Back to Archived Journals » Clinical Oncology in Adolescents and Young Adults » Volume 6
Clinical trial enrollment of adolescent and young adult patients with cancer: a systematic review of the literature and proposed solutions
Authors Friend BD, Baweja A, Schiller G, Bergman J, Litwin MS, Goldman JW, Davies S, Casillas J
Received 30 October 2015
Accepted for publication 25 March 2016
Published 19 January 2017 Volume 2016:6 Pages 51—59
DOI https://doi.org/10.2147/COAYA.S70375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mark Kieran
Brian D Friend,1 Abinav Baweja,2 Gary Schiller,3 Jonathan Bergman,4 Mark S Litwin,4 Jonathan W Goldman,3 Simon Davies,5 Jacqueline Casillas1
1Department of Pediatrics, Hematology-Oncology, University of California, Los Angeles, David Geffen School of Medicine, CA, 2Department of Medicine, New York University, New York, NY, 3Department of Medicine, Hematology-Oncology, University of California Los Angeles, David Geffen School of Medicine, 4Department of Urology, University of California, 5Teen Cancer America, Los Angeles, CA, USA
Abstract: Although there has been significant progress in the survival of children and older adults with cancer in the last few decades, this has not been the case for adolescent and young adults (AYAs). Although there are multiple reasons why outcomes for AYAs have not improved over time, it is evident from studies that AYAs fare better when enrolled in clinical trials, yet they are much less likely to participate in trials when compared with children and older adults. The goal of this review is to understand the trends in clinical trial enrollment of AYA patients over time, recognize facilitators and barriers to enrollment, and evaluate methods that have been utilized in order to improve clinical trial enrollment for this group of patients. A systematic review of the literature was performed to search for articles related to AYA oncology and clinical trials. The articles that met all inclusion criteria were then subject to full-text review, which yielded 17 articles that used quantitative, qualitative, or mixed methods. The articles reviewed demonstrated that for AYA patients, survival and clinical trial enrollment have shown some improvement over time, yet participation in clinical trials is still relatively poor, particularly for older patients within this group. Major barriers to enrollment in clinical trials include limited access and availability of trials, as well as patient knowledge. We have introduced the 4 Ps Conceptual “Onion Skin” Model, which includes patient, partners, providers, and policies, with the goal of reducing barriers to trial enrollment. Interventions utilizing this model include the establishment of more AYA centers, increased collaboration among cooperative groups with the goal of creating more AYA-specific trials, and educating patients and providers about the benefits of participating in clinical trials. Further studies are needed to understand which interventions would be most successful in encouraging AYA patients to enroll in clinical trials.
Keywords: adolescent and young adult, clinical trials, cancer
Background
In the US, over the past 35 years, marked improvement in survival for patients with cancer has increased the number of cancer survivors from 3 million to ~12 million.1 Yet the population of adolescent and young adult (AYA) patients, defined by the National Cancer Institute (NCI) as those between ages 15 years and 39 years at the time of diagnosis, has seen very little progress in survival outcomes when compared with children or older adults with cancer.2 From 1975 to 1997, the average annual 5-year survival rate for children <15 years of age and adults >50 years of age increased by at least 1.5% per year, while in AYAs, there was little or no improvement at all.2
Although the reasons that outcomes for AYAs with cancer remain inferior are multifactorial, some studies have shown that outcomes are better for those who are enrolled in clinical trials.3 Yet compared with children and older adults, a smaller proportion of AYA patients have been enrolled in clinical trials over time. For children <15 years of age, 40%–70% of these patients are enrolled in clinical trials compared with only 14% of AYA patients.4,5 As a group, 15- to 19-year-old participants are best represented in clinical trials, while only 1%–2% of 20- to 39-year olds participate in clinical trials, which is similar to the rate in older adults with cancer.3
One of the major reasons that enrollment of AYA patients in clinical trials has been low historically is regulatory barriers, which limit the availability and accessibility of trials to patients. These policies have originated primarily from the cooperative groups that organize trials, such as the Children’s Oncology Group (COG) and several adult cooperative groups, and the governing bodies, which include the federal Office for Human Research Protections and the NCI’s Cancer Therapy Evaluation Program (CTEP), which preside over these groups.6 A significant barrier related to trial availability is age eligibility, which traditionally has been set at >18 years of age for adult trials and <16–22 years of age for pediatric trials.7 Yet most studies have found that these age limits are arbitrary, and many health-care policy statements have discouraged the use of arbitrary age limits when opening clinical trials.7 Other requirements related to the affiliation of the institution’s institutional review board and its providers may prevent oncologists from enrolling younger patients in a clinical trial, and thereby limiting accessibility. These policies are fortunately in the process of changing, which is discussed in our review.
