Back to Archived Journals » Reports in Electrochemistry » Volume 4
Characterizing molecular junctions through the mechanically controlled break-junction approach
Authors Hamill J, Wang K, Xu B
Received 7 March 2014
Accepted for publication 9 April 2014
Published 28 May 2014 Volume 2014:4 Pages 1—11
DOI https://doi.org/10.2147/RIE.S46629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Joseph M Hamill,1 Kun Wang,1 Bingqian Xu1,2
1Single Molecule Study Laboratory, College of Engineering and Nanoscale Science and Engineering Center, 2College of Engineering, University of Georgia, Athens, GA, USA
Abstract: Mechanically controlled break-junction techniques, which emerged right after the invention of scanning tunneling microscopy, have enabled substantial progress in characterizing single-molecule junctions toward the ultimate goal of molecular devices. Dramatic advances have been made in design, fabrication, control, and understanding of the measurements of single-molecule junctions over the past decade. In this overview, we present the evolution of some of the recent issues, and an outlook for further developments in mechanically controlled break-junction techniques for characterizing molecular junctions. Topics of recent interest include contact geometry, electrochemical redox experiments, external bias effect, and environmental influences. Each will need further investigation to thoroughly understand the experimental information revealed from a molecular junction.
Keywords: mechanically controlled molecular break junction, scanning probe microscopy
Introduction
The desire to overcome the prediction of Moore’s law led researchers to single-molecule-scale devices.1–3 Molecular junctions emerged from the late 1990s,4 catalyzed by the invention of scanning tunneling microscopy (STM) and inspired by a paper by Aviram and Ratner describing molecular-sized p–n junctions.5,6 In the search for effective ways to couple a molecule between electrodes, researchers produced two efficient options: scanning probe microscopy (SPM) using the newly invented STM, and a microfabricated, electromigration piezoelectric three-point arching method. Both techniques provided a reliable platform to conduct measurements at a single-molecule level by utilizing piezoelectric transducers to mechanically open and close a break junction in which a molecule had become bonded. The key feature of each of these methods, and all other mechanically controlled break junctions (MCBJs), is a molecule in contact with two or more electrodes where one of the electrodes is mechanically controlled to allow it to create, break, and reform junctions with the molecule incorporated. In this way, the four main features of study in MCBJs are 1) the electrodes (and the macroscopic system), 2) the electrode–molecule junction, 3) the molecule, and 4) the environment in which the junction takes place. Often, this environment is in solution,4,7 sometimes in vacuum,8–10 and sometimes at cryogenic temperatures.11–13
MCBJ methods have come a long way since the early stages of the last decades. Experiments using MCBJs to measure the conductance of single molecules began in earnest in the late 1990s, and the first years of experiments resulted in many discrepancies among the measured and simulated results of single-molecule conductance from different labs. As the techniques matured, and as more labs took part in the experiments, the reproducibility of MCBJ experiments became more reliable.14 Today, MCBJ techniques are used in conjunction with other measurement techniques,15,16 either simultaneously or back to back, to derive still more information about the behavior of molecules at the nanoscopic level.
Some of the more detailed challenges that troubled the field in those early years, such as the strong sensitivity of MCBJs to contact geometry, behaviors relating to redox reactions in molecules in MCBJs, and the phenomenon of negative differential resistance (NDR), as well as other nonlinear current-voltage behaviors of molecular junctions, have been studied at length in the last decade, and we know much more about these issues today. We hope to discuss these advancements here, as well as discuss new directions for the future.
MCBJ experimental challenges
Before anything resembling single molecular electronics becomes possible, reliable molecule-electrode junctions using chemical bonds are essential. Without these, it had been necessary to rely on approaching the molecule within proximity to the electrodes using increasingly clever ways: using mercury drops as electrodes,17 using Lorentz force to cross metallic wires,18 and trapping molecules in a nanopore.19,20 Other successful techniques involved sandwiching the ends of very robust molecules between layers of electrode material, usually by thermal evaporation, and using lithographic techniques to etch away undesirable quantities of the substrate and electrodes; these techniques worked well for long and strong carbon nanotubes, but were too destructive for smaller molecules.21 A different method involved crisscrossing nanowires by overlaying layer after layer. This, however, usually only produced large arrays of nanojunction; the single-molecule junction was still out of reach.22,23 It was still necessary to find ways to bond one end of the molecule to one electrode, and the other end to the second electrode.
Ways to bond the molecule chemically to the electrodes were finally developed using amine and thiol-functionalized molecules, which bond readily to metal electrodes. Thiol-functionalized molecules were used in the crossed-wire technique,24 and the first attempts to use an STM to probe molecular conductance were attempted by functionalizing one side of the molecule and scanning the other end in a traditional STM or conducting atomic force microscopy (C-AFM) method,25,26 or with the probe scanning a gold nanoparticle atop the molecule.27,28 Finally, both ends of the molecule were functionalized and successfully incorporated into a mechanical break junction. One of the first truly powerful break-junction techniques used a piezoelectric transducer to arch a platform onto which a metallic wire had been anchored.4 By lithographically etching the metallic wire until it was nanoscopically thin, the arching of the platform allowed complete control of the breaking and reforming of nanojunctions repeatedly with subangstrom control.29,30 This three-point arching technique provides experimentalists today with a way to perform complementary measurements, such as optical spectroscopy alongside MCBJ-conductance measurements, because the design of the apparatus leaves the top exposed.31–33
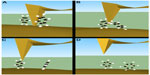
Another powerful method for creating MCBJs was created by Xu and Tao.7 This method involved forcing a gold tip into a gold substrate (Figure 1A) starting from a traditional STM setup, where a liquid cell was placed such that the molecule in solution was on top of the gold substrate. When the gold tip was withdrawn, many molecules were incorporated into the gap between the tip and substrate (Figure 1B), but as the tip was withdrawn further, the molecules broke one by one until there was only one left in the break junction (Figure 1C). All the while, a bias was applied between the tip and the substrate, and the current across the junction was recorded. As the molecules broke away, individual steps in the conductance were observed, each step differing from the next by a unique value of conductance. For atomically thin metal junctions, these quantized steps were predicted, and experimentally shown, to have a conductance of G0 = n × 2e2/h, where n is an integer, e is the electronic charge, and h is Planck’s constant.34 After the last metallic junction, new steps were observed for conductance values much smaller than the conductance quantum of G0. These steps corresponded to integer numbers of molecules in the junction. The last step before the conductance fell to zero (Figure 1D) was the conductance across a single molecule.
