Back to Journals » Journal of Blood Medicine » Volume 10
Clinical and laboratory manifestations of the prothrombin gene mutation in women of reproductive age
Authors Momot AP, Nikolaeva MG, Yasafova NN , Zainulina MS , Momot KA, Taranenko IA
Received 18 April 2019
Accepted for publication 3 July 2019
Published 2 August 2019 Volume 2019:10 Pages 255—263
DOI https://doi.org/10.2147/JBM.S212759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
AP Momot,1 MG Nikolaeva,2 NN Yasafova,3 MS Zainulina,4 KA Momot,3 IA Taranenko3
1Altai Branch of FSBI, National Research Center for Hematology, Barnaul, Russia; 2Obstetrics and Gynecology Department with The Course in Supplementary Vocational Education at FSBI of Higher Education, Altai State Medical University, Barnaul, Russia; 3Altai Branch of FSBI, National Research Center for Hematology, Ministry of Healthcare of the Russian Federation, Barnaul, Russia; 4Saint Petersburg State-Financed Health Institution, “Maternity Hospital № 6 Named after Professor V.F. Snegirev”, Obstetrics, Gynecology and Reproductive Medicine Department, Pavlov First Saint Petersburg State Medical University, Saint Petersburg, Russia
Research objective: To research the association of prothrombin (factor II) activity given the prothrombin G20210A mutation carriage with its clinical manifestations as thrombotic complications.
Materials and methods: A prospective clinical cohort study of 290 women of reproductive age was conducted. Two cohort groups were identified: the study group of 140 patients with prothrombin mutation G20210A genotype and the control group of 150 women with G20210G genotype.
Results: The prothrombin G20210A mutation carriage is associated with the risk of thrombotic complications compared to the wild G20210G type (RR =17.1; p<0.0001) and is characterized by thrombosis localized both in the venous (66.7%) and arterial (33.3%) vascular pools. The threshold value of prothrombin activity (174.8%) for G20210A genotype was calculated, making it possible to conclusively predict the risk of thrombotic events with the accuracy of 90.4%.
Conclusion: The phenotypic manifestation of the prothrombin G20210A mutation in the form of venous and arterial thromboses in women of reproductive age is associated with a super-threshold increase in prothrombin (factor II) activity, which makes it possible to stratify the patients into the group of high risk of thromboses.
Keywords: prothrombin gene mutation, prothrombin mutation G20210A genotype, venous and arterial thromboses, prothrombin activity
Introduction
Despite significant advances in clinical practice and pharmacology, thrombotic events of any localization remain the primary cause of deaths and disabilities in developed countries and represent a global medical and social problem.1,2 It has been determined that 6–20% of verified thrombotic events are conditioned by the prothrombin G20210A mutation,3–5 which is characterized by autosomal dominant inheritance and manifests itself in replacing the guanine nucleotide (G) with the adenine nucleotide (A) in the 20210 position. Due to increased minor gene expression, the level and activity of prothrombin encoded by the gene can be 1.5–2 times higher than normal.3 Prothrombin, or factor II, refers to vitamin K-dependent blood coagulation factors, being the precursor of the key coagulation enzyme, thrombin.6,7
Prothrombin G20210A mutation is found in 1–6% of the population,8,9 increasing the risk of venous thrombosis by 2–4.10
As concluded by experts, heterozygous carriage of the prothrombin G20210A mutation is seen as a “low risk” factor for the development of venous thromboembolic complications (VTECs),11–14 establishing the isolated relative risk of thrombotic complications as OR =2–3.15,16 However, with additional factors, the magnitude of thrombogenic risk varies significantly. It is reported that combined hormonal contraceptives (CHCs) increase the risk of venous thromboembolic complications (VTECs) for prothrombin G20210A mutation carriers by 16,17–20 and menopausal hormone therapy increases the risk of DVT by 2–4.19,21–23 Along with this, a number of authors have shown that the risk of developing VTECs during pregnancy and postpartum in patients with prothrombin G20210A mutation constitutes OR of 3–15, compared to the time outside of pregnancy.24–26
Thus, the risk of thrombosis in prothrombin G20210A mutation carriers can be considered cumulative and dependant on temporary (relatively controlled) factors, such as pregnancy and administration of estrogen containing medications.27 Nevertheless, despite a statistically significant association between the prothrombin G20210A mutation carriage and the risk of thrombosis in the setting of additional risk factors (administration of CHCs, MHT, and pregnancy), it is not possible to predict the likelihood of thrombotic events.