Given that AYA patients have poor outcomes compared with children and older adults with cancer and that improved survival appears to be linked to participation in clinical trials, we sought to review the literature on the trends of enrollment of AYA patients in clinical trials over time.
The goals of this review were to determine the overall trend of clinical trial enrollment for AYA patients over time, to identify the facilitators and barriers to trial enrollment of AYA patients, and to present the strategies that have been used to target these specific barriers.
Methods
A medical review of the literature was conducted utilizing the PubMed database, including all peer-reviewed journals. The search terms included the following phrases or keywords: “adolescent and young adult” OR “AYA oncology” OR “teen and young adult oncology” AND “clinical trials” OR “enrollment”. The following selection criteria were applied: articles published from January 1995 to May 2015 and included patients aged 15–39 years who were diagnosed with cancer during this time period. We required that all studies were published in the English language. Outcome variables included the number of clinical trials available to AYA patients and the number of AYA patients enrolled in clinical trials over a given time period. In addition, articles that discussed barriers and facilitators to clinical trial enrollment of AYA patients were selected. The reference list of all articles was manually searched for additional relevant articles. This review included quantitative, qualitative, and mixed methods articles. Articles that met all inclusion criteria were then subject to full-text review.
The methodological quality of the articles was judged using a validated set of guidelines for qualitative research. A reviewer assessed each article for the following items: well-defined aims and objectives, information on data collection and analysis, the context of the study, clear presentation of results, interesting discussion of results, study limitations included, and conclusion summarized the paper and mentioned the importance of further research.8 For quantitative research, articles were judged on the following items: appropriate study design and sample, valid and reliable data collection tools, and proper statistical methods.9 A total of 17 articles met these criteria and were included in this review.
Results
Table 1 shows the methodology and major findings of the reviewed studies. These studies used many different age groupings to define the AYA population. The majority of studies included a range of 15–39 years. Three studies used an upper age limit of 25 years. Sixteen studies used quantitative methods and one study used mixed methods. Most studies (eleven) examined all cancer types. Five studies looked at several common cancer types. One study focused specifically on bone and soft tissue tumors. Twelve studies reported data from the US, four reported data from Europe, and one reported data from Australia.
Of the articles reviewed, 12 showed that compared with children and older adults, enrollment in clinical trials for AYA patients is significantly lower.2,5,10–12 In particular, four studies demonstrated that patients aged 20–39 years were less likely to be enrolled in a clinical trial.2,5,11,12 The AYA Health Outcomes and Patient Experience (HOPE) Study, which has followed 524 AYA patients, reported that only 7% of respondents had participated in a clinical trial.13 Most troubling is that there has been a downward trend in clinical trial enrollment from 1997 to 2003 for patients aged 15–29 years in clinical trials sponsored by the NCI.2
Figure 1 illustrates that overall survival trends are improving for malignant cancers among all age groups in AYAs. However, for certain cancer types, this is not the case. Most concerning is that for both brain tumors and cervical cancer, 5-year survival has actually decreased over time in 15- to 19-year olds and 30- to 34-year olds. Among 20- to 24-year-old participants, survival for patients with testicular cancer appears to have plateaued.
