Back to Journals » Drug Design, Development and Therapy » Volume 13
Cinobufagin Suppresses The Characteristics Of Osteosarcoma Cancer Cells By Inhibiting The IL-6-OPN-STAT3 Pathway
Received 23 July 2019
Accepted for publication 22 October 2019
Published 4 December 2019 Volume 2019:13 Pages 4075—4090
DOI https://doi.org/10.2147/DDDT.S224312
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
This paper has been retracted.
Chuan Zhang,* Kun Ma,* Wu-Yin Li
Luoyang Orthopaedic-Traumatological Hospital and Henan Orthopaedic Hospital, Luoyang, Henan 471002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kun Ma; Wu-Yin Li
Luoyang Orthopaedic-Traumatological Hospital, 82 QiMing Road, Luoyang, Henan 471002, People’s Republic of China
Email [email protected]; [email protected]
Background: Current clinical treatments for osteosarcoma are limited by disease recurrence and primary or secondary chemoresistance. Cancer stem-like cells have been proposed to facilitate the initiation, progression, recurrence and chemoresistance of osteosarcoma. Furthermore, previous studies have reported that IL-6-STAT3 pathway is overexpressed in various types of cancer and contributes to cell proliferation, apoptosis, invasion/migration, chemoresistance and modulation of stemness features.
Aim: To examined the effect of cinobufagin on cancer progression and modulation of stemness features in osteosarcoma, and investigated the molecular mechanisms underlying such effects.
Methods: Human osteosarcoma cell lines U2OS/MG-63 were recruited in this study. Cell proliferation, migration, and invasion were determined by MTT assay, colony formation assay,wound healing assay, and cell invasion assay respectively. Its effect on stemness was assessed by flow cytometry and mammosphere formation. The protein expression levels of related proteins were detected by Western blot. The xenograft model, immunofluorescence staining and immunohistochemistry were used to determine the effect of cinobufagin on tumorigenicity in vivo experiment.
Results: We found that cinobufagin suppressed the viability of U2OS/MG-63 spheroids/parent cells in a time-and dose-dependent manner. Notably, cinobufagin had no effect on the viability of hFOB 1.19 cells. Moreover, cinobufagin induced apoptosis, increased the width of wounds, reduced invasive osteosarcoma spheroids/parent cell numbers and reduced EMT phenotype and OPN levels in U2OS/MG-63 spheroids as well as U2OS/MG-63 parent cells lines. Noticeablely, we found that OPN levels were higher in spheroids group than that in parent cells. In addition, cinobufagin ameliorated the proportion of CD133-positive cells, the size of spheroids and Nanog, Sox-2 and Oct3/4 protein levels. Our in vivo experiments showed that cinobufagin consistently reduced tumor volume,the expressions of OPN, Sox-2, Oct3/4, Nanog and p-STAT3 by the immuno histochemistry staining as well as CD133 expression in tumor tissues by immunofluorescence analysis. From a mechanistic point of view, cinobufagin was shown to inhibit IL-6-OPN-STAT3 signaling pathway. Exogenous IL-6/OE-OPN/overexpression STAT3 attenuated the induction of cinobufagin-mediated apoptosis and the suppression of stemness properties respectively.
Conclusion: Collectively, our data demonstrated that cinobufagin inhibited the viability and tumorigenesis capability of osteosarcoma cells by blocking IL-6- OPN-STAT3 signaling pathway. Cinobufagin may therefore represent a promising therapeutic agent for osteosarcoma management.
Keywords: osteosarcoma, cancer stem cells, cinobufagin, OPN, IL-6-STAT3 signaling pathway
Introduction
Osteosarcoma is one of the most common malignant bone tumors worldwide, occurs primarily in children and adolescents, and affects 1–3 individuals per million.1 The best form of treatment to improve the 5-year survival rate of patients with osteosarcoma is adjuvant chemotherapy, including cisplatin (cis-dichlorodiammine platinum), adriamycin, and localized surgery. Nevertheless, the most significant limitations to the current forms of clinical management for osteosarcoma are disease recurrence and either primary or secondary chemoresistance.2 Consequently, there is an urgent need to identify new therapeutic options for the treatment of osteosarcoma. The existing literature shows considerable acceptance of the fact that cancer stem cells (CSCs) play a key role in the development and progression of cancer. It is evident that these cell types are the source of increasing attention amongst researchers with regards to their potential as a novel therapeutic option.
CSCs are small subpopulations of cells with several key properties, including self-renewal, differentiation, increased rates of tumorigenesis, and sustained tumor growth. These are properties that traditional cancer therapies are not able to fully eradicate.3 Consequently, the development of CSC-targeted therapies has the potential to prevent tumor recurrence and increase the survival rate of patients with osteosarcoma.
Osteopontin (OPN), a phosphorylated glycoprotein, binds to cell surface components, such as integrins/CD44, growth factor/receptors and proteases.4 OPN is involved in many biological processes, such as the activation of regulatory and structural proteins, the regulation of metastatic spread, as well as the manipulation of tumor phenotype.5,6 Elevated OPN levels participated in many cancers, including breast, prostate, liver, cervical, lung and osteosarcoma.7–13 However, the relationship between OPN and cancer stem cells of osteosarcoma is still obscure.
