Back to Journals » Drug Design, Development and Therapy » Volume 12
Can dexmedetomidine reduce atrial fibrillation after cardiac surgery? A systematic review and meta-analysis
Authors Zhu ZP , Zhou HM, Ni YJ, Wu C, Zhang CJ, Ling XY
Received 12 October 2017
Accepted for publication 26 January 2018
Published 12 March 2018 Volume 2018:12 Pages 521—531
DOI https://doi.org/10.2147/DDDT.S153834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Zhipeng Zhu,1 Hongmei Zhou,1 Yunjian Ni,1 Cheng Wu,1 Caijun Zhang,1 Xiaoyan Ling2
1Department of Anesthesiology, the Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China; 2Outpatient-Nursing Department, the Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
Purpose: Cardiac surgery patients always present with atrial fibrillation (AF) after admission to the intensive care unit, leading to high mortality and lengthy hospitalization. Dexmedetomidine (DEX) is a popular medication used for sedation in the intensive care unit; however, whether it can reduce AF needs to be analyzed.
Materials and methods: Three primary databases, Medline, Embase (Ovid SP) and the Cochrane Central Register of Controlled Trials (CENTRAL), were searched. All English language and randomized control designed clinical publications comparing DEX to control medicines for sedation after elective cardiac surgery were included. Two independent colleagues conducted the data extraction and quality assessments. The subgroup analysis was performed according to the medicine used, age, AF history, and whether previous beta-blocker premedication and cardiopulmonary bypass (CPB) were applied. The overall incidence of AF was analyzed.
Results: A total of 1,295 patients in nine studies met the selection criteria among 2,587 studies screened from the database. After quantitative synthesis, our results revealed that the DEX group was not associated with a decreased incidence of AF compared with the placebo (risk ratio [RR] 0.76, 95% CI 0.37, 1.55, P=0.44) and morphine groups (RR 0.86, 95% CI 0.56, 1.31, P=0.48). Subgroup analysis also indicated that the DEX vs propofol comparison exhibited no difference: 1) for patients of age >60 years (P=0.69) or ≤60 years (P=0.69); 2) under CPB surgery (P=0.45) or without CPB surgery (P=0.88); 3) with beta-blocker premedication (P=0.32) or without beta-blocker premedication (P=0.90); and 4) with AF history (RR 1.07, 95% CI 0.85, 1.36, P=0.57) or without AF history (P=0.30).
Conclusion: This meta-analysis revealed that DEX could not reduce the incidence of AF compared to control medicines following cardiac surgery. DEX may have an increased influence on AF occurrence if patients had a history of AF. However, cautious interpretation should be made due to high clinical heterogeneity.
Keywords: dexmedetomidine, sedation, cardiac surgery, atrial fibrillation
Introduction
Postoperative arrhythmias, which are included among various complications, are complications that can occur following cardiac surgery. Atrial tachyarrhythmia occurs most frequently and includes postoperative atrial fibrillation (POAF).1
It has also been reported that up to 40%–50% of patients will develop POAF during hospitalization among those undergoing cardiac surgery.2 The occurrence of atrial fibrillation (AF) not only prolongs hospitalization but also increases the cost.3 Furthermore, overcoming these arrhythmias remains a challenge due to ineffective antiarrhythmic therapies and significant adverse effects of existing drugs. The development of antiarrhythmic drugs has always been challenging and limited. Most importantly, some prophylactic therapy drugs are usually used, except for some conventional antiarrhythmic medicines and overdrive pacing, which include amiodarone, statin and colchicine.
Dexmedetomidine (DEX) is a highly selective α-2 receptor agonist that is currently applied safely and efficiently in perioperative cardiac surgery. Since its approval by the US Food and Drug Administration,4 DEX has been a popular medication for cardiac surgery patients. Based on some randomized controlled studies, DEX was demonstrated to provide safe and effective sedation,5,6 facilitate extubation,5 and reduce delirium7,8 and renal9 and myocardial injury.10 Some reviews also indicated that use of this drug was safe and efficient with post-cardiac surgery patients.11,12
Ettema et al thought that preadmission interventions was necessary to prevent postoperative complications via preadmission interventions for older cardiac surgery patients.13 Based on their theory, rate and rhythm control strategies are usually the focus for prevention of AF. As an assumed first-line sedation medicine,14 DEX seems to be a promising candidate for postoperative cardiac patients; however, most of the present studies have some limitations because of small numbers of patients or single-center study designs. Eventually, different points of view on the influence of DEX on AF occurrence emerged.5,7,15,16 Some randomized controlled trials (RCTs) and observation studies asserted that DEX could reduce the incidence of AF,15,17–19 but some others drew a higher incidence in the control group.5,7,16 Therefore, efforts must be taken to include enough data to draw a conclusion, and the lack of relevant reviews regarding this aspect is noteworthy.