  | Figure 1 Surveillance, Epidemiology, End Results (SEER) data showing the change in 5-year survival of the most common cancer types from 1975 to 2011 in different age ranges among the AYA population. |
Figure 2 displays the trends in clinical trial enrollment of AYAs with cancer among the articles reviewed for this paper. Overall, clinical trial enrollment has increased over time in every age group. The enrollment was much higher in 15- to 19-year-old participants when compared with all other age groups. In addition, older age seemed to correlate with lower enrollment in clinical trials.
  | Figure 2 Trends in clinical trial enrollment of AYAs with cancer for the articles reviewed.5,7,12,21,27 |
The purpose of understanding why enrollment in clinical trials is lower for AYA patients is because multiple studies have shown that patients who participate in clinical trials tend to have better outcomes.2,3 In addition, Potosky et al19 showed that AYA patients who were placed on clinical trials were more likely to receive appropriate therapy. From our systematic review, we found five studies that showed an association between better survival and clinical trial enrollment in the AYA population.2,11 Hence, it is important to understand the barriers to clinical trial enrollment of this population and to analyze the results of any previous interventions aimed at attempting to facilitate participation in clinical trials. Significant barriers to clinical trial enrollment discovered in this review included limited access and availability of clinical trials, as well as inadequate patient education, which will be detailed here.
A major reason why enrollment in clinical trials is poor for the AYA population is because of the lack of available trials. Shaw et al showed that from 2001 to 2006, a majority (57%) of AYAs aged 15–22 years at the Children’s Hospital of Pittsburgh were not enrolled in a clinical trial because one was not available. This is compared with 41% of patients <15 years old who were not enrolled due to trial unavailability (P=0.04).14 This is partially because many clinical trials are designed with arbitrary age criteria that may not represent the biological age range of the disease being studied.12 In the UK, Fern et al found that only six of 49 clinical trials of cancers common in the AYA population had appropriate age criteria for this group.12
In addition to the limited availability of clinical trials to AYA patients, another related problem is reduced access to trials, which is determined at least partially by the referral patterns of primary care providers. Recent evidence suggests that most newly diagnosed oncology patients >15 years of age are being treated at community oncology facilities instead of NCI-designated comprehensive cancer centers that typically offer the most clinical trials.15,16 Studies have shown that when referred to tertiary care centers, AYA patients are much more likely to enroll in clinical trials if treated by a pediatric oncologist rather than an adult oncologist.10 Yet, of adolescents with cancer treated in Ohio, only 36% of 17-year olds and 23% of 18-year olds were treated by pediatric oncologists compared with 76% of 15-year olds.17 Similar results have been reported in Utah and Georgia.16,18
While barriers to clinical trial enrollment are substantial, our review also yielded facilitators such as the formation of AYA centers and collaboration among large cooperative groups. One strategy used to improve enrollment in clinical trials for AYA patients has been the creation of AYA centers involving both adult and pediatric oncology departments. Shaw et al found that for the first 5 years after their joint AYA Oncology Program opened, clinical trial enrollment increased from 4% to 32%.20 Besides departments at individual institutions working more closely together, adult and pediatric national cooperative groups are now collaborating more to help enroll more AYA patients in clinical trials. For example, in 2000, the Southwest Oncology Group (SWOG), which typically has only enrolled patients >18 years of age in clinical trials, opened a COG trial for metastatic Ewing sarcoma available to children and adults. Over the next 5 years, the number of sarcoma patients <40 years of age enrolled in clinical trials increased from 5.3% to 19.3%.21 Similar examples have been seen in the UK from 2005 to 2010, with enrollment increasing from 18% to 39% for 20-to 24-year-old sarcoma patients and from 6% to 35% for 15-to 19-year-old Hodgkin lymphoma patients.12
