Back to Journals » Clinical Interventions in Aging » Volume 15
Characteristics and Treatments of Patients Aged 65 Years or Over with Cervical Cancer
Authors Xie S, Pan S, Zou S, Zhu H, Zhu X
Received 24 March 2020
Accepted for publication 11 May 2020
Published 3 June 2020 Volume 2020:15 Pages 841—851
DOI https://doi.org/10.2147/CIA.S255305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Shangdan Xie,1 Shuya Pan,1 Shuangwei Zou,1 Haiyan Zhu,2 Xueqiong Zhu1
1Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Haiyan Zhu
Department of Gynecology, Shanghai First Maternity and Infant Hospital, No. 2699 Gaoke West Road, Shanghai, Pudong 200126 People’s Republic of China
Tel/ Fax +86 13758465255
Email [email protected]
Xueqiong Zhu
Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Wenzhou Medical University, No. 109 Xueyuan Xi Road, Zhejiang, Wenzhou 325027, People’s Republic of China
Tel/ Fax +86 577 88002796
Email [email protected]
Purpose: Although the mortality of elderly women with cervical cancer is high, their characteristics and prognosis have not attracted sufficient attention. This study aims to clarify the prognostic factors of cervical cancer patients aged ≥ 65.
Patient and Methods: The incidences and characteristics of patients diagnosed with cervical cancer (aged ≥ 65 and < 65) during 2004– 2015 were obtained through the Surveillance, Epidemiology, and End Results Program (SEER) database. The differences of distributions of characteristics between two age groups were compared by chi-squared (χ2) test. Kaplan–Meier survival method, Log-rank test, Cox-regression and visual nomogram were utilized for survival analysis.
Results: The annual incidences of two age groups with cervical cancer were (5.5– 7.5)/100,000 and (3.4– 3.9)/100,000, respectively, during 2004– 2015. The 1-year and 5-year cancer-specific survival rates of old patients were both lower than those of young patients (P < 0.001). The proportions of unmarried state and advanced International Federation of Gynecology and Obstetrics (FIGO) stage in old patients were higher than those in relatively young patients, and fewer elderly patients received surgery. Univariate and multivariate survival analysis showed non-squamous cell carcinoma, poor differentiation and late FIGO stage were independent poor prognostic factors for patients aged ≥ 65. Treatments improved the outcomes of elderly patients, and the effect of surgery was better than non-surgical treatment on elderly patients with FIGO I. Besides, geriatric score and survival probability could be accomplished by our nomogram with a c-index of 0.7945.
Conclusion: Delayed diagnosis and insufficient treatment were two distinct features of elderly patients and correlated with their poor clinical outcomes. More attention and active treatments should be adopted in elderly women based on their general condition.
Keywords: cervical cancer, elderly, database analysis, prognosis
Introduction
Cervical cancer is the fourth most common malignancy and threatens the health of women worldwide.1 There are approximate 570,000 new cases and 310,000 dead cases every year. At present, various biomarkers, such as p16l4a and microRNAs, were found to help the diagnosis and treatment of patients.2,3 Widespread human papilloma virus (HPV) vaccination inoculation decreases the number of young patients with cervical cancer, while the incidence and mortality of elderly patients are not significantly reduced with the advent of an aging society.4,5 A second incidence peak of cervical cancer appears between 60 and 70 years old,6 although the average age of the patients at diagnosis is about 53 years old.7
In fact, cervical cancer that occurs in elderly patients over 65 has received an increasing attention in recent years, with a proportion of approaching 25% and a dismal 5-year survival rate of 40.8% reported by epidemiologic studies.8,9 Older women with cervical cancer tend to be weaker and have multiple comorbidities such as diabetes or cardiac disease, which lead to rapid deterioration of the disease.10 Therapeutic dilemmas in the elderly are due to the underrepresentation of these patients aged ≥65 in clinical trials.11 Therefore, the treatment pattern of elderly patients is relatively conservative, which leads to a worse survival outcome compared with younger patients.12 However, the prognostic factors that are able to predict the survival of cervical cancer patients who are over 65 have not been investigated extensively. It’s imperative to acquaint the factors which influence the prognosis of elderly women diagnosed with cervical cancer.