Signal transducer and activator of transcription, (STAT)3, is a cytoplasmic transcription factor that can be activated by a number of different cytokines and growth factors, including interleukin (IL)-6, EGF, insulin-like growth factor, hepatocyte growth factor, colony-stimulating factor-1, and platelet derived growth factor.14 Previous research has demonstrated that while overexpression of the IL-6-STAT3 pathway contributes to cell survival, apoptosis, invasiveness/migration and angiogenesis, it also enhances the stemness features and chemoresistance of breast cancer, non-small cell lung cancer, colorectal cancer, and ovarian cancer.15–19 Further research has demonstrated that the attenuation of STAT3 phosphorylation promotes apoptosis and chemosensitivity in MG-63, U2OS and HOS cells.20 However, the precise role of the IL- 6/STAT3 pathway in modulating the stemness properties of osteosarcoma has yet to be elucidated.
“Chan su” is an established form of traditional Chinese medicine (TCM) in China and other countries in the Asia-Pacific region, containing bufalin, resibufogenin, and cinobufagin.21 Chan Su, and its derivatives, are widely utilized for the treatment of various types of cancer, including leukemia, hepatocellular cancer, lung cancer, prostate cancer, colon cancer, and cervical cancer.22–27 Previous research, including work performed in our own laboratory, revealed that cinobufagin and its analogues induce apoptosis in osteosarcoma cells by modulating the ROS/JNK/p38 pathway, the Notch pathway and the GSK-3β/NF-κB pathway in a mitochondrial-dependent manner.28–31 In addition, a study conducted by Chueh et al revealed that bufalin inhibited migration and invasion in human osteosarcoma U-2OS cells by suppressing the MMP-2 and ERK/JNK signaling pathways.32
In another study, Chang et al highlighted the fact that cinobufagin repressed the stemness features of osteosarcoma cells via mir-148.33 Data also shows that the elevation of OPN expression plays a role in osteosarcoma and therefore represents a potential biomarker for osteosarcoma.5,6 However, the role of OPN in modulating the traits of osteosarcoma spheroids has yet to be investigated. In the present study, we showed that cinobufagin suppressed cell viability, induced apoptosis, suppressed invasion/migration, and inhibited stemness features in osteosarcoma U2OS/MG-63 cells, both in vitro and in vivo. In addition, we revealed, for the first time, that the overexpression of OPN, and the hyperactivation of the IL-6-STAT3 pathway may be crucial for the cinobufagin-mediated suppression of stemness features and the induction of apoptosis.
Materials And Methods
Cell Culture And Reagents
U2OS and MG-63 cells were purchased from Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, Shanghai, China) and cultured in DMEM containing 10% fetal bovine serum (FBS), 100 μg/mL of penicillin and 100 μg/mL of streptomycin at 37°C in a 5% CO2 incubator. The human normal osteoblastic cell line, hFOB 1.19, was maintained in DMEM/F-12 (Gibco) supplemented with 10% FBS (Gibco) and 0.3 mg/mL of geneticin (G418; Gibco) at 37°C in a humidified atmosphere containing 5% CO2.
MTT Assay
Cells were maintained in 96-well flat-bottom microtiter plates overnight at a density of 1 × 104 cells/well and treated with cinobufagin at concentrations of 0, 10, 20, 40, and 80 nM for 24, 48, and 72 h. Subsequently, 20 μl of MTT solution (5 g/L) was added to the cells, followed by incubation for 4 h at 37°C. An automatic multi-well spectrophotometer was then used to calculate the absorbance value of each well at 570 nm. The rates of inhibition on U2OS/MG-63 adherent cells and U2OS/MG-63-derived spheroids, as well as the proliferative rate of hFOB1.19 cells, were then calculated according to the following formula: (1 − experimental absorbance value/control absorbance value) × 100%.
Sphere For Mation Assay
Sphere formation assays were carried out on cells from the third and fourth passages of cell culture. Osteosarcoma spheroids were cultured in a specialized growth medium (Celprogen, Inc, Torrance, CA, USA) containing 1% N2 supplement (Invitrogen), 2% B27 supplement (Invitrogen), 20 ng/mL of human platelet growth factor (Sigma-Aldrich), 100 ng/mL of epidermal growth factor (Invitrogen) and 1% antibiotic-antimycotic (Invitrogen). Osteosarcoma spheroids and hFOB 1.19 cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2. Spheres were subsequently counted by inverse microscopy and cell colonies with a diameter > 50 μm were measured.
FACS Analysis
Osteosarcoma cells were harvested with fresh 0.25% trypsin solution (Sigma–Aldrich) and suspended in phosphate-buffered saline (PBS). Cells were blocked on ice for 15 min and subsequently labelled with PE anti-human CD133 antibody (BioLegend) for 60 min. The cells were then washed twice with PBS and maintained on ice until analysis. Expression levels were determined by flow cytometry (FACS Calibur, BD Bioscience, USA) and data were analyzed using WinMDI software (Scripps Research Institute, La Jolla, CA,USA).
Western Blotting
Cells were washed in PBS and resuspended at room temperature. After incubation on ice for 30 min, cell lysates were centrifuged at 14,0009 ×g at 4°C. Protein concentrations were then measured using the Bradford protein assay using bovine serum albumin as a standard. Subsequently, membranes were incubated overnight at 4°C with a range of primary antibodies (all at a dilution of 1:1000) raised against the following molecules: phosphorylated- (p-)STAT3 or caspase 3 (Abcam, Cambridge, MA, USA); STAT3, GAPDH, α-SMA, E- cadherin (E-cad), vimentin, Nanog, OCT4 and SOX2 (Cell Signaling Technology, Danvers, MA, USA). Finally, membranes were incubated with secondary antibodies (1:5000) at room temperature for 2 h. GAPDH was used as a loading control.