Creating strong bonds with the gold electrode at both junctions was a significant improvement upon the previous methods, and created clear data (Figure 2A–D). Proving the molecule, in this case a 4,4′-bipyridine molecule, was indeed present in the junction was the next task. The new scanning probe microscopy (SPM) technique developed by Xu and Tao was very destructive, because it involved forcing the STM tip into the substrate before withdrawing it, and it was necessary to prove that during the withdrawal, a substrate–molecule–tip junction was indeed formed. Because the nitrogen was located at either end of the 4,4′-bipyridine molecule, it was able to bond simultaneously with both electrodes. By conducting the SPM measurement in solution without the molecule present, it was shown that the conductance fell off exponentially, without the presence of the signature steps at the conductance of the molecule (Figure 2E and F). Next, 2,2′-bipyridine was measured in solution. Because the nitrogen in 2,2′-bipyridine is not located at the end of the rod-like aromatic rings, it was not possible for it to bond simultaneously to both electrodes, and the conductance could not be measured, resulting once again with conductance traces that fell off exponentially without steps.
  | Figure 2 (A–J) Representative mechanically controlled break-junction data. (A) Au–Au junction-conductance trace with quantized conductance of step G0. (B) Conductance histogram from Au–Au conductance traces. (C) Au-4,4′-bipyridine-Au junction conductance traces with quantized conductance step ~0.01 G0. (D) Conductance histogram from multiple traces. (E) Conductance traces without molecule present, with no quantized steps. (F) Conductance histogram without peaks. (G) Conducting atomic force microscopy (C-AFM) schematic. (H) Example Au–Au junction conductance (note semilogarithmic scale for following conductance traces) and force traces resulting from C-AFM. (I) Example traces for 4,4′-bipyridine junction. (J) Example traces for 1,2-bis(4-pyridyl)ethylene junction. |
Because there was much thermal noise in measurements of this precision,35 it was beneficial to create 1-D histograms that revealed the statistical average of single-molecule conductance over thousands of repeated measurements (Figure 2B and D).36,37 Despite the reliance on statistical methods, this new SPM MCBJ technique resolved many standing debates surrounding MCBJs. SPM MCBJs showed that short strands of deoxyribonucleic acid (DNA) can be described most accurately as a semiconductor.38 Up until this point, there was much debate surrounding the electronic properties of DNA, with proponents arguing for insulator,39 large-band-gap semiconductor,40 conductor,41,42 and even superconductor.43 SPM MCBJs showed that DNA acted very much like a semiconductor, especially for short strands heavy in guanine.38
Reviews from the field of MCBJ written in the first decade of the twenty-first century all had one concern in common: reproducibility.14,44,45 The SPM technique resolved yet another debate, by confidently and repeatedly measuring the conductance of simple alkanedithiol chains, molecules that up until that point had discrepancies between different labs and between theory and experiment.7,46,47 Experiments on the single molecular conductance of octanedithiol conducted at different labs in 2001,48 2003,7 and 200449 appeared to have widely ranging results. After closer attention, these discrepancies have been resolved.14
As is so common in science, the resolution of one concern often is accompanied by new insight into other concerns. The resolution of the discrepancy in octanedithiol single-molecule conductance provided important information about the significance of contact geometry in MCBJs. It was shown that when the molecule is contacted to a planar crystalline electrode, it is of only minor importance whether it is contacted in a hill or valley of the crystal. Of significantly more importance is a contact geometry in which the molecule contacts a pyramid-shaped crystalline electrode instead of a planar electrode. In the pyramidal case, conductance is greatly decreased when compared to the planar case. Therefore, if the experimental apparatus has a preference for creating one contact geometry over another, then this can explain why different apparatuses produce different single-molecule conductance values.
Importance of contact geometry and evolution in MCBJs
At the most basic level, progress in controlling physical and chemical properties of a molecular junction started with the capability of making robust contact to form a single-molecule junction. This proving a difficult task, the role of the contact interfaces was omitted at the initial time point. Experiments without careful control of the contact parts revealed data hard to interpret.50,51
It has recently become apparent that electrode geometry and the specific orientation at which the molecule contacts the electrodes has a profound influence on the behavior of MCBJ measurements, and much effort has been devoted to understanding this phenomenon. Detailed quantum chemical studies have even shown allowed and forbidden states, based on phase and symmetry groups, influence molecular conductance.9,52
C-AFM (Figure 2G) is especially useful in measuring contact changes and flexibility during MCBJs. C-AFM uses a similar method as SPMBJs, but replaces the STM tip with an AFM cantilever. The result is an MCBJ in which force can be measured alongside conductance (Figure 2H–J).53 This technique was used to study contact phenomena, such as local heating, where it was predicted that inelastic scattering of electrons caused an increase in the temperature of the molecule.54,55 It has also been shown that gold contacts in a tetrahedral crystal structure have a quantized length slipping, allowing one, two, and three simultaneous slip events for each of the three tetrahedral faces slipping individually.56
Theoretical formalism describes the MCBJ system as a quantum scattering event, with the molecule in the junction performing the scattering much like any dielectric material.34,57 In this interpretation, the mechanism by which electrons are delivered to the junction via the contacts is of major significance, even when compared to the significance of the molecule in the junction. As stated earlier, the shape of the electrode where it contacts the molecule is of paramount importance. However, an MCBJ is a dynamic event that undergoes mechanical stretching, thermal fluctuations,58 local current-induced heating,53,55 and redox reactions.59 With this in mind, researchers have been focusing much attention on the progression of the MCBJ as it is formed, stretched, and finally broken. Computer simulations have been of immense help in this area, because as yet direct observation of contact-geometry evolution in experiments has been difficult.60,61 Simulations reported that with the same molecule bridged between electrodes with different shapes revealed totally different I–V characteristics and asymmetric binding sites of the anchoring group could also influence the resulting electronic properties.62–64 The same molecule could even have two or more probable orientations within a junction, each with a unique conductance signature (Figure 3A).65