These improvements in clinical trial participation are likely due to large systems changes, which promote collaboration among providers and increase access for patients. For instance, within the last year, the NCI has changed their clinical trials system, forming the National Clinical Trials Network (NCTN), which previously included nine adult cooperative groups, but now includes only four cooperative groups in addition to COG. The advantage of this system is that any provider who is a member of NCTN will have access to clinical trials from the other groups, and therefore improve trial availability.6 In the UK, the National Health Service created the National Cancer Research Networks in 2005, with the hope of improving recruitment for clinical trials. Through these regional networks, dedicated research nurses and data managers are able to assist providers in determining eligibility, managing informed consent, registering patients, and collecting data.22
However, although more trials are now available and accessible for AYA patients, providers and patients may still not be aware of this. Indeed, 62% of respondents in the AYA HOPE Study reported that they did not know whether clinical trials were available for their cancer type.13 NCI surveys of primary care providers revealed that 98% did not discuss clinical trials with patients of any age and 37% were not aware that clinical trials existed for their patients.23 Unpublished data from Gordon et al’s survey of primary care providers demonstrated that 78% of providers thought NCI-sponsored pediatric trials ended at age 21.24
Discussion
The results of our systematic review demonstrate that AYA patients continue to have low enrollment in clinical trials when compared with children and older adults. Reduced access, availability, and patient knowledge are major barriers to enrollment for this population.
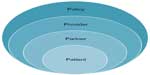
In order to address these barriers through specific interventions, we introduce the 4 Ps Conceptual “Onion Skin” Model (Figure 3). The idea is that there are multiple “layers” with barriers that relate directly to the patient’s ability to enroll in a clinical trial with the patient at the center. The “policy” layer is the closest to the surface but furthest from the patient as policy makers impact many patients yet their policies are not always aligned with the patients they serve. The layer closest to the center is the “partners” who are most closely related to the individual patient but not always the easiest to recognize. As we identify and target the problems related to these groups, we will be able to “peel” away the barriers to trial enrollment and hopefully this will lead to better patient outcomes.
This model originated with the idea that the care of AYA patients should be approached with the Donabedian model in mind. Through the 4 Ps, modifications can be made to the “structure” and “process” of recruiting AYA patients onto clinical trials and “outcomes” to these changes can be evaluated.30 Specific solutions will be discussed later and are outlined in Figure 4.
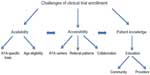  | Figure 4 Proposed strategies to improve clinical trial enrollment of AYA patients. |
Although recent studies have shown increases in trial participation overall, there is still ample room for improvement.5,12 As shown in Figure 2, even in the latest studies, most young adults are not being enrolled in clinical trials. Clearly, common cancer types in AYAs such as lymphoma are well represented in clinical trials.2,5 However, even relatively frequent diagnoses such as germ cell tumors, which represent ~15%–25% of invasive cancers in AYAs, continue to harbor low clinical trial accrual, as in one study only 0.3% of patients with germ cell tumors were enrolled in clinical trials.5 Unfortunately, while newer studies of patients with pediatric germ cell tumor have suggested that dose intensity may improve survival for high-risk patients, it is not known whether this benefit would also translate to AYA patients as similar trials have not been open to this group.31 This suggests the need for more AYA-specific trials that can better evaluate treatments for the common diagnoses that affect this population.
Throughout the AYA oncology literature is the corollary that treatment protocols should be more specific to tumor biology rather than age. In fact, some authors have argued that age should not be considered as a factor in defining treatment and that patients should be treated according to tumor type.27 Indeed, in the UK, two trials for bone sarcomas, EURAMOS-1 and EURO EWINGS-99, have opened the age eligibility to 0–40 years and 0–50 years, respectively.7 This led to significant increases in clinical trial enrollment rates across all AYA age groups in 2005–2008 when compared with data from 1997 to 2002, in the UK.7 Next-generation sequencing is starting to become a very useful tool, allowing providers to understand variations in cancer biology, which may affect individual treatment plans including whether a patient should be treated according to a pediatric or adult protocol.28,29 While widening age eligibility criteria can help increase clinical trial enrollments for AYAs, attention to adverse outcomes, especially among pediatric patients, will undoubtedly be important to monitor the benefits and pitfalls as more patients participate in clinical trials over time.