Materials and methods
We searched the electronic database and performed a handy search to identify literatures that compared the incidence of AF between DEX and control drugs, including morphine, propofol and placebo. All procedures were based on the Cochrane Review Methods.
Search strategy
Two independent coworkers searched the Embase (Ovid SP), the Cochrane Central Register of Controlled Trials (CENTRAL) and Medline databases. All eligible articles written in English were chosen between 1966 and May 2017. The search strategy used the words “Dexmedetomidine”, “Adrenergic alpha-Agonists”, “Precedex”, “Thoracic Surgery”, “Cardiac Surgical Procedures”, “cardiac Surgery”, “Arrhythmias, Cardiac” and “Atrial Fibrillation”. Various combinations of free words were also used, and different search strategies were developed for each database. The deadline for the search was June 2017 with two new interesting reports were found for further review.
Eligible studies
All eligible studies had to meet the following criteria: 1) RCTs of valve replacement surgery or coronary artery bypass surgery with or without cardiopulmonary bypass (CPB) that compared DEX to control drugs; 2) sedation time <48 h in the intensive care unit (ICU) since admission, regardless of when the sedation began and 3) patients older than 18 years. Non-RCT studies, American Society of Anesthesiologists ≥IV and deep hypothermic circulatory arrest surgery were excluded from this study.
Data extraction and quality control
After retrieving the full articles, two independent coworkers conducted quality assessment using Cochrane Risk Bias Assessment Tool in Cochrane Handbook for Systematic Reviews of Interventions.20 The assessment included the following: random sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, allocation concealment, selective reporting and other biases. The risk of bias for each study was presented in the percentage form. The third operator intervened if a conflict occurred. The main outcome was the incidence of AF, and the GRADE system was applied to evaluate the quality of the grade.
Statistical analysis
Review Manager (version 5.3)21 was used to pool and analyze the eligible studies when studies reported the outcomes of interest. A random-effects model was applied throughout the whole analysis, and the Mantel–Haenszel method was used to calculate the risk ratio (RR) of each study. The fixed-effect model was used only in the absence of clinically and statistically significant heterogeneity (P>0.1, I2<50%). RR was chosen as the effect measure for dichotomous outcomes. Subgroup analysis based on medication, age, administration of beta-blocker medicine and coexisting AF and CPB was used ahead of the analysis. We used funnel plots to check for publication bias for the incidence of AF. Sensitivity analyses were also performed by removing low-quality and small sample size studies to confirm the stability of the results after the analyses. Following meta-analysis of all of the outcomes, a summary of the findings for each outcome was created with the GRADE system to evaluate the quality of evidence.
Results
Included studies
The study flow was based on the PRISMA criteria. Two thousand five hundred and eighty records were discovered in the electronic databases, which included Medline (104), Embase (449) and CENTRAL (2,027). The other seven studies were identified through the reference list. After checking for duplication with endnote X7, 2,427 records remained for screening. Of these, 2,141 records were removed due to the titles or abstracts being totally incompatible with our criteria. Two hundred and twenty-three records were excluded for not being pertinent to the subject matter. The remaining 63 full-text articles were retrieved from a database purchased by our own unit. Fifty-four articles were excluded due to various reasons. Finally, the remaining nine studies were included in the qualitative and quantitative analyses5–10,15–17 (Figure 1).
  | Figure 1 Study flow diagram. |
Basic characteristics of the studies
Nine studies were included in this review. The total number of patients was 1,295, and the included patients across all studies ranged from 11 to 152 (Table 1). The main characteristics included age, surgery type, patient number and DEX intervention (Table 1). All studies were screened with the RCTs criteria. There were seven studies (449 in the DEX group, 461 in the control group) that compared DEX to propofol,5–7,10,15–17 accounting for ~70% of the patients. One study compared DEX to morphine8 and placebo,9 respectively. Five studies had completed cardiac surgery without a CPB assistant,5,6,9,10,16 while four studies used a CPB assistant.7,8,15,17 Three studies adopted a bolus of ~0.4 μg/kg/h in volume when DEX was infused,6,7,16 and two of these studies infused DEX before admission to ICU.6,16 Intravenous infusion of DEX began primarily upon admission to ICU in six studies. Only one study adopted low infusion rate (0.04 μg/kg/h) without a bolus upon admission to ICU.9 Only one study reported the main outcome of AF.15
Risk of bias
The ROB tool of the Cochrane library was adopted to identify the risk of bias in the nine studies (Figures 2 and 3).