  | Figure 3 (A–G) Example of geometric adjustments during mechanically controlled break-junction elongation. (A) 4,4′-bipyridine orients in two ways during junction elongation, resulting in two separate values for conductance. (B) Example slip of Au–Au bond. (C) Simulated changes in energy and force due to Au–Au bond slip. (D) Example slip of P–Au bond. (E) Simulated changes in energy, force, bond length, and conductance due to P–Au bond slip. (F) Example N–Au bond twist. (G) Simulated changes in energy, force, bond length, and conductance due to N-Au bond twist. |
Conductance across MCBJs as tunneling electron microscopy images are taken shows that as multiple atoms are individually added to the chain, the conductance oscillates in a consistent manner.60,66–69 Careful studies of similar chains occurring when a molecule is present in the junction have shown that metal chains of four atoms long can occur before the junction breaks.70 Other simulation work has shown the effects of slipping gold–gold bonds (Figure 3B and C) and molecule–electrode slipping (Figure 3D and E) and twisting (Figure 3F and G) on conductance in MCBJs.71,72 Studies like this reveal the molecular MCBJ to be flexible at the electrode junction, and this geometric flexibility has significant influence on molecular conductance as the junction evolves during the process of breaking. Some phenomena predicted for molecular electronics, it has been suggested, require this flexibility, and more.73 In cases where the junction is required to stretch still further, carbon nanotubes have been suggested.74
Theoretical advancements in electrochemical redox experiments
Most often, the overarching goal of MCBJ research is to understand and control the alignment of the molecules’ Fermi level with the conducting molecular orbitals (MOs) of the electrodes: the highest occupied molecular orbital (HOMO) for hole transfer, and the lowest unoccupied molecular orbital (LUMO) for electron transfer.75 Electrochemical gating of molecular conductance due to redox reactions is possibly the most powerful method to control this alignment.76–80 Adding a third terminal to the electrolytic solution within the liquid cell containing the MCBJ allows the experimentalist great control over the local potential at the molecule. For junctions with a large HOMO–LUMO gap, this gating has shown little effect, because the Fermi level of the molecule was never able to reach the conducting MOs.81 Some molecules exhibited reduction of nitrite groups, and although conductance was significantly increased when negative bias was applied to the gating terminal, the reaction was irreversible. The reaction did have the effect of causing a rectification feature in the current-bias (I–V) relationship of the molecule, and the reaction also caused the negative differential resistance (NDR) phenomenon.82 Of far greater interest were molecules that experienced reversible redox reactions, thus allowing the molecule to be gated on and off, resulting in ranges of conductance of as much as 15 magnitudes.83 Cyclic voltammetry revealed that the molecule switched between a low-conductance reduced state to a high-conductance oxidized state.84 There was great control over this reaction; however, stochastic switching between states was also observed. For most molecules, high bias is required to maintain one state or the other.14
Recently, transition voltage spectroscopy (TVS)85 was used to calculate the energy gap between the molecular Fermi level and the nearest frontier MOs in order to show quantitatively that the solution in which the molecule is immersed contributes to electron transfer, helping resolve questions about the role of solvent in MCBJs.7,86,87 In conjunction with a simplified model and the electrochemical techniques described in the previous paragraph, TVS was helpful in showing the effects of electrochemical gating on single-molecule conductance.88 The TVS approach was also helpful in comparing the relative importance of molecular length,89 contact geometry, energy-level alignment, and contact resistance, and showed that contact resistance is the dominant factor in MCBJ conductance.90 TVS can discern between the effects of Schottky barriers versus defect barriers at the molecule–electrode junctions.91 TVS analysis on experimental data, along with density-function theory simulations, identified the MOs, not merely the HOMO and LUMO, responsible for electron transport, and furthermore showed that these MOs can change as the probe–substrate distance is varied.92 TVS has shown potential to address many questions surrounding MCBJs, even while presenting new questions.93,94
The role of external bias
Since the electronic properties of a molecular junction, such as conductance and I–V characteristics, have to be measured under an external bias applied on one of the electrodes, this bias plays a prominent role in affecting the charge-transport process across the junction. Many studies have emphasized the importance of the explicit inclusion of bias effect in explaining the electrical results of molecular junctions.62,95,96 Given that the static conductance is usually measured under a fixed bias, I–V characteristics measured using a bias sweep are the key feature that reflects the influence of external bias. Rectification behavior and NDR have been studied as the main focus of molecular electronics, since the asymmetric current at the same bias magnitude but opposite bias polarity is the first step to ultimately realizing a molecular diode. To date, in most molecular rectification studies with MCBJ, the switch-on voltage, where rectification ratio starts going beyond unity, is postponed to a certain bias voltage11,64,97–99 instead of the ideal case for bulk diode, where the switch-on voltage is close to zero. Certain rectifying molecules do show immediate switch-on.100 Dhungana et al reported an electron–phonon interaction mode perpendicular to the direction of current flow that increased with the increase of external bias, and in turn destroyed part of the electrode–molecule–electrode current.62 Other simulations62,101 based on the transmission function suggested that the frontier MOs and the MOs away from the Fermi energy of the electrode respond differently to the applied bias: the transmission peaks may rise, fall, and shift as the bias increases (Figure 4A and C). All the effects induced by external bias were responsible for resulting electrical measurement properties.