This study comprehensively compared the epidemiological characteristics for cervical cancer between patient population ages ≥65 years and <65 years. Furthermore, the promising prognostic factors in the survival of older patients were analyzed. In addition, the impacts of various treatments on the survival of patients aged ≥65 years were explored.
Patients and Methods
Data Source
The Surveillance, Epidemiology, and End Results Program (SEER) online database was employed to obtain cancer statistic data from regional cancer registries for further analyses. SEER*Stat 8.3.6 was used to extract dataset from SEER 18 Regs Custom Data (with additional treatment fields). Since the data were de-identified, it was exempt from review by the local institutional review board and informed consent by patients.
Study Population
All cervical cancer patients aged 65 or over and below 65 diagnosed during 2004–2015 were included in our study. Using the SEER*Stat statistical software for Windows, we examined the incidence for cases diagnosed from 2004 to 2015 and its change over time. For analyzing characteristics of cervical cancer women, patients with missing information on International Federation of Gynecology and Obstetrics (FIGO) stage (according to the 2018 FIGO staging system), therapy or survival time were excluded. The flow chart of the patient selection process is shown in Figure 1.
 |
Figure 1 Patient selection flowchart. |
The following data were extracted for each case: patient ID, year and age at the time of diagnosis, race, marital status, histology, grade, FIGO stage, surgery, reasons of no cancer-directed surgery, radiotherapy, chemotherapy, survival months, cause-specific death classification and vital status.
Statistical Analysis
The incidence of patients aged ≥65 and <65 was compared by chi-squared (χ2) test. The differences of distributions of various characteristics between two age groups were compared by chi-squared (χ2) test. Kaplan–Meier method was utilized to display survival curves for age, race, marital status, histology, grade, FIGO stage, surgery, radiotherapy and chemotherapy as well as to estimate the average survival months of different treatment groups stratified by FIGO stage. The Log-rank test was conducted to compare the differences of various survival curves. Cox proportional hazards model was applied to perform univariate and multivariate analysis for the relationship between prognostic factors and cancer-specific survival. P values were two sided and P <0.05 were considered as a significant difference. Furthermore, employing the rms package in R version 3.6.2 (http://www.r-project.org/), a prognostic nomogram was generated according to the outcomes of multivariable analysis. Harrel’s concordance index (C-index) and calibration curve were used to measure the performance of this nomogram. All other data analysis was completed by SPSS 23 software (IBM Corp., USA).
Results
Incidence and Survival Rate
As shown in Figure 2A and Supplementary Table 1, there was an annual incidence of patients aged ≥65 and <65 with cervical cancer during 2004–2015. Overall, the incidence of cervical cancer in patients aged 65 or over was significantly higher than that of young patients (P <0.001). From the point of view of the trend, the incidence of cervical cancer in elderly women decreased slightly with the increase of year, but was in the rage of (5.5~7.5)/100,000. The incidence of younger women remained stable in the range of (3.4~3.9)/100,000.
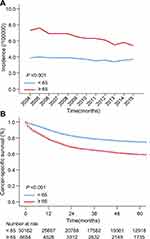 |
Figure 2 (A) The incidence of cervical cancer patients aged 65 or over and below 65; (B) the cancer-specific survival of cervical cancer patients aged 65 or over and below 65. |
The cancer-specific survival curves of cervical cancer patients in two age groups are shown in Figure 2B. The estimated 1-year survival rates of patients aged ≥65 and <65 were 78.50% and 90.25%, respectively (P <0.001), the estimated 5-year survival rates of two groups were 59.38% and 75.02% (P <0.001), respectively. Taken together, old patients showed worse cancer-specific survival compared with relatively young patients.
