Back to Journals » Drug Design, Development and Therapy » Volume 13
Coenzyme q10 liquid supplementation in dyslipidemic subjects with statin-related clinical symptoms: a double-blind, randomized, placebo-controlled study
Authors Derosa G , D'Angelo A , Maffioli P
Received 14 July 2019
Accepted for publication 7 August 2019
Published 21 October 2019 Volume 2019:13 Pages 3647—3655
DOI https://doi.org/10.2147/DDDT.S223153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Giuseppe Derosa,1–3 Angela D’Angelo,2 Pamela Maffioli1
1Center of Diabetes and Metabolic Diseases, Department of Internal Medicine and Therapeutics, University of Pavia and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 2Laboratory of Molecular Medicine, University of Pavia, Pavia, Italy; 3Center for Prevention, Surveillance, Diagnosis and Treatment of Rare Diseases, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Correspondence: Giuseppe Derosa
Department of Internal Medicine and Therapeutics, University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, P.le C. Golgi, 2, Pavia 27100, Italy
Tel +39 038 252 6217
Fax +39 038 252 6259
Email [email protected]
Introduction: Statin-associated myalgia occurs in about 1–3% of patients in the medical literature. Plasma CoQ10 levels are reduced in patients undergoing statin.
Objective: The primary outcome was the detection of clinical symptoms and the perception of pain evaluated throughout specific questionnaires. The secondary outcome was the variation in lipid profile and the variation in safety parameters.
Methods: We enrolled 60 Caucasian patients, intolerant to statins. During the run-in period, patients underwent a 1-month wash-out period during which statins were stopped. At the end of the wash-out period, if CPK and/or transaminases returned within an acceptable range, statins were re-introduced at half of the previously taken dose. After one month, patients were randomized to take either a liquid CoQ10 supplement or a placebo for three months at 100 mg/day.
Results: The Clinical Index Score for myalgia assessment was lower after 3 months with CoQ10, while it did not change with the placebo. The VAS score was lower after 3 months of CoQ10 supplementation, while no variation was recorded with the placebo. In the group treated with the dietary supplement, CoQ10 plasma concentrations were inversely correlated with CPK levels, Clinical Index Score absolute values, and VAS.
Conclusion: The addition of CoQ10 with half dosage statin in patients with previous intolerance to statins improves the perception of clinical symptoms such as asthenia, myalgia or pain.
Keywords: coenzyme Q10, myalgia, statins
Introduction
Coenzyme Q10 (CoQ10) is an intracellular anti-oxidant that prevents senescence and dysfunction caused by oxidative stress. It is an essential cofactor in the electron transport chain and the only endogenously synthesized lipophilic antioxidant. Coenzyme Q10 is also obtained from the diet, although dietary intake of CoQ10 only has a marginal effect on plasma CoQ10 concentrations.1 Coenzyme Q10 supplements are available over-the-counter in most countries, and supplementation has been reported to have a beneficial effect for patients with breast cancer,2 heart failure,3 and mitochondrial encephalomyopathy,4 among others. Plasma CoQ10 levels are reduced in patients undergoing statin (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor) therapy5,6 and in patients with heart failure.7 Inhibiting HMG-CoA reductase reduces tissue levels of cholesterol in humans by limiting the intrinsic biosynthesis of cholesterol, and also inhibits the intrinsic biosynthesis of CoQ10. Hydroxyl-methylglutaryl coenzyme A reductase inhibitors like statins interfere with the production of mevalonic acid, which is a precursor in the synthesis of CoQ10. Statin medications routinely result in lower CoQ10 levels in the serum,8–10 and some studies have also shown reduction of CoQ10 in muscle tissue.8–10 This kind of CoQ10 deficiency may be a cause for statin-induced myopathies.8–10 Statin-associated myalgia occurs in about 1–3% of patients in the medical literature, but that figure is likely not a true representation of the occurrence of muscle pain: in real-life situations, studies suggest that the occurrence is anywhere from 10% to 20%. The true definition of statin-associated myalgia is controversial; some physicians suggest that true myalgia is only present if muscle pain or cramping has occurred on multiple statins, and improves after each statin is discontinued. Less strictly defined, myalgia is present if a patient complains of muscle aching