  | Figure 4 (A–C) Bias-dependent electronic properties of molecular junctions. (A) Schematic of the strongly coupled Fe–terpyridine–gold junction (top left); calculated current and conductance as a function of applied bias (bottom-left); bias-dependent transmission as a function of injection energy E (right). (B) Schematic of interface Coulomb interaction (top); current (blue) and effective coupling (red) as a function of the bias voltage for the interface Coulomb interaction (bottom). (C) Calculated I–V characteristics for Ru–bis(terpyridine) molecular wires with different junction configurations (“d” refers to the interplanar distance between the sulfur and the nearest gold) (left); bias-dependent transmission as a function of injection energy for interfacial distances of 2.42 Å (1), 2.82 Å (2), and 3.02 Å (3) (right). |
NDR, defined as a nonmonotonic dependence of current on the bias voltage (for example, the current decreases with increasing bias), has been observed for various metal–molecule–metal junctions.19,102–105 However, the sources of this effect are still in controversy. The main sources are discussed to be bias-dependent electron–phonon interaction, potential-drop-induced shifting of MOs, nonlinear bias-dependent effective coupling between MOs and the electrodes (Figure 4B), and even image-charge effects. All possible mechanisms involve the significance of external applied bias.
Environmental influences
Besides the structure of a molecular junction, effects from the environment surrounding the MCBJ have been of considerable interest as well. To date, the dramatic improvement in design and fabrication of a molecular junction enables more precise measurements, but also begs further investigation into the non-negligible contribution from ionic transport in the solution, especially at room temperature. Recently, Doi et al reported on the transient electrical response of ions in the vicinity of biased electrodes.106 Their results showed that ions rapidly responded to the strong fields near the electrode surface after turning on an applied voltage that screened the field in the process. Ions subsequently translocated in the weak electric field, and slowly relaxed within the diffusion layer. The ionic current performed as a function of bias voltage and also as a function of ionic concentration (Figure 5A). The interaction between the ions in the solution and the molecule in the junction has been highlighted in studies focusing on DNA molecules.107–110 The interaction mainly consists of a binding between ions and molecules and a doping of ions into the space within the molecule, both of which are experimentally and theoretically expected to impact the charge-transport process. Lowering the temperature to cryogenic levels greatly reduced the influence of thermal fluctuation on conductance, and in turn made the sensitivity to ionic influences more pronounced.
  | Figure 5 (A and B) Environmental influence on molecular junction measurements with mechanically controlled break-junction (MCBJ) approach. (A) Transient current response of NaCl solution under an applied potential of 0.4 V for several salt concentrations (left); current response for a concentration of 200 mM NaCl at various bias voltages (right). (B) Schematic illustration of the experimental setup based on MCBJ at 77 K (left), junction lifetime tB versus junction-stretching rate Vd (middle), and junction-breakdown length LB versus junction-stretching rate Vd (right) for benzenedithiol (BDT) single-molecule junction. Results acquired at 293 K are also shown for comparison. |
Temperature also has influences on the molecule–electrode junction. Measurements conducted at 77 K revealed the temperature dependence of a benzenedithiol junction lifetime can be explained in terms of gold single-atom contact stability. This suggested that the molecular junction lifetime at 77 K started to become shorter than the lifetime at room temperature under low-strain-rate conditions (Figure 5B), where differences in the effects of thermal fluctuations on gold single-atom contact stability became notable.13 Otherwise, local heating, a common issue of current-carrying devices, is also observed for molecular junctions. A temperature increase of different degrees due to local heating was revealed for different molecular junctions. Electron–phonon scattering was suggested as the cause of local heating in MCBJs, which emerged at molecule–electrode interfaces and consequentially destabilized the junction.53,111 Therefore, severe electrical local heating has to be considered when discussing the resulting electronic properties.
Conclusion and outlook
There is still plenty of room for improvement in the techniques involved in MCBJs. The following suggestions are just some of the potential issues that may be addressed in the near future.
It is now beneficial to incorporate other measurement techniques into multivariate correlation schemes alongside MCBJ conductance. This is already being done with Raman scattering,15,16,112–115 but there is potential to use multivariate correlation to study magnetic phenomena, such as the Kondo effect.116,117 Optical manipulation of molecules in MCBJs is already a common practice;32,33,118 however, further optical manipulation and measurement is also possible using Fourier-transform infrared and further ultraviolet-visible techniques.22 Force is easily measured alongside conductance using C-AFM in much the same way SPMBJs are performed.53,119 New methods for analyzing multivariate data sets are just now emerging, with great potential for expansion and development.120–122 New coupled multivariate measurement techniques will have the ability to measure the behavior of MCBJs at finer resolutions, and retain the essential time-dependent nature of the evolving junction.44,123
Water is an essential part of organic molecule junctions when the molecule must be kept in a natural solution. It has been simulated that water, along with ions in solution, may incorporate into DNA grooves and alter the available conductance channels.124 However, the effects of water on the conductance of complex molecules have not been studied thoroughly in laboratory experiments.
Along the same lines, electrochemical gating too can have a strong effect on the molecule. Specifically, theoretical investigations into electron transfer and the effects of redox reactions on MO changes are beginning to show promise.94 The data-analysis method of TVS provides very simplified models, but far more fundamental models are necessary.
Many of the organic molecules researchers study (DNA and proteins) are too large for traditional Hartree–Fock ab initio simulations. In order to fully understand these molecular junctions, simulations are required, but often these simulations must be overly simplified. Better simulation methods for large systems will be required in the future.
For the development of far more stable junctions, it will become necessary to utilize semiconductor electrodes that provide the potential for C-C and Si-C bonds at the molecule–electrode junction.8,125,126 This will also pave the way for more advanced semiconducting molecular devices.127 It is also necessary to develop stable dielectric and electrolytic media to stabilize molecular junctions for use in future molecular electronics.128
In 1983, when Binnig and Rohrer were concluding the invention of the STM, they described the disadvantage of traditional tunnel junctions available at the time: once a metal–insulator–metal sandwich was manufactured, it was no longer possible to adjust the junction further.6 The STM they developed solved this problem by using the vacuum barrier between the tip and substrate along with a piezoelectric transducer to allow easy adjustments to the junction. MCBJs are a natural extension of the desire to provide dynamic, easily modified mesoscopic junctions for fundamental physical studies. The STM’s ability to scan the local density of states of the molecule is impressive, and when used as a probe to complete a single molecular circuit, it becomes a fundamental tool for physicists and chemists to explore the fundamentals of their fields.