Some of the difficulty of enrolling AYA patients on clinical trials is the informed consent process. Specifically for adolescents, providers must deal with patients of varying maturity and ultimately, only parents have the power to consent. This leads to many providers focusing their discussions on the parents, yet studies have shown that patients appreciated when they were given the facts directly and the opportunity to make decisions.32 Of course success in understanding the information related to a clinical trial is not only delivering the facts but also how those facts are delivered. Studies have shown that patients would prefer that the information could be individualized and given in different formats, such as an audio or video platform.33 Strategies such as these are likely to lead to better understanding and therefore, more willingness to participate in a clinical trial.
Besides a lack of understanding, some patients may mistrust the clinical trials system and its process. The AYA HOPE Study revealed the most common reason that AYAs did not participate in clinical trials was the fear that the medications that would be given to them were not sufficiently tested.13 Certainly, explaining what is known about the medication being studied including potential side effects may help alleviate these fears and lead to improved enrollment. More importantly, providers should demystify the concept of clinical trials with patients, and convey that these trials are our “gold standard” of research in medicine. This may be best accomplished through community-based participatory research, which involves the mutual transfer of knowledge and power sharing in decision making among community and academic partners.34 Providers could partner with groups such as Stupid Cancer, an organization that provides patient education and advocates for AYAs, and may be effective in informing AYA patients of the potential benefits of participating in clinical trials.
In addition to educating patients, primary care providers must also be educated about the advantages of enrolling patients on clinical trials in order to improve referral patterns. Although multiple studies have shown that a majority of AYA patients are referred to oncologists in the community, Wolfson et al showed that simply by receiving care at an NCI-designated cancer center, outcomes were improved for AYA patients with WHO grade II central nervous system tumors. Furthermore, patients aged 22–39 years were less likely to receive treatment at an NCI-designated cancer center due to insurance, low socioeconomic status, and distance >5 miles.35 Regarding insurance, the recent passing of the Affordable Care Act has allowed more AYAs to obtain health insurance and should translate into an increase in referrals to NCI-designated cancer centers.37 Still, more can be done to overcome these practical barriers.
There are limited studies examining interventions used to recruit AYA patients to enroll in clinical trials. Some data exist for racial minorities, given that many studies have shown decreased participation in clinical trials compared with Caucasians.37 One such example demonstrated increased accrual for Hispanic women by utilizing a media campaign when compared with a clinic registry.38 Given the different ways that AYAs communicate and interact with each other in contrast to older adults, it is likely that a similar campaign utilizing the Internet with delivery over multiple platforms may prove helpful. Many have had success with online surveys for the AYA population and acknowledge that the Internet is a useful tool to encourage AYAs to participate in clinical research.39 Another example showed that African Americans had increased accrual on clinical trials with the addition of a church-based project.40 A similar trend was seen in a study of adolescents with chronic diseases, demonstrating that they are more likely to be compliant with treatment if they have good support from family, friends, and nurses.41 As this relates to AYAs, this suggests that receiving information within a trusting and supportive environment may lead to increased participation in clinical trials.
The psychosocial challenges of this population and the success of Shaw’s program at Pittsburgh demonstrate that increasing the number of AYA centers will likely help with the enrollment of patients on clinical trials by improving availability, accessibility, and patient knowledge. Similar success has been seen in the UK, where the Teenage Cancer Trust has created several cancer units designed specifically for adolescents. They have also implemented “shared care” centers, which are hospitals with general pediatric services but also affiliated with cancer centers, and can provide many services to cancer patients. Teen Cancer America is now developing similar centers and programs in the United States. These centers allow patients to receive some of their care at institutions that are often closer to home.42 They also allow for collaboration between adult and pediatric providers, and will improve the availability of trials to this population. Furthermore, this environment is likely to make AYA patients feel more comfortable by providing them with an interdisciplinary team, which can better comprehend their needs, including their understanding of clinical trials.