Earlier, we conducted a research aimed at studying the association between the Leiden mutation laboratory phenotype and the risk of thrombosis in women of reproductive age.28,29 The research lasted 9 years and involved dynamic observation of the cohort of 500 FV Leiden G1691A (FVL G1691A) mutation carriers. It was established that in somatically healthy patients genotype FVL G1691A did not have any bearing on the risk of thromboses and was comparable to that in normal zygote patients [RR 1.5; 95% CI 0.03–76.5; р =0.8269]. With additional risk factors (co-morbid conditions, injuries, CHCs, pregnancy) the risk of VTEC comprised 9.3 [RR 9.3; 95% CI 4.7–18.5; р<0.0001] against the wild type FVL G1691G patients. At the same time, our study established that it is the degree of factor Va resistance to activated protein C (APC-R) that plays the key role in the manifestation of a thrombotic event in FVL G1691A patients. It was shown that at APC-R <0.49 the risk of acute VTEC is 27.5 times greater [RR =0.85; 95%CI 3.554–214.231; р <0.0015]. We therefore believe that the threshold value APC-R which determines the risk of thromboses can be viewed as a subclinical apparent phenotype of this mutation. At the same time, there are few data regarding the association of the subclinical manifestation of the prothrombin G20210A mutation with the risk of thrombotic events, which determined the purpose of this work.
Objective
to research the association of prothrombin (factor II) activity given the prothrombin G20210A mutation carriage with its clinical manifestations as thrombotic complications.
Materials and methods
According to the specified goal, a prospective clinical cohort study of 290 female patients was conducted from 2012 to 2017 based on clinical divisions of the FSBI HE Altai State Medical University of the Ministry of Healthcare of Russian Federation. There were two cohorts: the study group of 140 patients with prothrombin G20210A mutation genotype (mean age 31.2±4.7 years) and the control group of 150 women with the wild prothrombin mutation G20210A genotype (average age 32.3±3.9 years). The groups were comparable in age (p>0.05) and ethnicity: the study group was 92.9% of the Caucasian race, the control group was 91.9% of the Caucasian race (p>0.05). The observation period for both groups comprised no less than 6 years.
Study group inclusion criteria:
- female;
- Prothrombin G20210A mutation (GenBank 176930.0009) carriage;
- 18 to 45 years old.
Control group inclusion criteria were the same as for the study group, but the patients were not prothrombin G20210A mutation carriers.
Study group exclusion criteria:
- autoimmune diseases, including antiphospholipid syndrome;
- factor V Leiden mutation (GenBank 612309.0001) carriage;
- decrease in the functional activity of antithrombin III, proteins C or S.
The study was approved by the local ethics committee of the FSBI HE ASMU MOH Russia (protocol №5 of 06.25.12). In accordance with the recent revision of the World Medical Association’s Declaration of Helsinki, prior to the study all women granted their informed consent for their biological material to be used.
Presence of prothrombin G21210A mutation was identified by the PCR method using reagents by Litekh company (Russia). The study material was the human genomic DNA isolated from peripheral blood leukocytes. The analysis was based on the real-time polymerase chain reaction method (Real-Time PCR) using competing TagMan probes complementary to the polymorphic DNA sites. In all patients, prothrombin activity was tested using factor II-deficient plasma, Tromborel S reagent and BCS XP automatic coagulometer (Siemens, Germany). Prothrombin activity in the study group patients was tested 3–9 times throughout the period of observation. In case of the VTEC patients, the result prior to thrombosis was considered.
Statistics
Statistical data processing was performed using the MedCalc Version 17.9.7 statistical software package (license BU556-P12YT-BBS55-YAH5M-UBE51). Variation series were checked for normal distribution using the Shapiro-Wilk W-test. Laboratory values are presented as scatter plots with box plots (box-and-whisker plot). The box plot represents a median (Me) - middle of the sample, shown as a marker on the inside line of each box; interquartile range - interval between the 25th and 75th percentiles containing the central 50% of the sample’s observation, shown as a box; the 25th and 75th percentiles (the lower and upper quartiles) containing a quarter of the lowest and a quarter of the highest values in the sample, shown as straight lines (whiskers) coming out of the box. The upper whisker stretches from the upper border of the box to the maximal value. The lower whisker goes from the lower border of the box to the minimal value. The scatter plot representing all data points is superimposed on the box plot.