Acknowledgment
The authors thank the US National Science Foundation for funding this work (ECCS 0823849, ECCS 1231967).
Disclosure
The authors report no conflicts of interest in this work.
References
Feynman RP. There’s plenty of room at the bottom. Eng Sci. 1960;23(5):22–36. | |
von Hippel A. Molecular engineering. Science. 1956;123(3191):315–317. | |
Moore GE. Cramming more components onto integrated circuits. Electronics. 1965;38(8):114–117. | |
Reed MA, Zhou C, Muller CJ, Burgin TP, Tour JM. Conductance of a molecular junction. Science. 1997;278(5336):252–254. | |
Aviram A, Ratner MA. Molecular rectifiers. Chem Phys Lett. 1974;29(2):277–283. | |
Binnig G, Rohrer H. Scanning tunneling microscopy. Surf Sci. 1983;126(1):236–244. | |
Xu B, Tao NJ. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science. 2003;301(5637):1221–1223. | |
Guisinger NP, Yoder NL, Hersam MC. Probing charge transport at the single-molecule level on silicon by using cryogenic ultra-high vacuum scanning tunneling microscopy. Proc Natl Acad Sci U S A. 2005;102(25):8838–8843. | |
Taniguchi M, Tsutsui M, Mogi R, et al. Dependence of single-molecule conductance on molecule junction symmetry. J Am Chem Soc. 2011;133(30):11426–11429. | |
Tsutsui M, Taniguchi M, Kawai T. Quantitative evaluation of metal-molecule contact stability at the single-molecule level. J Am Chem Soc. 2009;131(30):10552–10556. | |
Elbing M, Ochs R, Koentopp M, et al. A single-molecule diode. Proc Natl Acad Sci U S A. 2005;102(25):8815–8820. | |
Reichert J, Weber HB, Mayor M, von Lohneysen H. Low-temperature conductance measurements on single molecules. Appl Phys Lett. 2003;82(23):4137–4139. | |
Tsutsui M, Taniguchi M, Kawai T. Atomistic mechanics and formation mechanism of metal-molecule-metal junctions. Nano Lett. 2009;9(6):2433–2439. | |
Lindsay SM, Ratner MA. Molecular transport junctions: clearing mists. Adv Mater. 2007;19(1):23–31. | |
Natelson D, Li Y, Herzog JB. Nanogap structures: combining enhanced Raman spectroscopy and electronic transport. Phys Chem Chem Phys. 2013;15(15):5262–5275. | |
Park TH, Galperin M. Correlation between Raman scattering and conductance in a molecular junction. Europhys Lett. 2011;95(2):27001. | |
Slowinski K, Chamberlain RV, Miller CJ, Majda M. Through-Bond and Chain-to-Chain Coupling. Two Pathways in Electron Tunneling through Liquid Alkanethiol Monolayers on Mercury Electrodes. J Am Chem Soc. 1997;119(49):11910–11919. | |
Gregory S. Inelastic tunneling spectroscopy and single-electron tunneling in an adjustable microscopic tunnel junction. Phys Rev Lett. 1990;64(6):689–692. | |
Chen J, Reed MA, Rawlett AM, Tour JM. Large on-off ratios and negative differential resistance in a molecular electronic device. Science. 1999;286(5444):1550–1552. | |
Zhou C, Deshpande MR, Reed MA, Jones L, Tour JM. Nanoscale metal/self-assembled monolayer/metal heterostructures. Appl Phys Lett. 1997;71(5):611–613. | |
Fischer CM, Burghard M, Roth S, Klitzing KV. Microstructured gold/Langmuir-Blodgett film/gold tunneling junctions. Appl Phys Lett. 1995;66(24):3331–3333. | |
McCreery RL, Bergren AJ. Progress with molecular electronic junctions: meeting experimental challenges in design and fabrication. Adv Mater. 2009;21(43):4303–4322. | |
Zhong Z, Wang D, Cui Y, Bockrath MW, Lieber CM. Nanowire crossbar arrays as address decoders for integrated nanosystems. Science. 2003;302(5649):1377–1379. | |
Kushmerick JG, Holt DB, Yang JC, Naciri J, Moore MH, Shashidhar R. Metal-molecule contacts and charge transport across monomolecular layers: measurement and theory. Phys Rev Lett. 2002;89(8):086802. | |
Bumm LA, Arnold JJ, Cygan MT, et al. Are single molecular wires conducting? Science. 1996;271(5256):1705–1707. | |
Wold DJ, Frisbie CD. Fabrication and characterization of metal-molecule-metal junctions by conducting probe atomic force microscopy. J Am Chem Soc. 2001;123(23):5549–5556. | |
Cui XD, Primak A, Zarate X, et al. Changes in the electronic properties of a molecule when it is wired into a circuit. J Phys Chem B. 2002; 106(34):8609–8614. | |
Dorogi M, Gomez J, Osifchin R, Andres RP, Reifenberger R. Room-temperature Coulomb blockade from a self-assembled molecular nanostructure. Phys Rev B Condens Matter. 1995;52(12):9071–9077. | |
Kolesnychenko OY, Shklyarevskii OI, van Kempen H. Calibration of the distance between electrodes of mechanically controlled break junctions using field emission resonance. Rev Sci Instrum. 1999;70(2):1442–1446. | |
Muller CJ, Krans JM, Todorov TN, Reed MA. Quantization effects in the conductance of metallic contacts at room temperature. Phys Rev B Condens Matter. 1996;53(3):1022–1025. | |
Dulic D, van der Molen SJ, Kudernac T, et al. One-way optoelectronic switching of photochromic molecules on gold. Phys Rev Lett. 2003;91(20):207402. | |
He J, Chen F, Liddell PA, et al. Switching of a photochromic molecule on gold electrodes: single-molecule measurements. Nanotechnology. 2005;16(6):695. | |
Kim Y, Hellmuth TJ, Sysoiev D, et al. Charge transport characteristics of diarylethene photoswitching single-molecule junctions. Nano Lett. 2012;12(7):3736–3742. | |