after a few weeks of initial statin therapy.11 Statin-induced myopathy covers a broader range of statin-associated muscle symptoms (SAMSs) and is subdivided by the presence or absence of creatine phosphokinase (CPK) elevation.12 For this reason, the National Lipid Association’s Muscle Safety Expert Panel was charged with examining the definitions for statin-associated muscle adverse events, the development of a clinical index to assess myalgia and the use of diagnostic neuromuscular studies to investigate muscle adverse events. Clinical index score was considered a reliable tool to assess myalgia.12 Although CoQ10 is widely used in clinical practice for the treatment of neuromuscular disorders induced by statins, scientific literature reports heterogeneous results that are not always positive. Such variability in effects might be due to the high lipophilicity of the molecule, which is insoluble in water and only slightly absorbed in the gut after oral intake. Moreover, its absorption is characterized by high variability (both inter-individual and inter-product), which affects both pharmacokinetics and pharmacodynamics. In order to overcome these bioavailability limits, some authors used high CoQ10 dosages, which exerts negative impact on patient compliance and the cost of therapy. Last, but not least, in many European countries, the highest amount of CoQ10 allowed by legislative bodies for use in dietary supplement formulations is 200 mg. Other authors prefer to emulsify the CoQ10 in oil or formulate it as a soft gel, using liquid formulations based on dispersion technology to ensure the highest bioavailability.13–16
In the current study, we use a liquid CoQ10 formulation based on micro-sphere dispersion technology (Q-Factor) at a low dosage (100 mg/day) since previous studies14–16 showed that its bioavailability was about 3-fold higher than other CoQ10-based products available on the market. Based on this, we planned a study aimed at evaluating whether supplementation with highly bioavailable CoQ10 at a relatively low dosage can be helpful in dyslipidemic patients with intolerance to statins at maximum dosage when LDL cholesterol levels are not adequately controlled. The primary outcome was the detection of clinical symptoms and the perception of pain evaluated throughout specific questionnaires. The secondary outcome was the variation in lipid profile and the variation in safety parameters.
Materials and methods
Study design
This 3-month, double-blind, randomized, placebo-controlled clinical trial was conducted at the Department of Internal Medicine and Therapeutics at the University of Pavia (Pavia, Italy) with patients visiting the Center for Prevention, Surveillance, Diagnosis and Treatment of Rare Diseases.
The study protocol was approved by the local institutional ethical committee and was conducted in accordance with the 1994 Declaration of Helsinki and its amendments, and the Code of Good Clinical Practice. All patients provided written, informed consent to participate in this study after a full explanation of the study was given.
Materials and methods
We enrolled 60 Caucasian patients, aged ≥18 of either sex, whose LDL cholesterol levels were not adequately controlled and who were intolerant to statins. Patients were considered intolerant if they had experienced an increase in CPK greater than 3 until 10 times the upper limits of the laboratory (ULN) and/or a rise in the value of transaminases greater than 3 until 5 times the ULN and/or the onset of clinical symptoms such as asthenia, myalgia or rhabdomyolysis.
Suitable patients, identified from review of case notes and/or computerized clinical registries, were contacted by the investigators in person or by telephone.
Patients were excluded if they had any of the following: secondary dyslipidemia or type 2 diabetes mellitus; impaired renal function (defined as a serum creatinine level higher than the ULN for age and sex); endocrine disorders or gastrointestinal disorders; current or previous evidence of ischemic heart disease, heart failure or stroke; weight change of >3 kg during the preceding 3 months; malignancy or significant neurological or psychiatric disturbances, including alcohol or drug abuse. Excluded medications (within the previous 3 months) included anorectic agents, laxatives, β-agonists (other than inhalers), diuretics, cyproheptadine, anti-depressants, anti-serotonergics, phenothiazines, barbiturates, oral corticosteroids and anti-psychotics. Women who were pregnant, breastfeeding or of childbearing potential and not taking adequate contraceptive precautions were also excluded.