Patient Characteristics
A total of 36,816 patients met the inclusion criteria for analyzing characteristics, 6654 of them were 65 years old or over and 30,162 of them were under 65 years old. The demographic data, tumor characteristics and treatments of patients are shown in Table 1. In terms of demographic characteristics, both patients aged ≥65 and <65 with cervical cancer were white race in predominance, while the proportion of unmarried women (64.25%) in the elderly group was significantly higher than that in a relatively young group (49.28%, P <0.001).
 |
Table 1 Patient Demographic, Clinical, and Tumor Characteristics |
In tumor characteristics, squamous cell carcinoma, Grade II and III, and negative lymph node were prevalent in both two age groups. According to FIGO, the proportions of FIGO II, III and IV in elderly patients were significantly higher than those in relatively younger patients (P <0.001). With regard to treatments, patients aged ≥65 tended to refuse surgery (P <0.001) but adopt radiotherapy more (P <0.001) compared with patients under 65. There was no statistical difference in the distributions of chemotherapy between the two groups (P =0.741).
Univariate and Multivariate Survival Analysis
In order to analyze the prognosis of elderly patients, all characteristics were analyzed by Kaplan–Meier method as shown in Figure 3. The results demonstrated that the patients with non-black race, married state, low grade, early FIGO stage, negative lymph node and receiving surgery had better cancer-specific survival time (P <0.001). However, there existed a crossed phenomenon in the survival curves of patients who received chemotherapy and did not receive chemotherapy, which also appeared in the survival curves of patients who received radiotherapy and did not receive radiotherapy and suggested there might be interference among the various elements.
As shown in Table 2, univariable and multivariable COX regression was utilized to determine the factors affecting the prognosis of elderly patients. The results of univariate analysis showed that black race, unmarried state, non-squamous cell carcinoma, poor differentiation, late FIGO stage and lymph node metastasis were associated with elevated death risks in elderly patients with cervical cancer. Multivariate analysis further supported that non-squamous cell carcinoma, poor differentiation and late FIGO stage were independent poor prognostic factors in elderly patients with cervical cancer. Besides, patients receiving surgery or radiotherapy or chemotherapy had better outcomes than patients refusing corresponding therapy, the HRs were 0.342 (95% CI: 0.296–0.396, P <0.001), 0.595 (95% CI: 0.519–0.683, P <0.001) and 0.507 (95% CI: 0.450–0.571, P <0.001), respectively. As shown in Supplementary Table 2, the results of multiple analysis demonstrated that race, marital status, histology, grade, FIGO stage, surgery and chemotherapy were independent prognostic factors in cervical cancer patients aged <65.
 |
Table 2 Univariable and Multivariable COX Regression for Elderly Patients with Cervical Cancer |
The Different Impacts of Various Treatments on Prognosis of Elderly Patients Stratified by FIGO
According to the above-mentioned clinical characteristics of cervical cancer women aged ≥65, the proportions of receiving surgery, radiotherapy and chemotherapy in elderly patients existed significant difference, thereby the correlation between treatment strategy and prognosis among old cervical cancer patients was evaluated in this study. In early-stage (FIGO I–II) group, cervical cancer patients were divided into three groups: surgical treatment (surgery with or without chemotherapy or radiotherapy, 1513 patients in FIGO I, 365 patients in FIGO II), non-surgical treatment (chemoradiotherapy or chemotherapy or radiotherapy alone, 459 patients in FIGO I, 916 patients in FIGO II) and no treatment (180 patients in FIGO I, 82 patients in FIGO II). In late–stage (FIGO III–IV) group, since few patients performed surgery, those patients were classified into two groups: treatment (1452 patients in FIGO III and 1078 patients in FIGO IV) and no treatment (173 patients in FIGO III and 436 patients in FIGO IV). As shown in Supplementary Table 3, elderly cervical cancer patients with each FIGO stage underwent insufficient treatment compared with the corresponding patients aged <65.