Landauer R. Spatial variation of currents and fields due to localized scatterers in metallic conduction. IBM J Res Dev. 1957;1(3):223–231. | |
Wassel RA, Fuierer RR, Kim N, Gorman CB. Stochastic variation in conductance on the nanometer scale: a general phenomenon. Nano Lett. 2003;3(11):1617–1620. | |
González MT, Wu S, Huber R, van der Molen SJ, Schönenberger C, Calame M. Electrical conductance of molecular junctions by a robust statistical analysis. Nano Lett. 2006;6(10):2238–2242. | |
Mayor M, Weber HB. Statistical analysis of single-molecule junctions. Angew Chem Int Ed Engl. 2004;43(22):2882–2884. | |
Xu B, Zhang P, Li X, Tao N. Direct conductance measurement of single DNA molecules in aqueous solution. Nano Lett. 2004;4(6):1105–1108. | |
Braun E, Eichen Y, Sivan U, Ben-Yoseph G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature. 1998; 391(6669):775–778. | |
Porath D, Bezryadin A, De Vries S, Dekker C. Direct measurement of electrical transport through DNA molecules. Nature. 2000;403(6770):635–638. | |
Fink HW, Schönenberger C. Electrical conduction through DNA molecules. Nature. 1999;398(6726):407–410. | |
Okahata Y, Kobayashi T, Tanaka K, Shimomura M. Anisotropic electric conductivity in an aligned DNA cast film. J Am Chem Soc. 1998;120(24):6165–6166. | |
Kasumov AY, Kociak M, Gueron S, et al. Proximity-induced superconductivity in DNA. Science. 2001;291(5502):280–282. | |
Nitzan A, Ratner MA. Electron transport in molecular wire junctions. Science. 2003;300(5624):1384–1389. | |
Tao N. Electron transport in molecular junctions. Nature Nanotechnol. 2006;1(3):173–181. | |
He J, Sankey O, Lee M, Tao N, Li X, Lindsay S. Measuring single molecule conductance with break junctions. Faraday Discuss. 2006;131:145–154. | |
Tomfohr J, Sankey OF. Theoretical analysis of electron transport through organic molecules. J Chem Phys. 2004;120(3):1542–1554. | |
Cui X, Primak A, Zarate X, et al. Reproducible measurement of single-molecule conductivity. Science. 2001;294(5542):571–574. | |
Haiss W, Nichols RJ, van Zalinge H, Higgins SJ, Bethell D, Schiffrin DJ. Measurement of single molecule conductivity using the spontaneous formation of molecular wires. Phys Chem Chem Phys. 2004;6(17):4330–4337. | |
Kushmerick JG. Metal-molecule contacts. Mater Today. 2005;8(7):26–30. | |
Salomon A, Cahen D, Lindsay S, Tomfohr J, Engelkes VB, Frisbie CD. Comparison of electronic transport measurements on organic molecules. Adv Mater. 2003;15(22):1881–1890. | |
Yoshizawa K. An orbital rule for electron transport in molecules. Acc Chem Res. 2012;45(9):1612–1621. | |
Huang Z, Xu B, Chen Y, Ventra MD, Tao N. Measurement of current-induced local heating in a single molecule junction. Nano Lett. 2006;6(6):1240–1244. | |
Chen YC, Di Ventra M. Effect of electron-phonon scattering on shot noise in nanoscale junctions. Phys Rev Lett. 2005;95(16):166802. | |
Segal D, Nitzan A. Heating in current carrying molecular junctions. J Chem Phys. 2002;117(8):3915–3927. | |
Marszalek PE, Greenleaf WJ, Li H, Oberhauser AF, Fernandez JM. Atomic force microscopy captures quantized plastic deformation in gold nanowires. Proc Natl Acad Sci U S A. 2000;97(12):6282–6286. | |
Büttiker M, Imry Y, Landauer R, Pinhas S. Generalized many-channel conductance formula with application to small rings. Phys Rev B Condens Matter. 1985;31(10):6207–6215. | |
Dubi Y. The effect of fluctuations, thermal and otherwise, on the temperature dependence of thermopower in aromatic chain single-molecule junctions. J Chem Phys. 2013;138(11):114706. | |
Xu B, Xiao X, Yang X, Zang L, Tao N. Large gate modulation in the current of a room temperature single molecule transistor. J Am Chem Soc. 2005;127(8):2386–2387. | |
Lagos MJ, Autreto PAS, Galvao DS, Ugarte D. Correlation between quantum conductance and atomic arrangement of atomic-size silver nanowires. J Appl Phys. 2012;111(12):124316–124316. | |
Pérez-Jiménez AJ. Uncovering transport properties of 4,4′-bipyridine/gold molecular nanobridges. J Phys Chem B. 2005;109(20):10052–10060. | |
Dhungana KB, Mandal S, Pati R. Switching of conductance in a molecular wire: role of junction geometry, interfacial distance, and conformational change. J Phys Chem C Nanomater Interfaces. 2012;116(32):17268–17273. | |
Hong W, Manrique DZ, Moreno-García P, et al. Single molecular conductance of tolanes: experimental and theoretical study on the junction evolution dependent on the anchoring group. J Am Chem Soc. 2012;134(4):2292–2304. | |
Zhang GP, Hu GC, Song Y, Li ZL, Wang CK. Modulation of rectification in diblock co-oligomer diodes by adjusting anchoring groups for both symmetric and asymmetric electrodes. J Phys Chem C Nanomater Interfaces. 2012;116(41):22009–22014. | |
Aradhya SV, Frei M, Hybertsen MS, Venkataraman L. Van der Waals interactions at metal/organic interfaces at the single-molecule level. Nat Mater. 2012;11(10):872–876. | |
Lagos MJ, Sato F, Galvão DS, Ugarte D. Mechanical deformation of nanoscale metal rods: when size and shape matter. Phys Rev Lett. 2011;106(5):055501. | |