To compare the levels of prothrombin activity in two independent samples, the Mann-Whitney nonparametric statistical U-test was used. To determine the prognostic value of prothrombin activity index for the development of thromboses in prothrombin G20210A mutation carriers, the ROC curve was used, with subsequent AUC calculation.
For qualitative features, the total and relative values were given in percentage; verification of statistical hypotheses on the coincidence of the observed and expected frequencies was performed using the χ2 criterion and Fisher’s exact test. For binary features, the relative risk (RR) and 95% confidence interval (95% CI) were calculated. The critical significance level of discrepancies (p) was defined as p<0.05.
Study results
In accordance with the data obtained from 140 prothrombin G20210A mutation carriers, thrombotic events over a 6-year observation period were recorded in 32 women (22.8% of 140), while with the wild G20210G type - in 2 (1.3% of 150) [RR17.1; 95% CI: 4.2–70.1; p<0.0001].
In the control group, two instances of thrombosis were represented by VTECs in the deep veins of the lower extremities. Both were defined outside of pregnancy: one episode of thrombosis was induced by taking combined hormonal contraceptives (CHC); the other one was induced by locked intramedullary nailing in the settings of a tibial diaphysis fracture (on the 2nd day of the postoperative period).
In total, 39 episodes of thrombotic events were recorded in 32 patients with prothrombin G20210A mutation: 26 (81.3% of 32) had a single episode of thrombosis; 5 (15.6% of 32) had an episode of rethrombosis; 1 (3.1% of 32) had two cases of rethrombosis.
Thrombosis structure as per localization is shown in Figure 1.
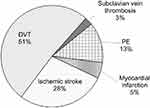 |
Figure 1 Thrombosis structure by localization in women (aged 18–45) with the prothrombin G20210A mutation carriage. Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism. |
In 66.7% (26 of 39) instances observed, the thromboses are found to have had venous localization, while the average age of acute thrombosis occurrence was 30.7±4.3 years. Arterial thromboses in the form of acute myocardial infarction and ischemic stroke were diagnosed in 33.3% of cases (13 of 39), the average age of patients in that group being 31.8±3.8 years. Thus, it can be concluded that the age at which a thrombotic event occurs with prothrombin G20210A mutation carriage is comparable (p>0.05) for both arterial and venous localizations.
In order to determine potential risk factors that initiate the development of a thrombotic event, we have carried out a comparative analysis of demographic and clinical history characteristics of patients in both the study and control groups (Table 1).
 |
Table 1 Characteristics of patients in comparison groups |
The analysis has shown that the patients in comparison groups are comparable by age, most of them being 18–35 years old. Body Mass Index across the groups was also identical. Extra weight (BMI >25) was found in 27.9% of prothrombin G20210A mutation carriers and in 24% of women with the wild prothrombin mutation G20210G type, which does not present any statistical difference. For birth control, CHCs were taken by 20.7% of women in the study group and 20.0% of patients in the control group (p=0.88), 109 (77.9%) of patients in the group of prothrombin G20210A mutation carriers and 115 (76.7%) of women in the control group had delivery in history. By delivery parity, there was no statistically significant difference between the comparison group respondents. It is noteworthy that family history burdened with thromboses before 50 years of age in prothrombin G20210A mutation carriers was found in 20.0% of instances, which is statistically significantly more frequent than in the wild G20210G type group – 4.7% [OR5.1; 95%CI 2.2–12.1; p=0.0002].
On the whole, comparative analysis of potential risk factors triggering thrombotic events has shown that the patients in comparison groups are comparable in major parameters analyzed except the family history of thromboses, which suggests inherited predisposition (in our situation, prothrombin G20210A mutation carriage). We were not able to assess the “smoking” factor in comparison groups since some respondents refused to answer this question.