In FIGO I group, cancer-specific survival of patients receiving surgical treatment was superior compared with that of patients adopting non-surgical treatment (P <0.001) as shown in Table 3. In the FIGO II group, there was no difference in cancer-specific survival between groups of surgical treatment and non-surgical treatment (P =0.137). In late–stage (FIGO III–IV) group, as shown in Table 3, cervical cancer women receiving treatments showed significantly favorable prognosis than those receiving no treatment (P <0.001), suggesting treatments improved prognosis of elderly patients with advanced cervical cancer.
 |
Table 3 Effect of Various Treatments on Cancer-Specific Survival Time of Elderly Patients with Various FIGO Stages |
Nomogram
As shown in Figure 4, a nomogram for forecasting the cancer-specific survival rate of elderly patients with cervical cancer was established and illustrated according to the Cox regression results. The C-index of the nomogram was 0.7945 (95% CI, 0.7827–0.8063) in the internal validation set. The calibration curve for the 1-,3- and 5-year CSS indicated favorable concordance (Figure 5). For instance, an elderly cervical cancer patient, was a white and married female, had grade III squamous cell carcinoma with FIGO stage III and negative regional lymph node, and underwent radiotherapy and chemotherapy but not surgery. The total points of the patient were 134, and the 1-, 3- and 5-year CSS probability was about 80%, 60% and 50%, respectively.
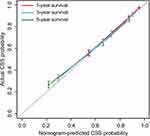 |
Figure 5 The calibration curves of 1-, 3- and 5-year CSS for cervical cancer patients aged ≥65. Abbreviation: CSS, cancer-specific survival. |
Discussion
The incidence of cervical cancer in women aged ≥65 was significantly elevated compared with that in relatively young women aged <65. Interestingly, the majority of elderly cervical cancer patients occurred in white and unmarried women with squamous cell carcinoma.
Cervical cancer patients aged ≥65 had conspicuously worse prognosis compared with those aged <65. Non-squamous cell carcinoma, poor differentiation and late FIGO stage were independent poor prognostic factors for elderly patients with cervical cancer. Radiotherapy and chemotherapy did not improve the prognosis of patients aged <65 in univariable analysis, which might be due to the bias by completely different treatment patterns in different FIGO stages. In the subsequently multivariable analysis, chemotherapy was an independent prognostic factor of cervical cancer patients aged <65. By further analyzing the clinical characteristics and treatment of elderly patients, the result demonstrated that patients aged ≥65 tended to be diagnosed with advanced stage disease, the proportion of non-treatment in them was relatively high and a few of them adopted surgery actively as main treatment. Thus, we hypothesized that a conservative treatment strategy and advanced diagnosis might be a possible explanation for poor prognosis among elderly cervical cancer patients. Berraho et al13 reported that unmarried patients presented an increased risk of late diagnosis compared with married patients. In our study, unmarried women account for the bulk of old patients with cervical cancer, which indicated that patients aged ≥65 were prone to a lack of partners. Inapparent early clinical symptoms partly resulting from decreased sex life brought out insufficient attention paid by elderly patients, who possibly sought medical advice after massive hemorrhage. Besides, the inadequate early cervical screening in patients aged ≥65 possibly delayed the diagnosis and deteriorate the prognosis.14,15 Regular cervical screening in patients above 65 years old helps reduce the incidence of cervical cancer by 75% and the mortality of the disease.16 Therefore, deficiency of typical clinical features and cervical screening might contribute to late diagnosis.