Conclusion
Although, over the last few decades, survival for patients with cancer has improved greatly, the same progress has not been made for AYA patients. For those patients who have been enrolled in clinical trials, they appear to have higher 5-year survival rates than those who have not participated in trials. Yet AYAs as a group have much lower enrollment in clinical trials when compared with children and older adults. Improved trial enrollment will likely lead to improved understanding of cancers in this population, and hopefully translate to better outcomes.
Our systematic review demonstrates that clinical trial enrollment for AYAs has made little progress in recent years, likely due to low trial availability and accessibility, as well as a lack of patient knowledge. In order to overcome these barriers, we have introduced the 4 Ps Conceptual “Onion Skin” Model. More specifically, we have discussed opening more AYA centers, improving collaboration among cooperative groups to open more AYA-specific trials, and better educating patients and providers about the benefits of participating in clinical trials. More studies need to be performed to better understand which specific interventions may help influence AYA patients to enroll in clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
Centers for Disease Control and Prevention. Cancer survivors – United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60(9)269–272. | |
Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7 suppl):1645–1655. | |
Nachman J, Sather HN, Buckley JD, et al. Young adults 16–21 years of age at diagnosis entered on Children’s Cancer Group acute lymphoblastic leukemia and acute myeloblastic leukemia protocols. Results of treatment. Cancer. 1993;71(Suppl 10):3377–3385. | |
Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321–2332. | |
Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–4053. | |
Felgenhauer J, Hooke MC. Regulatory barriers to clinical trial enrollment of adolescent and young adult oncology patients. Pediatrics. 2014;133(Suppl 3):S119–S122. | |
Fern LA, Whelan JS. Recruitment of adolescents and young adults to cancer clinical trials – international comparisons, barriers, and implications. Semin Oncol. 2010;37(2):e1–e8. | |
Kuper A, Lingard L, Levinson W. Critically appraising qualitative research. BMJ. 2008;337:a1035. | |
Leonard J, Hayes SC, Scharalda JG, et al. Appraising quantitative research in health education: guidelines for public health educators. Health Promot Pract. 2010;11(2):161–165. | |
Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J Pediatr Hematol Oncol. 2009;31(12):927–929. | |
Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891–1897. | |
Fern LA, Lewandowski JA, Coxon KM, Whelan J; National Cancer Research Institute Teenage and Young Adult Clinical Studies Group, UK. Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 2014;15(8):e341–e350. | |
Harlan LC, Lynch CF, Keegan TH, et al. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. J Cancer Surviv. 2011;5(3):305–314. | |
Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children’s hospital. J Pediatr Hematol Oncol. 2007;29(12):811–814. | |
Shaw PH, Hayes-Lattin B, Johnson R, Bleyer A. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133(Suppl 3):S109–S113. | |
Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25(29):4616–4621. | |
Yeager ND, Hoshaw-Woodard S, Ruymann FB, Termuhlen A. Patterns of care among adolescents with malignancy in Ohio. J Pediatr Hematol Oncol. 2006;28(1):17–22. | |
Howell DL, Ward KC, Austin HD, Young JL, Woods WG. Access to pediatric cancer care by age, race, and diagnosis, and outcomes of cancer treatment in pediatric and adolescent patients in the state of Georgia. J Clin Oncol. 2007;25(29):4610–4615. | |
Potosky AL, Harlan LC, Albritton K, et al. Use of appropriate initial treatment among adolescents and young adults with cancer. J Natl Cancer Inst. 2014;106(11):1–9. | |
Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2012;118(14):3614–3617. | |