Treatment
During the run-in period, patients underwent a 1-month wash-out period during which statins were stopped. At the end of the wash-out period, if CPK and/or transaminases returned within an acceptable range (below three times the ULN) and clinical symptoms were mitigated, statins were re-introduced at half of the previously taken dose (Tables 1 and 2). After one month, patients were randomized to take either a liquid CoQ10 supplement (Q-Factor, a water-soluble CoQ10 based on micro-sphere dispersion technology; Giellepi S.p.A, Italy) or a placebo for three months using a double-blind, randomized, placebo-controlled study design (Figure 1). Patients with a further increase in CPK and/or transaminases and the absence of clinical symptoms were excluded.
 |
Table 1 Dosage of statins taken at the study enrollment before addition of CoQ10 supplementation |
 |
Table 2 Dosage of statins taken at the study enrollment before addition of placebo |
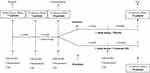 |
Figure 1 Design of the study. |
Patients were randomized to take the placebo or CoQ10 at 100 mg/day for 3 months. CoQ10 and the placebo were self-administered once a day (before, with or after a meal) at 1 mL/day using oral drops. Both CoQ10 and the placebo were supplied as identical, opaque, coded bottles to ensure the blind status of the study.
Randomization was done by drawing envelopes containing randomization codes prepared by a statistician. Medication compliance was assessed by counting the number of bottles returned at the time of specified clinical visits. Throughout the study, we instructed patients to take their first dose of new medication on the day after they were given the study medication. At the same time, all unused medication was retrieved for inventory. All medications were provided free of charge (Figure 1).
Assessments
Before starting the study, all patients underwent an initial screening assessment that included a medical history, physical examination, vital signs (blood pressure and heart rate), a 12-lead electrocardiogram, measurements of height and body weight, calculation of BMI, abdominal circumference (Abd. Cir.), waist circumference (Waist Cir.), hip circumference (Hip Cir.), assessment of plasmatic CoQ10, total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), triglycerides (Tg), transaminases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] and CPK. Patients also answered two questionnaires:
- Clinical Index Score for myalgia assessment
- Visual Analogue Scale (VAS) for Pain
All parameters were assessed at baseline and after 1 and 3 months since the study start. The questionnaires were administered at baseline and after 1 and 3 months.
All parameters were determined from plasma taken after a 12 hr overnight fast. Venous blood samples were taken from all patients between 8 and 9 am, and were drawn from an antecubital vein using a 19-gauge needle without venous stasis.
We used plasma obtained by adding Na2-EDTA (1 mg/mL) and centrifuging at 3000 g for 15 mins at 4 °C. Immediately after centrifugation, the plasma samples were frozen and stored at −80 °C for no more than 3 months. All measurements were performed in a central laboratory.
BMI was calculated as weight in kilograms divided by height squared in meters.
Total cholesterol and Tg levels were determined by fully enzymatic techniques17,18 on a clinical chemistry analyzer (HITACHI 737; Hitachi, Tokyo, Japan). Intra- and interassay CsV were 1.0 and 2.1 for TC measurement, and 0.9 and 2.4 for Tg measurement, respectively. High density lipoprotein-cholesterol levels were measured after precipitation of plasma apo B-containing lipoproteins with phosphotungstic acid.19 Intra- and interassay CsV were 1.0 and 1.9, respectively. LDL-C level was calculated using the Friedewald formula.20
Coenzyme Q10 was determined using a previously validated high-performance liquid chromatography procedure with electrochemical detection. Intra- and interassay CsV were 1.2 and 4.9, respectively.21
Safety measurements
Investigators assessed treatment tolerability by carefully interviewing patients during study visits and by comparing clinical and laboratory values with baseline levels. Safety monitoring included physical examination, vital sign assessment, weight assessment, adverse events assessment and laboratory tests. Liver and muscle function were evaluated by measuring transaminases and CPK, and all adverse events were recorded. Creatinine levels were also assessed.