Age played an important role in allocating treatments, and poor prognosis of elderly patients mainly accounted for advanced stage and treatment allocation instead of age.10,17,18 Shen et al19 demonstrated that the proportion of delayed treatments in patients aged ≥65 was significantly higher than that in patients of other ages, especially in patients over 75 years old, the proportion of delayed treatments was as high as 9.91%. Cervical cancer patients whose treatments were delayed presented worse prognosis than those with timely treatments. However, the effect of adjuvant therapy on the prognosis of elderly patients was still controversy. Albert et al20 found that older patients with cervical cancer received less standard treatments and lower overall survival compared with younger patients. Adopting a conservative treatment may be concerned about the unbearable side effects on the elderly. Wang et al suggested that older women with cervical cancer tended to have higher radiation-related proctitis than younger patients.21 A previous study demonstrated that elderly patients over 70 years old presented more chronic gastrointestinal toxicities than those under 60 years old after receiving radiotherapy or concurrent chemoradiotherapy.22 Yanazume et al23 showed that intracavitary brachytherapy accelerated the mortality of elderly patients over 70 years old due to its incomplete treatment. However, multiple studies found that adjuvant therapies were well tolerated in elderly patients24 and extended their lives.25–28 It’s even safe and effective to apply radiation therapy to cervical cancer patients aged ≥80.27 Brachytherapy or concurrent chemoradiotherapy for most older patients improved their prognosis and represented a standard clinical choice.29,30 Xiang et al26 suggested that cervical cancer women aged ≥65 benefited much from cisplatin with definitive radiotherapy. In our study, radiotherapy and chemotherapy were both independent prognostic factors of elderly patients. As a result, adjuvant therapy should be applied more to patients aged ≥65 since the number of old patients receiving chemotherapy or radiotherapy was still small. As for surgery, its impact on the prognosis of elderly women presents controversy. Elderly patients were less likely to undergo surgery, over 80% of patients who did not receive surgery result from no recommendation. First, surgery is not the standard treatment for cervical cancer patients with FIGO IIB-IV,4 while older patients are more likely to present advanced FIGO stage than younger patients. Besides, the purpose of cure also dropped with increasing age, some elderly patients even forego therapy.31 In addition, Radical hysterectomy may increase perioperative morbidity and mortality of older patients.32 A recent study found that non-infectious complication was more likely to occur in cervical cancer patients aged ≥60 compared with relatively young patients after undergoing minimally invasive surgery or laparotomy.33 However, Elisabeth et al12 suggested that undergoing surgery was connected tightly with high overall survival of patients aged ≥65 years old. Laparoscopic and open radical hysterectomy were tolerated well in Cervical cancer women aged ≥65 with stage IA2-IIA2.34 In our study, surgery was an independent prognostic element of elderly patients. For elderly patients with early FIGO stage (FIGO I and II), non-surgical treatment and surgical treatment both improved the prognosis of patients; in patients with earlier FIGO stage (FIGO I), surgery was better than non-surgical treatment. For patients with advanced FIGO stage (FIGO III–IV), the prognosis of patients with treatment was significantly better than that of patients without treatment. Besides, new immunotherapy and hypoxic modifiers are new methods to decrease treatment-related complications.10 As a result, elderly patients could also benefit from standard treatment, in spite of the huge burden of comorbidities.17 Since they are more vulnerable and have lower life expectancy than younger counterparts, patients aged ≥65 years are supposed to be in individual management based on their general condition and background, should be encouraged more to raise life expectations and actively receive treatments.31 HPV vaccination use has decreased the incidence of cervical cancer in multiple developed countries and has been promoted in China since 2016. However, elderly women are no longer eligible for the HPV vaccine due to poor preventive outcomes. A multicentral study with large population and long follow-up period is still needed to clarify whether early administration of HPV vaccination can reduce the incidence of cervical cancer in women when they become older. Thus, cervical screening such as smear test is more essential and must be conducted as routine physical examination programs for elderly women.
So far, there have been multiple nomograms to evaluate the outcomes of cervical cancer patients,35,36 while few research has focused on scoring elderly patients with cervical cancer based on their characteristics and treatments. In our study, since the prognosis of old patients with cervical cancer was disappointing, an easy-to-use nomogram model was proposed to conduct geriatric scores, make an individual prophecy for their cancer-specific survival and assist clinicians to lay down a rational follow-up plan by including various survival factors.
There are some limitations to our research that should be interpreted. First, most of the women included in the SEER database are white, the proportion of other races is small. The number of included women with surgery remains minor and reduces the representativeness of the results. Besides, our nomogram is only verified internally but lack external verification. Last, we cannot collect the information from the SEER database about general condition, complications or living habits of elderly patients, which has a great influence on the treatment choice of patients and may cause our results to be biased.
In conclusion, elderly patients with cervical cancer remain a serious issue with high morbidity and poor prognosis. Delayed diagnosis and insufficient treatment are two distinct features of these patients and correlated with their poor clinical outcomes. Besides, our nomogram can predict 1-, 3- and 5-year cancer-specific survival rates based on the characteristics and treatments of elderly patients. Researches and physicians should pay more attention to elderly cervical cancer patients and some novel personalized therapies or preventive strategies should be developed in the future.