Ferrari A, Montello M, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50(5 suppl):1101–1104. | |
Sinha G. United Kingdom becomes the cancer clinical trials recruitment capital of the world. J Natl Cancer Inst. 2007;99(6):420–422. | |
Crosson K, Eisner E, Brown C, Ter Maat J. Primary care physicians’ attitudes, knowledge, and practices related to cancer clinical trials. J Cancer Educ. 2001;16(4):188–192. | |
Albritton KH, Coccia P. Influencing referral of adolescents and young adults with cancer to sites with higher rates of trial enrollment. Pediatrics. 2014;133(Suppl 3):S104–108. | |
Mitchell AE, Scarcella DL, Rigutto GL, et al. Cancer in adolescents and young adults: treatment and outcome in Victoria. Med J Aust. 2004;180(2):59–62. | |
Fern L, Davies S, Eden T, et al. Rates of inclusion of teenagers and young adults in England into National Cancer Research Network clinical trials: report from the National Cancer Research Institute (NCRI) Teenage and Young Adult Clinical Studies Development Group. Br J Cancer. 2008;99(12):1967–1974. | |
Ferrari A, Bleyer A. Participation of adolescents with cancer in clinical trials. Cancer Treat Rev. 2007;33(7):603–608. | |
Silva JG, Corrales-Medina FF, Maher OM, et al. Clinical next generation sequencing of pediatric-type malignancies in adult patients identifies novel somatic aberrations. Oncoscience. 2015;2(2):187–192. eCollection 2015. | |
Subbiah V, Bupathi M, Kato S, et al. Clinical next-generation sequencing reveals aggressive cancer biology in adolescent and young adult patients. Oncoscience. 2015;2(7):646–658. eCollection 2015. | |
Donabedian A. Continuity and change in the quest for quality. Clin Perform Qual Health Care. 1993;1(1):9–16. | |
Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110(11):2385–2393. | |
Palmer S, Mitchell A, Thompson K, Sexton M. Unmet needs among adolescent cancer patients: a pilot study. Palliat Support Care. 2007;5(2):127–134. | |
Baker JN, Leek AC, Salas HS, et al. Suggestions from adolescents, young adults, and parents for improving informed consent in phase 1 pediatric oncology trials. Cancer. 2013;119(23):4154–4161. | |
Jones L, Wells K. Strategies for academic and clinician engagement in community-participatory partnered research. JAMA. 2007;297(4):407–410. | |
Wolfson J, Sun CL, Kang T, Wyatt L, D’Appuzzo M, Bhatia S. Impact of treatment site in adolescents and young adults with central nervous system tumors. J Natl Cancer Inst. 2014;106(8):1–10. | |
Bleyer A, Ulrich C, Martin S. Young adults, cancer, health insurance, socioeconomic status, and the Patient Protection and Affordable Care Act. Cancer. 2012;118(24):6018–6021. | |
Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. | |
Brewster WR, Anton-Culver H, Ziogas A, et al. Recruitment strategies for cervical cancer prevention study. Gynecol Oncol. 2002;85(2):250–254. | |
Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer. Oncologist. 2015;20(2):186–195. | |
Ford M, Havstad HL, Davis SD. A randomized trial of recruitment methods for older African-American men in the prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial. Clin Trials. 2004;1(4):343–351. | |
Kyngas H, Rissanen M. Support as a crucial predictor of good compliance of adolescents with a chronic disease. J Clin Nurs. 2001;10(6):767–774. | |
National Institute for Health and Care Excellence [homepage on the Internet]. Guidance on Cancer Services – Improving Outcomes in Children and Young People with Cancer. London: National Institute for Health and Clinical Excellence; 2005. Available from: http://nice.org.uk. Accessed August 5, 2015. | |
Surveillance, Epidemiology, and End Results Program [webpage on the Internet]. SEER Cancer Statistics Review, 1975–2011. Table 32.20. Bethesda: National Cancer Institute; 2014. Available from: http://seer.cancer.gov/archive/csr/1975_2011/browse_csr.php?sectionSEL=32&pageSEL=sect_32_table.20.html. Accessed August 5, 2015. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.