Statistical analysis
An intention-to-treat (ITT) analysis was conducted in patients who received ≥1 dose of study medication with subsequent efficacy observation. Patients were included in the tolerability analysis if they received ≥1 dose of trial medication after randomization and underwent subsequent tolerability observation. The comparison of variables between two groups was performed with the Student t-test for independent data, while paired t-test was used to compare values obtained before and after treatment administration. Statistical analysis of data was performed using the Statistical Package for Social Sciences software version 21.0 (SPSS Inc., Chicago, Illinois, USA). Data were presented as the mean (± SD). For all statistical analyses, a p-value <0.05 was considered statistically significant.22
Results
Study sample
A total of 60 patients were enrolled in this trial. Of these, 30 were randomly selected to take CoQ10 supplementation and 30 were randomly selected to take the placebo. Fifty-six subjects completed the study; there were 4 patients who did not complete the study and the reasons for their premature withdrawal included non-compliance with treatment or lack of follow-up. The dosage list for statins taken by patients during enrollment is given in Tables 1 and 2. The types of adverse events recorded for patients prior to enrollment are listed in Table 3 according to statin subgroup.
 |
Table 3 Type of adverse events recorded for enrolled patients at baseline |
Coenzyme Q10
Coenzyme Q10 levels were significantly higher after 3 months in the CoQ10 group, both compared to the baseline and the placebo (p<0.05 for both) (Table 4).
 |
Table 4 Baseline, 1 and 3 months data of patients during CoQ10 supplementation and placebo |
Clinical symptoms
The Clinical Index Score for myalgia assessment was lower after 3 months with CoQ10 (7.3±1.6 vs 10.3±2.1 at baseline, p<0.05), while it did not change with the placebo. The difference between the CoQ10 and placebo groups was significant after 3 months (p<0.05; Figure 2). Fourteen patients (46.6%) in the CoQ10 group reported a reduction of muscular pain compared to 2 patients (6.6%) in the placebo group.
The VAS score was lower after 3 months of CoQ10 supplementation (3.1±1.2 vs 5.3±2.4 at baseline), while no variation was recorded with the placebo. The difference between the CoQ10 and placebo groups was significant after 3 months (p<0.05). In the group treated with the dietary supplement, CoQ10 plasma concentrations were inversely correlated with CPK levels (r=−0.57; p<0.01), Clinical Index Score absolute values (r=−0.26; p<0.05) and VAS (r=−0.44; p<0.05).
Lipid profile
No variation in the lipid profile was recorded for CoQ10 group, while there was an increase in both TC and LDL-C with the placebo at 1 month (p<0.05 vs baseline) and 3 months (p<0.01 vs baseline). Patients treated with CoQ10 had lower TC and LDL-cholesterol compared to those taking the placebo (p<0.05 at 3 months) (Table 4).
Safety parameters
Transaminases and CPK values were lower after 3 months of CoQ10 supplementation compared to the baseline (p<0.05) and the placebo (p<0.05 compared to 1 month of taking the placebo) (Table 4). No variations in creatinine levels were recorded during the study.