Acknowledgment
The study was supported by funds from Science and Technology Innovation Team of Wenzhou City-Gynecological Oncology (No. C20170004), Pudong Medical Union Fund (PDYLT201901-16) and grants from Science and Technology Planning Project of Wenzhou City (No. ZS2017006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Vitale SG, Valenti G, Rapisarda AMC, et al. P16INK4a as a progression/regression tumour marker in LSIL cervix lesions: our clinical experience. Eur J Gynaecol Oncol. 2016;37(5):685–688.
3. Gao C, Zhou C, Zhuang J, et al. MicroRNA expression in cervical cancer: novel diagnostic and prognostic biomarkers. J Cell Biochem. 2018;119(8):7080–7090. doi:10.1002/jcb.27029
4. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–182. doi:10.1016/S0140-6736(18)32470-X
5. Hammer A, Kahlert J, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002–2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98(8):1063–1069. doi:10.1111/aogs.13608
6. Kissel M, Rambeau A, Achkar S, Lecuru F, Mathevet P. Challenges and advances in cervix cancer treatment in elder women. Cancer Treat Rev. 2020;84:101976. doi:10.1016/j.ctrv.2020.101976
7. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203.
8. Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in massachusetts compared with younger women. J Low Genit Tract Dis. 2018;22(4):314–317. doi:10.1097/LGT.0000000000000435
9. Inoue S, Ito H, Hosono S, et al. Net survival of elderly patients with gynecological cancer aged over 75 years in 2006–2008. Asian Pac J Cancer Prev. 2019;20(2):437–442. doi:10.31557/APJCP.2019.20.2.437
10. Nogueira-Rodrigues A, de Melo AC, Garces AH, et al. Patterns of care and outcome of elderly women diagnosed with cervical cancer in the developing world. Int J Gynecol Cancer. 2016;26(7):1246–1251. doi:10.1097/IGC.0000000000000756
11. Venkatesulu BP, Mallick S, Rath GK. Patterns of care of cervical cancer in the elderly: a qualitative literature review. J Geriatr Oncol. 2017;8(2):108–116. doi:10.1016/j.jgo.2016.12.004
12. Diver EJ, Hinchcliff EM, Gockley AA, et al. Assessment of treatment factors and clinical outcomes in cervical cancer in older women compared to women under 65 years old. J Geriatr Oncol. 2018;9(5):516–519. doi:10.1016/j.jgo.2018.02.004
13. Berraho M, Obtel M, Bendahhou K, et al. Sociodemographic factors and delay in the diagnosis of cervical cancer in Morocco. Pan Afr Med J. 2012;12:14.
14. Hammer A, Soegaard V, Maimburg RD, Blaakaer J. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older-A national cohort study. Cancer Med. 2019;8(1):418–427. doi:10.1002/cam4.1926
15. Jorgensen SF, Njor SH. Higher cervical cancer mortality among older women in Denmark could be due to insufficient screening coverage. Acta Obstet Gynecol Scand. 2019;98(11):1489–1490. doi:10.1111/aogs.13685
16. Elit L. Role of cervical screening in older women. Maturitas. 2014;79(4):413–420. doi:10.1016/j.maturitas.2014.09.012
17. Shimamoto K, Saito T, Kitade S, et al. A study of treatments and outcomes in elderly women with cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2018;228:174–179. doi:10.1016/j.ejogrb.2018.06.032
18. Eggemann H, Ignatov T, Geyken CH, Seitz S, Ignatov A. Management of elderly women with cervical cancer. J Cancer Res Clin Oncol. 2018;144(5):961–967. doi:10.1007/s00432-018-2617-5
19. Shen SC, Hung YC, Kung PT, Yang WH, Wang YH, Tsai WC. Factors involved in the delay of treatment initiation for cervical cancer patients: a nationwide population-based study. Medicine (Baltimore). 2016;95(33):e4568. doi:10.1097/MD.0000000000004568