In order to identify potential risk factors triggering thrombotic events in prothrombin G20210A mutation carriers, within the group there was a single factor analysis carried out by specified risk factors (Table 2).
 |
Table 2 Demographic and clinical history characteristics of patients in the study group depending on the personal thrombotic history |
As per data received, age, BMI, and pregnancy parity have no bearing on the risk of thrombotic events in prothrombin G20210A mutation carriers. Administration of CHCs increases the risk of thromboses more than by 4 times. It has also been found that patients with prothrombin G20210A mutation having family history of thromboses present with personal thrombotic events significantly more often.
Furthermore, we have carried out analysis of clinical situations that initiate the development of a thrombotic event in the analyzed cohort of prothrombin G20210A mutation carriers (Figure 2).
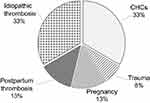 |
Figure 2 Causative factors preceding thrombotic events in patients of the study group. |
Upon closer examination, it was determined that of 13 (33%) instances of acute thrombosis occurred in prothrombin G20210A mutation carriers on CHCs, one 39-year-old patient was diagnosed with acute myocardial infarction, and two (24 and 33 years old) with ishemic stroke. Eight studies showed venous thrombosis localization in association with CHCs in the tibial deep veins (mean age 31.0±2.4 years), and in two cases PE (25 and 39 years old) was diagnosed. In the course of treatment, a cava filter was implanted in three patients. It should be noted that CHCs were prescribed solely for the purpose of contraception.
During pregnancy, 10 thrombotic events were diagnosed (25.6% of 39 episodes). In two cases, it was an ischemic stroke diagnosed in the first trimester of pregnancy; in 8 cases, it was thrombosis localized in the lower leg deep vein region that developed in 5 patients 2–3 days after delivery and in 3 patients during the first and second trimester.
In 13 studies (33.3% of 39 thrombosis episodes) with prothrombin G20210A mutation carriage, the cause of thrombosis could not be established. In 4 patients with idiopathic acute thrombosis, there was a rethrombosis episode on CHCs during the first year (n=2) and during pregnancy (n=2).
Further on, development of thromboses with prothrombin G20210A mutation carriage was juxtaposed with its previous laboratory phenotype. For this purpose, prothrombin activity (factor II) in the study groups was tested. In patients with the wild prothrombin mutation G20210G type, the median (Me) of this factor’s coagulation activity was determined as 108.0% [95% CI: 103.0–111.2], which is 1.4 times less than in prothrombin G20210A mutation carriers without a thrombosis episode in history - 148.5% [95% CI: 145.3–156.9%]. With VTECs (n=26), the median prothrombin activity was 178.2% [95% CI: 151.5–190.3%], and with arterial thrombosis (n=13) - 180.1% [95% CI: 172.4–201.3%]. It was also found that the median prothrombin activity is comparable for venous and arterial thromboses (p=0.1469), and its performance differs significantly from that in women with prothrombin G20210A mutation without a thrombotic episode (p<0.0001) (Figure 3).
Designations: median — marker; box — interval between the 25th and the 75th percentiles containing the central 50% of excerpt’s observations; whiskers – values corresponding to the 25th and 75th percentiles.
Taking into account the data obtained, a ROC analysis was performed to determine the critical cut-off threshold for prothrombin activity (%) to predict the development of thrombosis in prothrombin G20210A mutation carriers regardless of thrombosis localization (Figure 4).
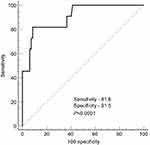 |
Figure 4 ROC curve model to predict the development of thrombotic events as per prothrombin activity (%) in prothrombin G20210A mutation carriers. |
The area under the curve (AUC) of the prothrombin activity index was defined as 0.904 (95% CI: 0.825–0.955) with the level of significance at p<0.0001, which determines good informational content of the analyzed diagnostic method. The maximal diagnostic significance is determined with prothrombin activity at 174.8% (sensitivity: 81.8; specificity: 91.5).