Transwell Assay
Cells were seeded in 10-mm diameter transwell plates with polycarbonate filters (with a pore size of 8-μm). The upper and lower compartments of the plates were separated by a filter coated with 25 mg of Matrigel that formed a reconstituted basement membrane at 37°C. After treatment with 0–80 nM of cinobufagin for 48 h, cells were seeded into the upper well, while the lower well was filled with DMEM containing 10% FCS. After incubation in the presence of 5% CO2, cells were fixed for 30 min in 4% formaldehyde and stained for 15 min. Non-migrating cells were then removed from the upper surface of the transwell using a wet cotton swab, and the number of cells that had migrated or invaded the bottom surface of the filter was determined. For each well, six evenly spaced fields of cells were counted using an inverted phase-contrast microscope.
Wound Healing Assay
Cells were seeded in six-well plates at a density of 5 × 105 cells/well in DMEM supplemented with 10% FBS. Twelve hours after seeding, cells were treated with IL-6 (20 μM) or an equivalent volume of DMSO for 48 h. A scratch was subsequently made in the cell monolayer, and the rate of wound closure was observed at 24 h and 48 h. The resultant data were analyzed using Image J software.
Immunofluorescence Staining
Tumor tissues were fixed in 4% paraformaldehyde at 4 °C, and then frozen sections were permeabilized with 0.5%Triton X-100 for 10 min at room temperature, washed with PBS, and incubated with primary antibodies against CD133 (1:500, Proteintech Group, Chicago, USA) overnight, followed by fluorescence-tagged secondary antibodies against rabbit IgG (1:500).
Plasmid Transfection And Knockdown
The plasmid was transiently transfected into U2OS/MG-63 spheroids using Lipofectamine 3000 in accordance with the manufacturer’s instructions. Twenty-four hours after transfection, we added cinobufagin and incubated the cells for 48 h.
Tumor Xenografts
All the studies were approved by the medical ethical committee of Luoyang Orthopaedic-Traumatological Hospital and Henan Orthopaedic Hospital. Animal care and experiments were carried out according to the guidelines of the Institutional Animal Care and Use of Luoyang Orthopaedic-Traumatological Hospital and Henan Orthopaedic Hospital (The interests of animals should be fully considering; The experimental animals should be treated kindly; To prevent or reduce the stress, suffering and injury of the animals; To respect the animal life, stop the barbaric act and take the least painful method to dispose of the animal). Twenty 4–6 week-old female BALB/c mice were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). Osteosarcoma cells (2 × 106 cells in 200 μl of PBS) were subcutaneously injected into each mouse. After 2 weeks, the mice were randomly divided into four groups (three treatment groups and one control group; n = 5 per group). The three treatment groups received 10, 20, and 40 mg/kg of cinobufagin every 3 days for 4 weeks, whereas the control group received an equal volume of saline. Every 3 days, we determined the body weight of each mouse, and measured the length and width of tumors. In addition, we calculated tumor volume using the following formula: volume = 1/2 (width2 × length). After 4 weeks, the mice were sacrificed. Tumors were then excised and weighed.
Immunohistochemistry
Immunohistochemical staining was performed to investigate OPN, Nanog, Sox-2, Oct3/4 and p-STAT3 protein levels. The tumor tissue was embedded in paraffin and then cut into 4 μm sections which were then heated overnight at 60°C. Next, the sections were dewaxed in xylene and sequentially rehydrated with100, 95, 80 and 75% alcohol baths. The slides were then incubated overnight with primary antibodies to CD133 and p-STAT3 at 4°C. The following morning, the sections were incubated with appropriate secondary antibodies for 1 h at 37°C. Data were then analyzed using a light microscope (Olympus Corporation).
Statistical Analysis
Data are presented as means ± standard deviation (SD). Differences between groups were analyzed using the Student’s t-test. Statistical analyses were performed using SPSS software version 16.0 (SPSS, Inc.). P < 0.05 was considered to indicate statistical significance.
Results
Cinobufagin Suppressed Cell Prolifer Ation In Osteosarcoma Cell Lines And Had No Effect On Normal Human Osteoblastic hFOB 1.19 Cells
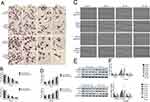
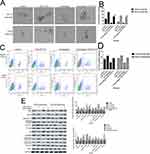
To investigate the effect of cinobufagin on the viability of MG-63/U2OS cells, we treated cells with 0–80 nM of cinobufagin for 0–72 h and tested their viability using 3-(4,5-dimethyl-2- thiazolyl)-2,5-diphenyl- 2H-tetrazolium bromide (MTT) assays. As shown in Figure 1A and B, and as expected, cinobufagin gradually reduced the growth of both parent cells and spheroids in a dose- and time-dependent manner (P < 0.05). The suppressive effect of cinobufagin was generally higher in the U2OS/MG-63 adherent group of cells than in the corresponding group of U2OS/MG-63 spheroids. However, there was no significant effect on the viability of hFOB 1.19 cells (Figure 1C).