Discussion
It is well known that statins are effective in the treatment of dyslipidemia, but often their side effects are dose limiting. Furthermore, 10–15% of patients are intolerant to statins, and after 1 year almost one-third of patients discontinue the treatment.11 The main result of our study was the significant decrease in asthenia, myalgia and muscle pain in patients taking CoQ10 compared to the placebo, in line with the meta-analysis published by Qu et al23. In particular, our results, obtained with 2 different tests for the evaluation of myopathy, confirm the positive results also obtained previously by 4 different clinical trials.24–27 It should be noted that, similar to our study, both Caso24 and Skarlovnik26 used 100 mg/day and achieved an average reduction of 30–40% in the severity of myalgia. According to a recent meta-analysis, statin therapy, regardless of whether it is carried out with lipophilic or hydrophilic molecules, significantly reduces the plasma levels of CoQ10 by 16–54%.28 In fact, statins reduce farnesyl pyrophosphate synthesis, which is a precursor of CoQ10, and they interfere with the intestinal absorption of dietary CoQ10. Intestinal microbiota dysbiosis is likely involved in this effect.29
As an intracellular antioxidant, CoQ10 protects the cell membrane phospholipids and mitochondrial membrane proteins against free radical-induced damage; intramuscular CoQ10 deficiency reduces the phosphorylation potential of ADP and causes mitochondrial complex I and IV dysfunction.30 For these reasons, CoQ10 supplementation should be considered as a substitutive and integrative therapy rather than a pharmacological therapy in patients undergoing statin therapy. In our study, patients treated with CoQ10 showed a significant reduction in liver enzymes, likely as a consequence of its anti-steatosis effect, which has been described by other authors.31 Our results confirm the lipid-lowering action of CoQ10, which enabled us to reach the therapeutic target for LDL cholesterol in a higher percentage of patients treated with the dietary supplement in comparison to the placebo group at half dosage of statin. The administration of CoQ10 in patients with intolerance to statins is able to both improve the quality of life (QoL) and reduce the cardiovascular risk. In fact, in addition to the beneficial effects on the liver and lipid parameters, CoQ10 administration could improve myocardial energy metabolism and decrease physical exertion, as has been shown for patients with chronic heart insufficiency.32,33 Moreover, a great number of intolerant patients interrupt the lipid-lowering treatment and/or significantly reduce physical activity because of fatigue and myalgia. This leads to a significant increase in adverse cardiovascular events.34,35 Such a situation could be reduced with CoQ10 supplementation, which should also increase the persistence of statin therapy.
Differently from other previous studies,29 we found a greater reduction in CPK levels after CoQ10 supplementation and half dosage of statin. This result is very interesting, although the plasma levels of CPK are not considered sensitivity markers for statin myopathy.
At first sight, the results of our study appear to suggest that CoQ10 has a neutral effect on the lipid profile, but this is not true. It is important to remember that in our study, patients were taking a half dosage of statin at baseline, so a worsening of the lipid profile would be expected. This happened in the placebo group, but it did not get worse in the CoQ10 group. Moreover, the lipid profile recorded in the CoQ10 group was better than the one recorded in the placebo group. The safety of the CoQ10 dietary supplement was demonstrated by no side effects or alterations in laboratory values.
There is evidence in the literature that low plasma levels of CoQ10 are associated with increased arterial stiffness in hypercholesterolemic subjects.36 This is improved by dietary supplementation with CoQ10 containing nutraceuticals.37 In the literature, supplementation with CoQ10 resulted in a decrease in blood pressure levels and in significant improvement of glycemic control.38 In our study, despite the concomitant use of statins, we observed a significant increase in plasmatic CoQ10 values after 3 months in the group treated with CoQ10, but not in patients in the placebo group. Given that it takes months to replenish CoQ10 stores and reach therapeutic levels, we hypothesize that doubling the dosage of CoQ10 would allow for reaching higher blood levels quicker, thus having a meaningful impact in the short-term management of symptoms.
The meta-analysis conducted by Jorat et al39 suggested that the differences in the type and dosage of CoQ10 used may help explain the discrepancies in the results obtained in previous published studies. Another reason for the discrepant results may be the low bioavailability of CoQ10; the hydrophobicity exhibited by the CoQ10 molecule leads to reports of poor absorption profiles. Thus, the optimization of formulations and methods of delivery is an ever-evolving therapeutic goal.