Discussion
During the 6-year observation of the prothrombin G20210A mutation cohort, we recorded thrombosis of both venous (66.7%) and arterial beds (33.3%). It should be noted that the evidence base regarding the risk of thrombotic events with heterozygous form of the prothrombin G20210A mutation in previously published documents was determined only for thrombosis in the venous basin.2,9,30 Nevertheless, in our study, every fourth case of thrombosis is described as an ischemic stroke, and in 5% of cases myocardial infarction was diagnosed. These data are consistent with the previously published results from other authors, where the prothrombin G20210A mutation is also considered as a proven risk factor for arterial thrombosis in patients under the age of 45: in particular, ischemic stroke (OR 1.6; 95% CI 1.3–2.0) and myocardial infarction (OR 2.6; 95% CI 1.1–6.3).31–36
It is known that the prothrombin G20210A mutation is a key risk factor for thrombosis; however, the latter is most likely to occur with additional triggers, such as CHCs, pregnancy and trauma.25,32,38,39 In the present work, CHCs are defined as an additional factor leading to a thrombotic event in the settings of the prothrombin G20210A mutation. Estrogen-containing drugs are known to be absolutely contraindicated for patients with the prothrombin G20210A mutation.14,37,38 Nevertheless, in our study, 29 patients with the prothrombin mutation G20210A genotype were administered estrogen-containing contraceptives, which was clinically manifested as thrombosis in 44.8% of cases (13 out of 29) during the first month of intake. Taking into account that estrogen-containing drugs are widely used in the gynaecological practice (contraception, menopausal hormone therapy, ovulation induction cycles, etc.), we calculated the risk of thrombosis with CHCs for patients with the prothrombin G20210A mutation as 13.9 [RR 13.9; 95% CI: 1.9–96.3; p=0.0097]. It can be noted that the obtained data do not contradict previous studies.18,20
A number of articles have identified the role of pregnancy and the postpartum period in increasing the risk of thrombosis in congenital thrombophilia.25,39 In the present work, with prothrombin G20210A mutation the risk of developing thrombosis during pregnancy and the first 6 weeks after delivery is identical and is calculated as 14.1 [RR 14.1; 95% CI: 0.78–254.47; p=0.0727], which is consistent with the previously published data.
It is known that both pregnancy40–42 and combined oral contraceptives43–45 are accompanied by an increase in prothrombin activity in blood plasma. It is likely that the primary increase in this factor (associated with the prothrombin G20210A mutation) in combination with the secondary one (during pregnancy or CHC administration) is more likely to manifest itself as thrombosis. In our work, the critical threshold value of prothrombin activity was calculated as 174.8% and higher, which allows, as our analysis has shown, to predict the development of thrombosis in 90.4% of cases.
Conclusion
The phenotypic manifestation of the prothrombin G20210A mutation can be considered as two interrelated phenomena: a thrombosis-preceding suprathreshold increase in the activity of prothrombin (Factor II) and thrombosis itself. The significance of our study is primarily seen in highlighting the key role of high prothrombin activity in patients with the prothrombin G20210A mutation. It can serve as an objective laboratory criterion that allows stratification of patients into the group at high risk of thrombosis.
Abbreviation list
AUC, area under the curve; APCR, activated protein C resistance; VTECs, venous thromboembolic complications; СHСs, combined hormonal contraceptives; 95% CI, 95% confidence interval; DVT, deep vein thrombosis; Ме, median; NR, normalized ratio; р, the significance level of differences; PE, pulmonary embolism; OR, Оdds Ratio; ROC, receiver operating characteristic; RR, Relative Risk.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5–12. doi:10.1097/AOG.0000000000000564
2. Royal College of Obstetricians and Gynaecologists (2015) Green-top Guideline No. 37b. Thromboembolic disease in pregnancy and the puerperium: acute management. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg37b/. Accessed March 1, 2019.
3. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variant in the 30-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increased in venous thrombosis. Blood. 1996;88:3698–3703.
4. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism - pooled analysis of 8 casecontrol studies including 2310 cases and 3204 controls. Study group for pooledanalysis in venous thromboembolism. Thromb Haemost. 2001;86(3):809–816.