Cinobufagin Induced Apoptosis In Osteosarcoma Spheroids And Parent Cells
Hyperactivated proliferation and chemoresistance are the hallmarks of CSCs. As shown in Figure 2A and B, we observed that cinobufagin increased the apoptotic ratio of spheroids and parent cells in a dose-dependent manner. Chang et al previously revealed that bufalin induced the expression of cleaved caspase-3 in CIOS-CSCs in a time-dependent manner.33 In our research, compared with the untreated group, the group of cells treated with 20 nM cinobufagin showed significant upregulation of caspase-3, cleaved PARP and Bax (P < 0.05). As shown in Figure 2C, The expression of Bax, caspase-3 and cleaved PARP was highest in the 80 nM cinobufagin group, indicating that cinobufagin induced mitochondrial-dependent cell death in U2OS/MG-63 derived spheroids/parent cells. In the 80nm cinobufagin treatment group, the levels of Bax, caspase-3, and cleaved PARP, were all significantly higher in the parent cells than in spheroids (P<0.05). Furthermore, cinobufagin reduced the levels of Bcl-2 in osteosarcoma CSCs and parent cell lines in a dose-dependent manner (P<0.05). These findings agreed partly with Dass’ opinion (P<0.05), at least in part.
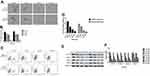
Cinobufagin Inhibited The Migration/Invasion Capacity And EMT Phenotype Of Cells
Previous research reported that CSCs can facilitate the migration and invasion of tumor cells. As shown in Figure 3A and B, the invasive ability of osteosarcoma cells generally decreased in a concentration-dependent manner. Moreover, we observed fewer invasive cells in osteosarcoma parent cells than in corresponding CSCs. Furthermore, the administration of cinobufagin increased the width of wounds in osteosarcoma cells (Figure 3C and D). In the context of oncogenicity, EMT phenotype has been linked to the acquisition of stem cell-like properties via the downregulation of E-cad and the upregulation of vimentin.34 As demonstrated in Figure 3E and F, the administration of cinobufagin downregulated the expression of α-SMA and vimentin in osteosarcoma CSCs/parent cells but upregulated the expression of E-cad in a dose-dependent manner. These data indicated that cinobufagin has the potential to suppress the migration/invasion capacity of osteosarcoma CSCs and therefore inhibit EMT (P<0.05).
Cinobufagin Attenuated Stemness Features In CSCs Derived From MG-63/U2os In Vitro
As shown in Figure 4A and B, compared with the control group, the administration of cinobufagin resulted in a reduction in the number and size of spheroids in a dose-dependent manner. CD133 has been proven to represent a reliable marker for stem-like properties in SAOS2, MG63, and U2OS cells.35 In a previous study, Qu et al revealed that exposure to melatonin may downregulate the proportion of CD133+ cells.36
Peng et al also reported that treatment with 40 μM of resveratrol reduced the expression of CD133, a surface marker of CSCs, in the spherical tumor colonies of MG-63 and MNNG/HOS cells.37 As expected, the administration of cinobufagin reduced the proportion of CD133-positive osteosarcoma cells in a dose-dependent manner (Figure 4C and D). Similarly, the protein levels of genes related to self-renewal (Nanog, Sox-2 and Oct3/4) were decreased (Figure 4E and F). Collectively, these results indicated that cinobufagin suppressed the stemness properties of MG-63/U2OS spheroids in vitro.
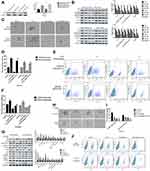
IL-6 Rescued The Cinobufagin-Induced Repression Of Stem-Like Properties In Osteosarcoma CSCs
Dass and Choong38 found that the reduced expression of OPN might cause a reduction in cell proliferation, tumor growth and the inhibition of osteosarcoma metastasis, thus suggesting that OPN plays a positive role in the development of osteosarcoma. Remarkably, we also observed that the expression of OPN (Figure 5A) was significantly higher in the spheroid group than the parent cell group (P<0.05).
Next, we found that the administration of cinobufagin led to a reduction in the levels of IL-6, p-STAT3 (Tyr727) and p-STAT3 (Ser705). The expression of OPN was also reduced in a dose-dependent manner (Figure 5B), indicating that the overexpression of OPN, and the constitutive activation of the IL-6-STAT3 pathway, may participate in the cinobufagin-induced regulation of stemness in osteosarcoma cells.
To further verify our hypothesis, we treated MG-63/U2OS spheroids with different concentrations of cinobufagin in the presence or absence of 20 μg/mL IL-6. We found that cinobufagin-treated MG- 63/U2OS spheres were larger in size (Figure 5C and D) and had an increased proportion of CD133-positive cells in the presence of IL-6 (Figure 5E and F) than that in the absence of IL-6. Interestingly, the administration of cinobufagin also downregulated the levels of IL-6-stored OPN, p-STAT3 (Tyr727) and p-STAT3 (Ser705) in U2OS/MG-63 spheroids (Figure 5F). Furthermore, the addition of IL-6 attenuated the cinobufagin-induced restoration of the levels of Bax, caspase-3 and cleaved PARP, and restored the EMT phenotype. In contrast, pretreatment with si-IL-6 reduced the size of the U2OS/MG-63 spheroids (Figure 5H and I) and attenuated the number of CD133-positive cells (Figure 5J and K). As expected, si-IL-6 downregulated the expression of CD133, vim, a-SMA, and OPN. In the si-IL-6+cinobufagin group, we observed increased levels of Bax, Bcl- 2, caspase-3, E-cad and cleaved PARP, thus indicating that OPN was the downstream target of IL-6 in our experiments. Moreover, the overexpression of IL-6 was partly responsible for the retention of stemness features in osteosarcoma spheroids (P<0.05).
 |
Figure 5 (Continued). |
Overexpression Of OPN Contributed To The Maintenance Of Osteosarcoma CSCs
In the above experiments, we detected that OPN levels was higher in U2OS/MG-63 spheroids (Figure 5A). And cinobufagin treatment indeedly decreased OPN levels(Figure 5B) and IL-6-elevated OPN expressions (Figure 5G). So, we suspected that whether OPN could participate in cinobufagin-modulated osteosarcoma CSCs features. As shown in Figure 6A and B, OE-OPN obviously upregulated spheroids were larger in size than cinobufagin-treated MG-63/U2OS spheroids in the absence of OE-OPN(P<0.05).