Rodrigues V, Ugarte D. Real-time imaging of atomistic process in one-atom-thick metal junctions. Phys Rev B Condens Matter Mater Phys. 2001;63(7):073405. | |
Tavazza F, Barzilai S, Smith DT, Levine LE. The increase in conductance of a gold single atom chain during elastic elongation. J Appl Phys. 2013;113(5):54316–54316. | |
Thiess A, Mokrousov Y, Blügel S, Heinze S. Theory and application of chain formation in break junctions. Nano Lett. 2008;8(8):2144–2149. | |
Vélez P, Dassie SA, Leiva EP. Role of metal contacts in the mechanical properties of molecular nanojunctions: comparative ab initio study of Au/1,8-octanedithiol and Au/4,4-bipyridine. Phys Rev B Condens Matter Mater Phys. 2010;81(23):235435. | |
Kamenetska M, Koentopp M, Whalley AC, et al. Formation and evolution of single-molecule junctions. Phys Rev Lett. 2009;102(12):126803. | |
Pérez-Jiménez ÁJ, Sancho-García JC, Pérez-Jordá JM. Torsional potential of 4,4′-bipyridine: ab initio analysis of dispersion and vibrational effects. J Chem Phys. 2005;123(13):134309. | |
del Valle M, Gutiérrez R, Tejedor C, Cuniberti G. Tuning the conductance of a molecular switch. Nat Nanotechnol. 2007;2(3):176–179. | |
Zhang C, He Y, Cheng HP, et al. Current-voltage characteristics through a single light-sensitive molecule. Phys Rev B Condens Matter Mater Phys. 2006;73(12):125445. | |
Tsutsui M, Taniguchi M. Single molecule electronics and devices. Sensors. 2012;12(6):7259–7298. | |
Chen F, Nuckolls C, Lindsay S. In situ measurements of oligoaniline conductance: linking electrochemistry and molecular electronics. Chem Phys. 2006;324(1):236–243. | |
Gittins DI, Bethell D, Schiffrin DJ, Nichols RJ. A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature. 2000;408(6808):67–69. | |
Haiss W, Zalinge H van, Higgins SJ, et al. Redox state dependence of single molecule conductivity. J Am Chem Soc. 2003;125(50):15294–15295. | |
Tao NJ. Probing potential-tuned resonant tunneling through redox molecules with scanning tunneling microscopy. Phys Rev Lett. 1996;76(21):4066–4069. | |
Xu B, He H, Tao NJ. Controlling the conductance of atomically thin metal wires with electrochemical potential. J Am Chem Soc. 2002;124(45):13568–13575. | |
Li X, Xu B, Xiao X, Yang X, Zang L, Tao N. Controlling charge transport in single molecules using electrochemical gate. Faraday Discuss. 2006;131(0):111–120. | |
Xiao X, Nagahara LA, Rawlett AM, Tao N. Electrochemical gate-controlled conductance of single oligo(phenylene ethynylene)s. J Am Chem Soc. 2005;127(25):9235–9240. | |
Chen F, He J, Nuckolls C, Roberts T, Klare JE, Lindsay S. A molecular switch based on potential-induced changes of oxidation state. Nano Lett. 2005;5(3):503–506. | |
Xiao X, Brune D, He J, Lindsay S, Gorman CB, Tao N. Redox-gated electron transport in electrically wired ferrocene molecules. Chem Phys. 2006;326(1):138–143. | |
Beebe J, Kim B, Gadzuk J, Frisbie CD, Kushmerick J. Transition from direct tunneling to field emission in metal-molecule-metal junctions. Phys Rev Lett. 2006;97(2):026801. | |
Bâldea I. Transition voltage spectroscopy reveals significant solvent effects on molecular transport and settles an important issue in bipyridine-based junctions. Nanoscale. 2013;5(19):9222–9230. | |
Quek SY, Kamenetska M, Steigerwald ML, et al. Mechanically controlled binary conductance switching of a single-molecule junction. Nat Nanotechnol. 2009;4(4):230–234. | |
Song H, Kim Y, Jang YH, Jeong H, Reed MA, Lee T. Observation of molecular orbital gating. Nature. 2009;462(7276):1039–1043. | |
Ho Choi S, Kim B, Frisbie CD. Electrical resistance of long conjugated molecular wires. Science. 2008;320(5882):1482–1486. | |
Guo S, Hihath J, Díez-Pérez I, Tao N. Measurement and statistical analysis of single-molecule current-voltage characteristics, transition voltage spectroscopy, and tunneling barrier height. J Am Chem Soc. 2011;133(47):19189–19197. | |
Chiu PW, Roth S. Transition from direct tunneling to field emission in carbon nanotube intramolecular junctions. Appl Phys Lett. 2008;92(4):042107. | |
Lennartz MC, Atodiresei N, Caciuc V, Karthäuser S. Identifying molecular orbital energies by distance-dependent transition voltage spectroscopy. J Phys Chem C Nanomater Interfaces. 2011;115(30):15025–15030. | |
Artés JM, López-Martínez M, Giraudet A, Díez-Pérez I, Sanz F, Gorostiza P. Current-voltage characteristics and transition voltage spectroscopy of individual redox proteins. J Am Chem Soc. 2012;134(50):20218–20221. | |
Bâldea I. Important insight into electron transfer in single-molecule junctions based on redox metalloproteins from transition voltage spectroscopy. J Phys Chem C Nanomater Interfaces. 2013;117(48):25798–25804. | |
Dubi Y. Dynamical coupling and negative differential resistance from interactions across the molecule-electrode interface in molecular junctions. J Chem Phys. 2013;139(15):154710. | |
Pal PP, Pati R. Charge Transport in strongly coupled molecular junctions: “in-phase” and “out-of-phase” contribution to electron tunneling. J Phys Chem C Nanomater Interfaces. 2011;115(35):17564–17573. | |