5. Gehring NH, Frede U, Neu-Yilik G, et al. Increased efficiency of mRNA 3ʹ end formation: a new genetic mechanism contributing to hereditary thrombophilia. Nat Genet. 2001;28(4):389–392. doi:10.1038/ng578
6. Magnusson S.The Enzymes.
7. Crawley JT, Zanardelli S, Chion CK, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007;5(Suppl 1):95–101. doi:10.1111/j.1538-7836.2007.02500.x
8. Spector EB, Grody WW, Matteson CJ, et al. Technical standards and guidelines: venous thromboembolism (Factor V Leiden and prothrombin 20210G > A testing): a disease-specific supplement to the standards and guidelines for clinical genetics laboratories. Genet Med. 2005;7(6):444–453. doi:10.109701.GIM.0000172641.57755.3A
9. Dziadosz M, Baxi LV. Global prevalence of prothrombin gene mutation G20210A and implications in women’s health: a systematic review. Blood Coagul Fibrinolysis. 2016;27(5):481–489. doi:10.1097/MBC.0000000000000562
10. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369–384. doi:10.1007/s004390100593
11. Klein L, Bhardwaj V, Gebara B. Cerebral venous sinus thrombosis in a neonate with homozygous prothrombin G20210A genotype. J Perinatol. 2004;24:797–799. doi:10.1038/sj.jp.7211187
12. Martinelli I, Battaglioli T, Razzari C, Mannucci PM. Type and location of venous thromboembolism in patients with factor V Leiden or prothrombin G20210A and in those with no thrombophilia. J Thromb Haemost. 2007;5:98–101. doi:10.1111/j.1538-7836.2006.02291.x
13. Jacobsen AF, Dahm A, Bergrem A, Jacobsen EM, Sandset PM. Risk of venous thrombosis in pregnancy among carriers of the factor V Leiden and the prothrombin gene G20210A polymorphisms. J Thromb Haemost. 2010;8(11):2443–2449. doi:10.1111/j.1538-7836.2010.04038.x
14. Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism. International consensus statement. Int Angiol. 2013;32(2):111–260.
15. Bank I, Libourel EJ, Middeldorp S, et al. Prothrombin 20210A mutation: a mild risk factor for venous thromboembolism but not for arterial thrombotic disease and pregnancy-related complications in a family study. Arch Intern Med. 2004;164:1932–1937. doi:10.1001/archinte.164.17.1932
16. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472–2485. doi:10.1001/jama.2009.853
17. Williamson MA, Snyder LM, Wallach JB. Wallach’s Interpretation of Diagnostic Tests.
18. Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527–1535. doi:10.1056/NEJM200105173442007
19. Gomes MP, Deitcher SR. Risk of venous thromboembolic disease associated with hormonal contraceptives and hormone replacement therapy: a clinical review. Arch Intern Med. 2004;164(18):1965–1976. doi:10.1001/archinte.164.18.1965
20. Wu O, Robertson L, Langhorne P, et al. Oral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: a systematic review. The thrombosis: risk and economic assessment of thrombophilia screening (TREATS) Study. Thromb Haemost. 2005;94(1):17–25. doi:10.1160/TH04-11-0759
21. Simone B, De Stefano V, Leoncini E, et al. Risk of venous thromboembolism associated with single and combined effects of factor V Leiden, prothrombin 20210A and methylenetethraydrofolate reductase C677T: a meta-analysis involving over 11,000 cases and 21,000 controls. Eur J Epidemiol. 2013;28(8):621–647. doi:10.1007/s10654-013-9825-8
22. Daly E, Vessey MP, Painter R, Hawkins MM. Case-control study of venous thromboembolism risk in users of hormone replacement therapy. Lancet. 1996;348:1027. doi:10.1016/S0140-6736(96)24041-3
23. Grodstein F, Stampfer MJ, Goldhaber SZ, et al. Prospective study of exogenous hormones and risk of pulmonary embolism in women. Lancet. 1996;348(9033):983–987. doi:10.1016/S0140-6736(96)07308-4
24. Varga EA, Moll S. Cardiology patient pages. Prothrombin 20210 mutation (factor II mutation). Circulation. 2004;110(3):e15–8. doi:10.1161/01.CIR.0000135582.53444.87
25. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med. 2000;342(6):374–380. doi:10.1056/NEJM200002103420602
26. Martinelli I, De Stefano V, Taioli E, et al. Inherited thrombophilia and first venous thromboembolism during pregnancy and puerperium. Thromb Haemost. 2002;87(5):791–795.