Furthermore, OE-OPN attenuated the cinobufagin-increased the levels of Bax, caspase-3 and cleaved PARP,EMT phenotype (Figure 6C). Significantly, CD133, p-STAT3(Tyr705), p-STAT3(Ser727) expressions were also promoted by OE-OPN treatment. Equally, si-OPN aggravated the inhibitive effect of cinobufagin on the size of MG-63/U2OS spheroids (Figure 6D and E). Moreover, si-OPN offsetted cinobufagin-increased the levels of Bax, caspase-3,cleaved PARP,CD133, EMT phenotype (Figure 6F).
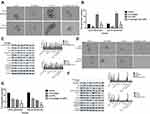
Hyperactivation Of The STAT3 Pathway Facilitated The Maintenance Of Osteosarcoma CSCs
STAT3 and its downstream signaling cascades are considered to be critical regulators of tumorigenesis. Research has shown that STAT3 promotes the growth of tumors through a multi-step process by interacting with tumor cells and surrounding tumor-associated cells.14 In order to verify whether activation of the STAT3 pathway is involved in cinobufagin/IL-6 crosstalk and the modulation of stem-like properties in MG-63/U2OS CSCs, we performed rescue therapy. As shown in Figure 7A and B, the overexpression of STAT3 rescued the cinobufagin-reduced size of MG-63/U2OS spheroids (P < 0.05). However, the overexpression of STAT3 clearly increased the ratio of CD133-positive cells (Figure 7C and D). We also detected lower expression levels of Bax, caspase-3 and cleaved PARP, along with higher expression levels of CD133, Nanog, Sox-2 and Oct3/4, in the cinobufagin plus STAT3-overexpression group (Figure 7E). Combined with the above results (Figures 5 and 6), we suspected that IL-6 was the upstream modulator of OPN expression and that STAT3 was the downstream modulator of OPN. Overall, the toxic effect of cinobufagin on MG-63/U2OS spheroids was attributed to down-regulation of the IL-6-OPN-STAT3 pathway.
Cinobufagin Suppressed The Tumor Igenesis Of MG-63/U2OS Spheroids, Along With Stemness, Apoptosis And Protein Expression Via The IL-6-OPN-STAT3 Pathway In Vivo
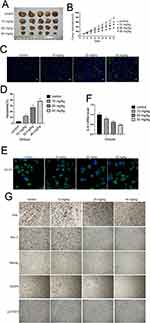
Finally, we investigated whether cinobufagin could prevent osteosarcoma-derived CSC progression in vivo. Tumor-bearing animals were randomly divided into four groups, followed by the administration of 10, 20, and 40 mg/kg of cinobufagin. As shown in Figure 8A, cinobufagin suppressed tumor growth in mice bearing osteosarcoma-derived CSCs. In addition, tumor volume (Figure 8B) in cinobufagin-treated mice was significantly reduced compared with those observed in the control group, particularly at doses of 20 and 40 mg/kg. The TUNEL assay demonstrated that cinobufagin caused a significant increase in the proportion of apoptotic cells in tumor tissues and that this effect occurred in a dose-dependent manner (Figure 8C and D). These observations were further confirmed by Qrt-PCR analysis (Figure 8E) and immunofluorescence revealed markedly decreased CD133 expression in tumor tissues treated with cinobufagin (Figure 8F). Moreover, immunohistochemistry analysis showed that the protein levels of OPN, Nanog, Sox-2, Oct3/4, and p-STAT3 were downregulated (Figure 8G).
Discussion
Cancer is a multifactorial disease associated with hyperactivated patterns of cell growth. The common consensus of opinion is that cancer stem cells play a crucial role in the uncontrolled proliferation of tumor cells and chemoresistance.39 Previous research conducted in our laboratory, and by other groups, showed that cinobufagin suppressed cell proliferation and induced apoptosis in osteosarcoma cells by inactivating Notch signaling and by downregulating MicroRNA-221 and the ROS/JNK/p38 signaling pathway.28–30 However, prior to this study, evidence regarding the role of cinobufagin in modulating the differentiation and stemness features of osteosarcoma CSCs was limited.
In a previous study, Chang et al reported that bufalin inhibited the differentiation and proliferation of hMG63- derived CSCs and induced cleaved caspase-3 in C1OS CSCs, respectively.33,40 In the present study, we found that cinobufagin efficiently inhibited the proliferation of U2OS/MG-63 spheroids and parent cells (Figure 1A and B). Furthermore, cinobufagin induced apoptosis in U2OS/MG-63 spheroids and parent cells and elevated the protein levels of caspase-3, cleaved PARP, Bax and Bcl-2 (Figure 2A–C).
Chueh et al reported that bufalin inhibited the invasion and migration of human osteosarcoma U2OS parent cells.32 We detected a lower number of invasive cells (Figure 3A and B) and increased the width of wounds(Figure 3C and D) in osteosarcoma parent cells than in corresponding CSCs which implied that cinobufagin possessed the ability to suppress the invasion/migration of osteosarcoma spheroids/parent cells. Moreover, we observed that levels of vim and a-SMA were gradually reduced as the expression of E-cad increased (Fg3EF); this was indicative of stem cell-like properties. The dimensions of spheroids(Figure 4A and B), the proportion of CD133-positive cells(Figure 4C and D), as well as stemness marker protein levels(Figure 4E and F) were also downregulated in response to cinobufagin-treatment. Collectively, these results suggested that cinobufagin induced apoptosis, repressed EMT and reduced the stemness properties of spheroids derived from U2OS/MG-63 cells.