Díez-Pérez I, Hihath J, Lee Y, et al. Rectification and stability of a single molecular diode with controlled orientation. Nat Chem. 2009;1(8):635–641. | |
Saraiva-Souza A, Souza FM de, Aleixo VF, et al. A single molecule rectifier with strong push-pull coupling. J Chem Phys. 2008;129(20):204701. | |
Zhao J, Yu C, Wang N, Liu H. Molecular rectification based on asymmetrical molecule-electrode contact. J Phys Chem C Nanomater Interfaces. 2010;114(9):4135–4141. | |
Tsuji Y, Yoshizawa K. Current rectification through π-π stacking in multilayered donor-acceptor cyclophanes. J Phys Chem C Nanomater Interfaces. 2012;116(50):26625–26635. | |
Pati R, McClain M, Bandyopadhyay A. Origin of negative differential resistance in a strongly coupled single molecule-metal junction device. Phys Rev Lett. 2008;100(24):246801. | |
Bürkle M, Viljas JK, Vonlanthen D, et al. Conduction mechanisms in biphenyl dithiol single-molecule junctions. Phys Rev B Condens Matter Mater Phys. 2012;85(7):075417. | |
Migliore A, Nitzan A. Nonlinear charge transport in redox molecular junctions: a Marcus perspective. ACS Nano. 2011;5(8):6669–6685. | |
Mishchenko A, Vonlanthen D, Meded V, et al. Influence of conformation on conductance of biphenyl-dithiol single-molecule contacts. Nano Lett. 2010;10(1):156–163. | |
Zhou J, Samanta S, Guo C, Locklin J, Xu B. Measurements of contact specific low-bias negative differential resistance of single metalorganic molecular junctions. Nanoscale. 2013;5(13):5715–5719. | |
Doi K, Tsutsui M, Ohshiro T, et al. Nonequilibrium ionic response of biased mechanically controllable break junction (MCBJ) electrodes. J Phys Chem C Nanomater Interfaces. 2014;118(7):3758–3765. | |
Barnett RN, Cleveland CL, Joy A, Landman U, Schuster GB. Charge migration in DNA: ion-gated transport. Science. 2001;294(5542):567–571. | |
Genereux JC, Barton JK. Mechanisms for DNA charge transport. Chem Rev. 2010;110(3):1642–1662. | |
Soler JM, Artacho E, Gale JD, et al. The SIESTA method for ab initio order-N materials simulation. J Phys Condens Matter. 2002;14(11):2745. | |
Zakjevskii VV, King SJ, Dolgounitcheva O, Zakrzewski VG, Ortiz JV. Base and phosphate electron detachment energies of deoxyribonucleotide anions. J Am Chem Soc. 2006;128(41):13350–13351. | |
Tsutsui M, Taniguchi M, Kawai T. Local Heating in metal-molecule-metal junctions. Nano Lett. 2008;8(10):3293–3297. | |
Nowak AM, McCreery RL. In situ Raman spectroscopy of bias-induced structural changes in nitroazobenzene molecular electronic junctions. J Am Chem Soc. 2004;126(50):16621–16631. | |
Tian J-H, Liu B, Li, et al. Study of molecular junctions with a combined surface-enhanced Raman and mechanically controllable break junction method. J Am Chem Soc. 2006;128(46):14748–14749. | |
Ward DR, Halas NJ, Ciszek JW, et al. Simultaneous measurements of electronic conduction and Raman response in molecular junctions. Nano Lett. 2008;8(3):919–924. | |
Ward DR, Scott GD, Keane ZK, Halas NJ, Natelson D. Electronic and optical properties of electromigrated molecular junctions. J Phys Condens Matter. 2008;20(37):374118. | |
Calvo MR, Fernandez-Rossier J, Palacios JJ, Jacob D, Natelson D, Untiedt C. The Kondo effect in ferromagnetic atomic contacts. Nature. 2009;458(7242):1150–1153. | |
Parks JJ, Champagne AR, Hutchison GR, Flores-Torres S, Abruña HD, Ralph DC. Tuning the Kondo effect with a mechanically controllable break junction. Phys Rev Lett. 2007;99(2):026601. | |
Zhou J, Chen F, Xu B. Fabrication and electronic characterization of single molecular junction devices: a comprehensive approach. J Am Chem Soc. 2009;131(30):10439–10446. | |
Xu BQ, Li XL, Xiao XY, Sakaguchi H, Tao NJ. Electromechanical and conductance switching properties of single oligothiophene molecules. Nano Lett. 2005;5(7):1491–1495. | |
Aradhya SV, Frei M, Halbritter A, Venkataraman L. Correlating structure, conductance, and mechanics of silver atomic-scale contacts. ACS Nano. 2013;7(4):3706–3712. | |
Frei M, Aradhya SV, Hybertsen MS, Venkataraman L. Linker dependent bond rupture force measurements in single-molecule junctions. J Am Chem Soc. 2012;134(9):4003–4006. | |
Makk P, Tomaszewski D, Martinek J, et al. Correlation analysis of atomic and single-molecule junction conductance. ACS Nano. 2012;6(4):3411–3423. | |
Natelson D. Mechanical break junctions: enormous information in a nanoscale package. ACS Nano. 2012;6(4):2871–2876. | |
Mallajosyula SS, Pati SK. Toward DNA conductivity: a theoretical perspective. J Phys Chem Lett. 2010;1(12):1881–1894. | |
Fang L, Liu J, Coulter S, et al. Formation of π-conjugated molecular arrays on silicon (001) surfaces by heteroatomic Diels-Alder chemistry. Surf Sci. 2002;514(1):362–375. | |
McCreery RL, Wu J, Kalakodimi RP. Electron transport and redox reactions in carbon-based molecular electronic junctions. Phys Chem Chem Phys. 2006;8(22):2572–2590. | |
Piva PG, DiLabio GA, Pitters JL, et al. Field regulation of single-molecule conductivity by a charged surface atom. Nature. 2005;435(7042):658–661. | |
Lindsay S. Molecular wires and devices: advances and issues. Faraday Discuss. 2006;131:403–409. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.