27. Hertzberg MS. Genetic testing for thrombophilia mutations. Semin Thromb Hemost. 2005;31:33–38. doi:10.1055/s-2005-863803
28. Momot AP, Nikolaeva MG, Elykomov VA, Momot KA. The role of APC-resistance for predicting venous thrombosis and pregnancy complicationsin carriers of factor V Leiden (1691) G/A mutation. In: Pregnancy and Birth Outcomes. Intech Open; 2018:33–57. doi:10.5772/intechopen.72210
29. Momot AP, Nikolaeva MG, Zainulina MS. The main causes of thrombotic events in carriage of Leiden mutation in women of reproductive age. J Hematol Thrombo Dis. 2018;6:296. doi:10.4172/2329-8790.1000296
30. National Institute for Health and Clinical Excellence. Venous Thromboembolism: Reducing the Risk. Reducing the Risk of Venous Thromboembolism (deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Hospital. NICE Clinical Guideline 92. London: NICE; 2010.
31. Rosendaal FR, Siscovick DS, Schwartz SM, et al. A common prothrombin variant (20210 G to A) increases the risk of myocardial infarction in young women. Blood. 1997;90(5):1747–1750. http://www.bloodjournal.org/content/90/5/1747.long?sso-checked=true.
32. Reiner AP, Siscovick DS, Rosendaal FR. Hemostatic risk factors and arterial thrombotic disease [review]. Thromb Haemost. 2001;85:584–595. doi:10.1055/s-0037-1615638
33. Casas JP, Hingorani AD, Bautista LE, Sharma P. Meta-analysis of genetic studies in ischemic stroke: thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61(11):1652–1661. doi:10.1001/archneur.61.11.1652
34. Bentley P, Peck G, Smeeth L, Whittaker J, Sharma P. Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants. PLoS One. 2010;5(2):e9136. doi:10.1371/journal.pone.0009136
35. Korchagin VI, Mironov KO, Dribnokhodova OP, et al. The role of genetic factors in the development of individual predisposition to ischemic stroke. Hum Physiol. 2017;43(8):886–897. doi:10.1134/S0362119717080047
36. Rallidis LS, Gialeraki A, Tsirebolos G, Tsalavoutas S, Rallidi M, Iliodromitis E. Prothrombotic genetic risk factors in patients with very early ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2017;44(2):267–273. doi:10.1007/s11239-017-1520-2
37. Bloemenkamp KWM, Rosendaal FR, Helmerhorst FM, Vandenbroucke JP. Higher risk of venous thrombosis during early use of oral contraceptives in women with inherited clotting defects. Arch Intern Med. 2000;160:49–52. doi:10.1001/archinte.160.1.49
38. Medical eligibility criteria for contraceptive use – 3rd ed. World Health Organization; 2004 ISNB 92 4 156266 8 (NLM classification: WP 630). Available from: http://bono-esse.ru/blizzard/Gyn/Contracep/MEC-merged_2004.pdf. Accessed March 1, 2019.
39. Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321–335. doi:10.1111/j.1365-2141.2008.07339.x
40. Stirllng Y, Woolf I, North WRS, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–182.
41. Thornton P, Douglas J. Coagulation in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2010;24:339–352. doi:10.1016/j.bpobgyn.2009.11.010
42. Momot AP, Semenova NA, Belozerov DE, Trukhina DA, Kudinova IY. The dynamics of the hemostatic parameters in physiological pregnancy and after delivery. J Hematol Blood Transfus Disord. 2016;3:005. doi:10.18411/d-2016-058
43. The Oral Contraceptive and Hemostasis Study Group. The effects of seven monphasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomised multicenter study. Contraception. 2003;67:173–185. doi:10.1016/S0010-7824(02)00476-6
44. Brummel-Ziedins KE, Vossen CY, Butenas S, Mann KG, Rosendaal FR. Thrombin generation profiles in deep venous thrombosis. J Thromb Haemost. 2005;3:2497–2505. doi:10.1111/j.1538-7836.2005.01584.x
45. Aldrighi JM, De Campos LS, Eluf Gebara OC, Petta CA, Bahamondes L. Effect of a combined oral contraceptive containing 20 microg ethinyl estradiol and 75 microg gestodene on hemostatic parameters. Gynecol Endocrinol. 2006;22(1):1–4. doi:10.1080/09513590500430328
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