Previously, Ouyang et al found that osteopontin promoted cancer cell drug resistance and that invasion was associated with a poor outcome in patients with advanced non-small-cell lung cancer.41 We therefore hypothesize that OPN may participate in the regulation of stemness features in U2OS/MG-63 spheroids as well as the progression of cinobufagin-suppressed stemness in osteosarcoma spheroids.
Numerous environmental conditions and multi-step processes are associated with the initiation and development of tumors.42,43 Previous research has shown that levels of IL-6 were increased in the serum of patients with osteosarcoma.44 Qin et al further reported that IL-6 acts as a major upstream molecule that triggers the induction of neoplastic OPN.45 In another study, Uchibori et al showed that IL-6 signaling was the upstream mediator responsible for upregulating OPN.46 Remarkably, we observed that OPN expression was higher in spheroids than in parent cells (Figure 5A. We also found that cinobufagin also reduced the expression of IL-6, p-STAT3 Tyr705, p-STAT3 Ser727 and OPN levels in spheroids/parent cells (Figure 5B). Figure 5C–F show that the addition of IL-6 impaired the cinobufagin-mediated suppression of spheroid size, the proportion of CD133-positive cells, and reduced the expression of stemness markers. However, si-IL-6 and cinobufagin treatment synergistically inhibited spheroid size, the proportion of CD133-positive cells, as well as the expression of stemness markers (Figure 5G–L), thus proving that IL-6 plays a role in the mechanism by which cinobufagin modulates the stemness of osteosarcoma spheroids.
However, exogenous IL-6 and si-IL-6 up/downregulated OPN levels in a similar manner (Figure 5F and K) which indicated that IL-6 acts as an upstream molecule to trigger an elevation in OPN levels. In contrast, Wang et al observed that IL-6 enhanced cancer stemness and promoted the metastasis of hepatocellular carcinoma by up-regulating the expression of osteopontin. It is possible that the mechanisms of mutual adjustment between IL-6 and OPN differs in different cell types.
OPN has been shown to exert a positive effect on the modulation of stemness traits in hepatocellular carcinoma, lung cancer, colorectal cancer, and pancreatic cancer.47–50 Figure 6 shows that OE-OPN neutralized the cinobufagin-induced reduction in spheroid size and p-STAT3(Tyr727) along with p-STAT3(Ser705), Bax, Bcl-2, caspase-3, cleaved PARP, Nanog, Sox-2 and Oct3/4 levels. We also found that si-OPN plus cinobufagin treatment synergistically downregulated the expression of these indicators which suggested that OPN participates in the cinobufagin-induced suppression of stemness features in osteosarcoma spheriods. Our results also indicated that STAT3 acts as a downstream target for OPN in osteosarcoma spheroids/parent cells (Figure 6C and F). In a previous study, Behera et al reported that activation of JAK2/STAT3 signaling by OPN promoted tumor growth in human breast cancer cells; these previous data were in accord with our present data.
Generally, IL-6 is known to modulate the self-renewal of stem cells by phosphorylating the STAT3, MAPK and Akt signaling pathways.51–54 Other research has shown that both constitutive and IL-6-inducible activation of STAT3 (Tyr705) and JAK2 can occur in osteosarcoma.55 In agreement with the opinion of Peng et al,27 Figure 7 shows that the overexpression of STAT3 offset the cinobufagin-induced reduction in CD133-positive cell ratios and the dimension of spheroids as well as the cinobufagin-induced increase in the expression of Bax, caspase-3, cleaved PARP, vimentin and α-SMA, and the lower levels of Bcl-2 and E-cad. Our studies in nude mice further confirmed the effect of cinobufagin in vivo:the reduced tumore volume(Figure 8A and B), the increased apoptotic ratio(Figure 8C and D), the CD133 flurescent expression(Figure 8E and F), the downregulated immunohistochemistry levels of OPN, Nanog, Sox-2, Oct3/4, and p-STAT3 (Figure 8G). Our data suggest that cinobufagin delayed the progression of cancer and reduced stemness properties in osteosarcoma by downregulating the IL-6-OPN-STAT3 pathway.
However, our reserach had some limits. No data showed the crosstalk between OPN expression and the clinicopathologic features of osteosarcoma patients. Whether IL-6-OPN-STAT3 pathway exerted the positive effect on modulation of osteosarcoma spheroids stemness features need more comfirmation. More efforts are still needed to elucidate the possible mechanisms in the future.
In conclusion, we observed that levels of OPN were higher in osteosarcoma spheroids than in parent cells. We also found that cinobufagin downregulated the stem-like properties of osteosarcoma cells, thus inhibiting the process of tumorigenesis by inhibiting the IL-6-OPN-STAT3 pathway. Therefore, the present findings define potential new mechanisms and identify novel molecular targets for the prevention and treatment of osteosarcoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895
2. Li S, Sun W, Wang H, et al. Research progress on the multidrug resistance mechanisms of osteosarcoma chemotherapy and reversal. Tumor Biol. 2015;36(3):1329–1338. doi:10.1007/s13277-015-3181-0
3. Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer. 2013;13:497–510. doi:10.1038/nrc3486
4. Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11(3):279–303. doi:10.1177/10454411000110030101
5. Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer. 2001;84(7):951–958
6. Luo X, Chen J, Song WX, et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest. 2008;88(12):1264–1277.
7. Gao YL, Xing LQ, Ren TJ, et al. The expression of osteopontin in breast cancer tissue and its relationship with p21ras and CD44V6 expression. Eur J Gynaecol Oncol. 2016;37(1):41–47.