As far as we know, we are the firsts to report effects of liquid CoQ10 formulation in statin intolerant patients, moreover, contrary to other studies reported in literature, we evaluated CoQ10 level at baseline and at 1 and 3 months. Of course, our study has some limitations given the small number of samples and parameters analyzed. A further limitation is the short duration of the study; a longer follow-up will be needed to see if the effects observed in three months will be maintained or even enhanced in the long term.
In conclusion, the addition of CoQ10 with half dosage statin in patients with previous intolerance to statins improves the perception of clinical symptoms such as asthenia, myalgia or pain. CoQ10 was safe and effective in preventing the worsening of the lipid profile that would be expected with a reduced dosage of statin.
Acknowledgments
We are grateful to all patients who participated the clinical trial. The authors did not receive any funding for carrying out the clinical study. The investigational products were provide by the manufacturer Giellepi S.p.A. (Italy) free of charge.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaikkonen J, Nyyssönen K, Tuomainen TP, Ristonmaa U, Salonen JT. Determinants of plasma coenzyme Q10 in humans. FEBS Lett. 1999;443:163–166. doi:10.1016/s0014-5793(98)01712-8
2. Lockwood K, Moesgaard S, Yamamoto T, Folkers K. Progress on therapy of breast cancer with vitamin Q10 and the regression of metastases. Biochem Biophys Res Commun. 1995;212:172–177. doi:10.1006/bbrc.1995.1952
3. Soja AM, Mortensen SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Mol Aspects Med. 1997;18:s159–s168. doi:10.1016/S0098-2997(97)00042-3
4. Ogasahara S, Engel AG, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci USA. 1989;86:2379–2382. doi:10.1073/pnas.86.7.2379
5. Folkers K, Langsjoen P, Willis R, et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci USA. 1990;87:8931–8934. doi:10.1073/pnas.87.22.8931
6. Päivä H, Thelen KM, Van Coster R, et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–68. doi:10.1016/j.clpt.2005.03.006
7. Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci USA. 1985;82:901–904. doi:10.1073/pnas.82.3.901
8. Deichmann R, Lavie C, Andrews S, Ochsner J. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010;10(1):16–21.
9. Derosa G, D’Angelo A, Franzetti IG, et al. Efficacy and safety of ezetimibe/simvastatin association on non-diabetic and diabetic patients with polygenic hypercholesterolemia or combined hyperlipidemia and previously intolerant to standard statin treatment. J Clin Pharm Ther. 2009;34(3):267–276.
10. Derosa G, Romano D, D’Angelo A, Maffioli P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis. 2015;239(1):87–92. doi:10.1016/j.atherosclerosis.2014.12.043
11. Harper CR, Jacobsen TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep. 2010;12(5):322–330. doi:10.1007/s11883-010-0120-9
12. Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. The national lipid association’s muscle safety expert panel. an assessment by the statin muscle safety task force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S58–S71. doi:10.1016/j.jacl.2014.03.004
13. Chopra RK, Goldman R, Sinatra SP, Bhagavan HN. Relative bioavailability of Coenzyme Q10 formulations in human subjects. Int J Nutr Res. 1998;68:109–113.
14. Miles MV, Horn P, Miles L, Tang P, Steele P, DeGrauw T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutr Res. 2002;919–929. doi:10.1016/S0271-5317(02)00402-5
15. Zmitek J, Smidovnik A, Fir M, et al. Relative bioavailability of two forms of a novel water-soluble coenzyme Q10. Ann Nutr Metab. 2008;52(4):281–287. doi:10.1159/000129661
16. Beg S, Javed S, Kohli K. Bioavailability enhancement of coenzyme Q10: an extensive review of patents. Recent Pat Drug Deliv Formul. 2010;4(3):245–255.
