446-4
32. Allegra A, Marino A, Coffaro F, et al. GnRH antagonist-induced inhibition of the premature LH surge increases pregnancy rates in IUI-stimulated cycles. A prospective randomized trial. Hum Reprod. 2007;22(1):101–108. doi:10.1093/humrep/del337
33. Lee A, Somkuti SG, Barmat LI, Smith SE, Schinfeld JS. Continued ovarian stimulation after premature LH surge with oocyte retrieval and embryo cryopreservation performed in the luteal phase for fertility preservation prior to breast cancer treatment. Fertil Steril. 2008;90:S449–S450. doi:10.1016/j.fertnstert.2008.07.876
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