8. Nakamura KD, Tilli TM, Wanderley JL, et al. Osteopontin splice variants expression is involved on docetaxel resistance in PC3 prostate cancer cells. Tumour Biol. 2016;37(2):2655–2663. doi:10.1007/s13277-015-4095-6
9. Ding K, Fan L, Chen S, et al. Overexpression of osteopontin promotes resistance to cisplatin treatment in HCC. Oncol Rep. 2015;34(6):3297–3303. doi:10.3892/or.2015.4306
10. Cho H, Hong SW, Oh YJ, et al. Clinical significance of osteopontin expression in cervical cancer. J Cancer Res Clin Oncol. 2008;134(8):909–917. doi:10.1007/s00432-007-0351-5
11. Zhang J, Yamada O, Kida S, et al. Identification of brefelamide as a novel inhibitor of osteopontin that suppresses invasion of A549 lung cancer cells. Oncol Rep. 2016;36(4):2357–2364. doi:10.3892/or.2016.5006
12. Velupillai P, Sung CK, Tian Y, et al. Polyoma virus-induced osteosarcomas in inbred strains of mice: host determinants of metastasis. PLoS Pathog. 2010;6(1):e1000733. doi:10.1371/journal.ppat.1000733
13. Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growthand activation of promatrix metalloproteinase-2 through NF-kappa B-mediated induction of membrane type 1 matrix metalloproteinase inmurine melanoma cells. J Biol Chem. 2001;276:44926–44935. doi:10.1074/jbc.M103334200
14. Carpenter RL, Lo HW. STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel). 2014;6:897–925.
15. Liu C, Dong L, Sun Z, et al. Esculentoside A suppresses breast cancer stem cell growth through stemness attenuation and apoptosis induction by blocking IL-6/STAT3 signaling pathway. Phytother Res. 2018;32(11):2299–2311. doi:10.1002/ptr.v32.11
16. Chung SY, Chen YH, Lin PR, et al. Two novel SHP-1 agonists, SC-43 and SC-78, are more potent than regorafenib in suppressing the in vitro stemness of human colorectal cancer cells. Cell Death Discov. 2018;4:25. doi:10.1038/s41420-018-0084-z
17. Lee SO, Yang X, Duan S, et al. IL-6 promotes growth and epithelial-mesenchymal transition of CD133+cells of non-small cell lung cancer. Oncotarget. 2016;7(6):6626–6638. doi:10.18632/oncotarget.6570
18. Wang Y, Zong X, Mitra S, et al. IL-6 mediates platinum-induced enrichment of ovarian cancer stem cells. JCI Insight. 2018;3. doi:10.1172/jci.insight.122360
19. Chang MT, Lee SP, Fang CY, et al. Chemosensitizing effect of honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3signaling. Environ Toxicol. 2018;33(11):1105–1112. doi:10.1002/tox.22587
20. Wang ZY, Wang CR, Zuo DQ, et al. Attenuation of STAT3 phosphorylation promotes apoptosis and chemosensitivity in human osteosarcoma induced by Raddeanin A. Int J Biol Sci. 2019;15(3):668–679. doi:10.7150/ijbs.30168
21. Qi F, Li A, Inagaki Y, et al. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int Immunopharmacol. 2011;11:342–349. doi:10.1016/j.intimp.2010.12.007
22. Zhu Z, Li E, Liu Y, et al. Bufalin induces the apoptosis of acute promyelocytic leukemia cells via the downregulation of survivin expression. Acta Haematol. 2012;128:144–150. doi:10.1159/000339424
23. Xie RF, Li ZC, Chen PP, et al. Bufothionine induced the mitochondria-mediated apoptosis in H22 liver tumor and acute liver injury. Chin Med. 2015;10:5. doi:10.1186/s13020-015-0033-1
24. Wang J, Jin Y, Xu Z, et al. Involvement of caspase-3 activity and surviving downregulation in cinobufocini-induced apoptosis in A 549 cells. Exp Biol Med (Maywood). 2009;234:566–572. doi:10.3181/0811-RM-326
25. Yu CH, Kan SF, Pu HF, et al. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Sci. 2008;99:2467–2476. doi:10.1111/j.1349-7006.2008.00966.x
26. Li C, Hashimi SM, Cao S, et al. The mechanisms of chansu in inducing efficient apoptosis in colon cancer cells. Evid Based Complement Alternat Med. 2013;849054.
27. Cao-Hong, Shibayama-Imazu T, Masuda Y, et al. Involvement of Tiam1 in apoptosis induced by bufalin in HeLa cells. Anticancer Res. 2007;27:245–249.