17. Klose S, Borner K. Enzymatische Bestimmung des Gesamtcholesterins mit dem [Enzymatic dosage of total cholesterolemia by Greiner Selective Analyzer (GSA II)]. J Clin Chem Clin Biochem. 1978;15:121–130.
18. Wahlefeld AW. Triglycerides determination after enzymatic hydrolysis. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis.
19. Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi:10.1172/JCI103182
20. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
21. Tang PH, Miles MV, DeGrauw A, Hershey A, Pesce A. HPLC analysis of reduced and oxidized coenzyme Q(10) in human plasma. Clin Chem. 2001;47(2):256–265.
22. Winer BJ. Statistical Principles in Experimental Design.
23. Qu H, Guo M, Chai H, Wang WT, Gao ZY, Shi DZ. Effects of coenzyme Q10 on statin-induced myopathy: an updated meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(19):e009835. doi:10.1161/JAHA.118.008528
24. Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–1412. doi:10.1016/j.amjcard.2006.12.063
25. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q10 and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165–170. doi:10.1139/cjpp-2012-0118
26. Skarlovnik A, Janic M, Lunder M, Turk M, Sabovic M. Coenzyme Q10 supplementation decreases statin-related mild-to-moderate muscle symptoms: a randomized clinical study. Med Sci Monit. 2014;20:2183–2188. doi:10.12659/MSM.890777
27. Toth S, Sajty M, Pekarova T, et al. Addition of omega-3 fatty acid and coenzyme Q10 to statin therapy in patients with combined dyslipidemia. J Basic Clin Physiol Pharmacol. 2017;28:327–336. doi:10.1515/jbcpp-2016-0149
28. Banach M, Serban C, Ursoniu S, et al. Statin therapy and plasma coenzyme Q10 concentrations: a systematic review and meta-analysis of placebo-controlled trials. Pharmacol Res. 2015;99:329–336. doi:10.1016/j.phrs.2015.07.008
29. Qu H, Meng YY, Chai H, et al. The effect of statin treatment on circulating coenzyme Q10 concentrations: an updated meta-analysis of randomized controlled trials. Eur J Med Res. 2018;23(1):57. doi:10.1186/s40001-018-0306-0
30. Mikashinovich ZI, Belousova ES, Sarkisyan OG. Impairment of energy dependent processes in the muscle tissue as a pathogenetic mechanism of statin-induced myopathy. Bull Exp Biol Med. 2017;162:433–435. doi:10.1007/s10517-017-3633-1
31. Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA. Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr. 2016;35(4):346–353. doi:10.1080/07315724.2015.1021057
32. Keogh A, Fenton S, Leslie C, et al. Randomised double-blind, placebo-controlled trial of coenzyme Q, therapy in class II and III systolic heart failure. Heart Lung Circ. 2003;12(3):135–141.
33. Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors. 2005;25(1–4):137–145.
34. Marrs JC, Kostoff MD. Discontinuation of statins: what are the risks? Curr Atheroscler Rep. 2016;18(7):41. doi:10.1007/s11883-016-0596-z
35. Young JM, Molyneux SL, Reinheimer AM, et al. Relationship between plasma coenzyme Q10, asymmetric dimethylarginine and arterial stiffness in patients with phenotypic or genotypic familial hypercholesterolemia on long-term statin therapy. Atherosclerosis. 2011;218(1):188–193. doi:10.1016/j.atherosclerosis.2011.04.034
36. Larijani VN, Ahmadi N, Zeb I, Khan F, Flores F, Budoff M. Beneficial effects of aged garlic extract and coenzyme Q10 on vascular elasticity and endothelial function: the FAITH randomized clinical trial. Nutrition. 2013;29(1):71–75. doi:10.1016/j.nut.2012.03.016
37. Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56(11):1137–1142. doi:10.1038/sj.ejcn.1601464
38. Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Hum Hypertens. 1999;13(3):203–208.
39. Jorat MV, Tabrizi R, Mirhosseini N, et al. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018;17(1):230. doi:10.1186/s12944-018-0876-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