20. Albert A, Lee A, Allbright R, Vijayakumar S. Impact of age on receipt of curative treatment for cervical cancer: an analysis of patterns of care and survival in a large, national cohort. J Geriatr Oncol. 2019;10(3):465–474. doi:10.1016/j.jgo.2018.10.005
21. Wang YM, Wang CJ, Fang FM, et al. Differences in the outcomes and complications between elderly and younger uterine cervical cancer patients treated by definitive radiotherapy - A propensity score-matched study. Gynecol Oncol. 2017;145(2):277–283. doi:10.1016/j.ygyno.2017.02.034
22. Wang W, Liu X, Meng Q, Zhang F, Hu K. Comparisons of survivals and toxicities between young and elderly patients with cervical cancer treated with definitive radiotherapy or concurrent chemoradiotherapy. Taiwan J Obstet Gynecol. 2019;58(3):364–369. doi:10.1016/j.tjog.2018.08.036
23. Yanazume Y, Yanazume S, Iio K, et al. Major causes of impractical brachytherapy in elderly patients with uterine cervical cancer. J Obstet Gynaecol Res. 2014;40(6):1725–1732. doi:10.1111/jog.12387
24. Nosaka K, Shibata K, Utsumi F, et al. Feasibility and benefit of concurrent chemoradiotherapy for elderly patients with uterine cervical cancer. Tumori. 2016;102(6):600–605. doi:10.5301/tj.5000530
25. You KY, Peng HH, Jiang YH, Bi ZF, Qiu XS. Selective use of concurrent chemotherapy in elderly cervical cancer patients treated with definitive radiotherapy: experience from two institutions. Cancer Manag Res. 2019;11:4815–4823. doi:10.2147/CMAR.S190025
26. Xiang M, Kidd EA. Benefit of cisplatin with definitive radiotherapy in older women with cervical cancer. J Natl Compr Canc Netw. 2019;17(8):969–975. doi:10.6004/jnccn.2019.7289
27. Hata M, Koike I, Miyagi E, et al. Radiation therapy for very elderly patients aged 80 years and older with squamous cell carcinoma of the uterine cervix. Am J Clin Oncol. 2017;40(2):178–182. doi:10.1097/COC.0000000000000125
28. Hata M. Radiation therapy for elderly patients with uterine cervical cancer: feasibility of curative treatment. Int J Gynecol Cancer. 2019;29(3):622–629. doi:10.1136/ijgc-2018-000077
29. Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019;18(1):29–37. doi:10.1016/j.brachy.2018.08.016
30. Wang W, Hou X, Yan J, et al. Outcome and toxicity of radical radiotherapy or concurrent Chemoradiotherapy for elderly cervical cancer women. BMC Cancer. 2017;17(1):510. doi:10.1186/s12885-017-3503-2
31. Vitale SG, Capriglione S, Zito G, et al. Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet. 2019;299(2):299–315. doi:10.1007/s00404-018-5006-z
32. George EM, Tergas AI, Ananth CV, et al. Safety and tolerance of radical hysterectomy for cervical cancer in the elderly. Gynecol Oncol. 2014;134(1):36–41. doi:10.1016/j.ygyno.2014.04.010
33. Dos Reis R, Andrade C, Frumovitz M, Munsell M, Ramirez PT. Radical hysterectomy and age: outcomes comparison based on a minimally invasive vs an open approach. J Minim Invasive Gynecol. 2018;25(7):1224–1230. doi:10.1016/j.jmig.2018.03.002
34. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic versus open radical hysterectomy for elderly patients with early-stage cervical cancer. Am J Obstet Gynecol. 2012;207(3):195e191–198. doi:10.1016/j.ajog.2012.06.081
35. Wang C, Yang C, Wang W, et al. A prognostic nomogram for cervical cancer after surgery from seer database. J Cancer. 2018;9(21):3923–3928. doi:10.7150/jca.26220
36. Wang W, Liu X, Meng Q, Zhang F, Hu K. Nomogram for predicting para-aortic lymph node metastases in patients with cervical cancer. Arch Gynecol Obstet. 2018;298(2):381–388. doi:10.1007/s00404-018-4829-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