28. Dai G, Zheng D, Guo W, et al. Cinobufagin induces apoptosis in osteosarcoma cells via the mitochondria-mediated apoptotic pathway. Cell Physiol Biochem. 2018;46(3):1134–1147. doi:10.1159/000488842
29. Ma K, Zhang C, Huang MY, et al. Cinobufagin induces autophagy-mediated cell death in human osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway. Oncol Rep. 2016;36(1):90–98. doi:10.3892/or.2016.4782
30. Cao Y, Yu L, Dai G, et al. Cinobufagin induces apoptosis of osteosarcoma cells through inactivation of Notch signaling. Eur J Pharmacol. 2017;794:77–84. doi:10.1016/j.ejphar.2016.11.016
31. Yin JQ, Wen L, Wu LC, et al. The glycogen synthase kinase-3β/nuclear factor-kappa B pathway is involved in cinobufagin-induced apoptosis in cultured osteosarcoma cells. Toxicol Lett. 2013;218(2):129–136. doi:10.1016/j.toxlet.2012.11.006
32. Chueh FS, Chen YY, Huang AC, et al. Bufalin-inhibited migration and invasion in human osteosarcoma U-2 OS cells is carried out by suppression of the matrix metalloproteinase-2, ERK, and JNK signaling pathways. Environ Toxicol. 2014;29(1):21–29. doi:10.1002/tox.20769
33. Chang Y, Zhao Y, Gu W, et al. Bufalin inhibits the differentiation and proliferation of cancer stem cells derived from primary osteosarcoma cells through Mir-148a. Cell Physiol Biochem. 2015;36(3):1186–1196. doi:10.1159/000430289
34. Ishiwata T. Cancer stem cells and epithelial-mesenchymal transition: novel therapeutic targets for cancer. Pathol Int. 2016;66(11):601–608. doi:10.1111/pin.2016.66.issue-11
35. Yang M, Yan M, Zhang R, et al. Side population cells isolated from human osteosarcoma are enriched with tumor-initiating cells. Cancer Sci. 2011;102:1774–1781. doi:10.1111/j.1349-7006.2011.02028.x
36. Qu H, Xue Y, Lian W, et al. Melatonin inhibits osteosarcoma stem cells by suppressing SOX9-mediated signaling. Life Sci. 2018;207:253–264. doi:10.1016/j.lfs.2018.04.030
37. Peng L, Jiang D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS One. 2018;13(10):e0205918. doi:10.1371/journal.pone.0205918
38. Dass CR, Choong PF. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol Cancer Ther. 2007;6(12 Pt 1):3263–3270. doi:10.1158/1535-7163.MCT-07-0546
39. Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234(6):8381–8395.
40. Chang Y, Zhao Y, Zhan H, et al. Bufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63-derived cancer stem cells. Tumour Biol. 2014;35(2):1075–1082. doi:10.1007/s13277-013-1143-y
41. Ouyang X, Huang Y, Jin X, et al. Osteopontin promotes cancer cell drug resistance, invasion, and lactate production and is associated with poor outcome of patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2018;11:5933–5941. doi:10.2147/OTT
42. Salvatore V, Teti G, Focaroli S, et al. The tumor microenvironment promotes cancer progression and cell migration. Ontarget. 2017;8(6):9608–9616.
43. Yadav AK, Desai NS. Cancer stem cells and their microenvironment: biology and therapeutic implications. Stem Cell Rev. 2019;15(3):331–355. doi:10.1007/s12015-019-09887-2
44. Xiao H, Chen L, Luo G, et al. Effect of the cytokine levels in serum on osteosarcoma. Tumor Biol. 2014;35(2):1023–1028. doi:10.1007/s13277-013-1136-x
45. Qin X, Yan M, Wang X, et al. Cancer-associated fibroblast-derived IL-6 promotes head and neck cancer progression via the osteopontin-NF-kappa B signaling pathway. Theranostics. 2018;8(4):
46. Uchibori T, Matsuda K, Shimodaira T, et al. IL-6 trans-signaling is another pathway to upregulate osteopontin. Cytokine. 2017;90:88–95. doi:10.1016/j.cyto.2016.11.006
47. Behera R, Kumar V, Lohite K, et al. Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis. 2010;31(2):192–200. doi:10.1093/carcin/bgp289
48. Ng L, Wan T, Chow A, et al. osteopontin overexpression induced tumor progression and chemoresistance to oxaliplatin through induction of stem like properties in human colorectal cancer. Stem Cells Int. 2015;2015:247892. doi:10.1155/2015/247892
49. Yang M-C, Wang H-C, Hou Y-C, et al. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol Cancer. 2015;14:179. doi:10.1186/s12943-015-0449-3
50. Cao. L, Fan. X, Jing W, et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-κB-HIF-1α pathway. Oncotarget. 2015;6(9):6627–6640. doi:10.18632/oncotarget.3113
51. Hideshima T, Nakamura N, Chauhan D, et al. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20(42):5991–6000. doi:10.1038/sj.onc.1204833
52. Zhang Y, Wang L, Deng Y, et al. Fraxetin suppresses proliferation of non-small-cell lung cancer cells via preventing activation of signal transducer and activator of transcription 3. Tohoku J Exp Med. 2019;248(1):3–12. doi:10.1620/tjem.248.3
53. Zhuo D, Wu Y, Luo J, et al. CSTP1 inhibits IL-6 expression through targeting Akt/FoxO3a signaling pathway in bladder cancer cells. Exp Cell Res. 2019;380(1):80–89. doi:10.1016/j.yexcr.2019.04.019
54. Balachander GM, Talukdar PM, Debnath M, et al. Inflammatory role of cancer-associated fibroblasts in invasive breast tumors revealed using a fibrous polymer scaffold. ACS Appl Mater Interfaces. 2018;10(40):33814–33826. doi:10.1021/acsami.8b07609
55. Zhang Y, Cheng H, Li W, et al. Highly-expressed P2X7 receptor promotes growth and metastasis ofhuman HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3-catenin and mTOR/HIF-1a/VEGF signaling. Int J Cancer. 2019. doi:10.1002/ijc.32207
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.