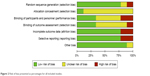  | Figure 2 Risk of bias presented as percentages for all included studies. |
  | Figure 3 Risk of bias: author’s judgment for all included studies. |
According to the Cochrane handbook, a high risk of bias accounted for <25% for most of the included studies (Figure 2). Based on a detailed evaluation of the single studies, we observed that four studies in this review could be considered as high quality because no high risk of bias was found.6–8,16 There was an “unknown risk” that came from some nonspecific details of randomization or insufficient information for confirmation. Three studies exhibited moderate quality due to incomplete data that caused disproportionate data loss.9,10,17 For example, one study had obvious high-risk bias because of the mentioned “respiratory function” indicators in the methods while providing no results in the later sections.9 The remaining two studies were classified as low quality because of multiple “high risk of bias” due to lack of randomization, attrition balance or intention-to-treat.5,15
Publication bias was visualized through funnel plots, and no significant publication bias was found (Figure 4).
  | Figure 4 Funnel plot of included studies for the incidence of AF. |
Summary of findings by the GRADE system
The main outcomes of AF were graded according to the GRADE system standard. Incomplete data and disproportionate data loss occurred in two studies, which equaled one level of risk of bias downgrade,5,15 while another level of downgrade was applied for imprecision bias due to a small number of studies with wide CIs (Table 2).
Outcomes analysis
After meta-analysis of the nine included studies, the incidence of AF was aggregated with 1,295 participants. The RR for AF in the DEX group was 0.76 (Mantel-Haenszel [M–H], random 95% CI 0.37, 1.55) compared to the placebo group (Figure 5) and 0.86 (M–H, random 95% CI 0.56, 1.31) compared to the morphine group (Figure 6). For the propofol group, which contained the majority of the participants, it was difficult to draw a conclusion because of the complex clinical heterogeneity before performing subgroup analysis.
  | Figure 5 Forest plot: the incidence of AF between DEX and placebo groups. |
  | Figure 6 Forest plot: the incidence of AF between DEX and morphine groups. |
Subgroup analysis and sensitivity analysis
Subgroup analysis also indicated that DEX vs the propofol subgroup had similar RR (RR 0.90, 95% CI 0.54, 1.51, I2=43%) for patients of age >60 years (Figure 7) and (RR 0.89, 95% CI 0.52, 1.54) ≤60 years (Figure 8); under CPB surgery (RR 0.81, 95% CI 0.47, 1.39, I2=69%; Figure 9) and without CPB surgery (RR 0.94, 95% CI 0.45, 1.97, I2=4%; Figure 10); for beta-blocker premedication (RR 0.72, 95% CI 0.37, 1.39, I2=66%; Figure 11) and without beta-blocker premedication (RR 0.97, 95% CI 0.57, 1.63, I2=0%; Figure 12); and for AF history (RR 1.07, 95% CI 0.85, 1.36, I2=0%; Figure 13) and without AF history (RR 0.67, 95% CI 0.32, 1.43, I2=33%; Figure 14).
  | Figure 7 Forest plot: the subgroup analysis for age >60 years. |
  | Figure 8 Forest plot: the subgroup analysis for age ≤60 years. |
  | Figure 9 Forest plot: the subgroup analysis for CPB surgery. |
  | Figure 10 Forest plot: the subgroup analysis without CPB surgery. |
  | Figure 11 Forest plot: the subgroup analysis for beta-blocker premedication. |
  | Figure 12 Forest plot: the subgroup analysis without beta-blocker premedication. |
  | Figure 13 Forest plot: the subgroup analysis with AF history. |
  | Figure 14 Forest plot: the subgroup analysis without AF history. |
Sensitivity analysis was carried out based on the high statistical heterogeneity for AF under CPB surgery (I2=69%; Figure 9) and beta-blocker premedication (I2=66%; Figure 11). After excluding the low-quality and small-sample studies, the heterogeneity disappeared, and similar results affirmed the incidence of AF with beta-blocker premedication (Figure 15) and CPB surgery (I2=0%; Figure 16), respectively.
  | Figure 15 Forest plot: the sensitivity analysis for beta-blocker premedication. |
  | Figure 16 Forest plot: the sensitivity analysis for CPB surgery. |
Discussion
With the popularity of DEX sedation for ICU patients, an increasing number of functions for this drug have been found; however, controversies exist that need to be solved. Our current meta-analysis with limited resources indicated that sedation with DEX following cardiac surgery not only could not reduce the incidence of AF compared with control drugs but may also promote the incidence of AF in patients with a history of AF.
Currently, the risk factors for postoperative arrhythmias have been proposed to include hypoxia, ischemia, trauma, inflammation, catecholamines and electrolyte abnormalities; however, some patients present with different features.22 It is well known that tachyarrhythmias can lead to decreased diastolic filling time and reduced cardiac output, resulting in possible myocardial ischemia and hypotension. Related research has indicated that the excitation of the sympathetic nerve is the primary pathogenesis of tachyarrhythmia following cardiac surgery.23 AF following cardiac surgery is a thoroughly studied postoperative tachyarrhythmia.1,24–27 Specifically, new-onset AF has been the focus of current research.25 According to Banach et al’s meta-analysis, the incidence of AF in patients after cardiac surgery doubles the risk of death.28 The ACCP Guidelines for the Prevention and Management of Postoperative Atrial Fibrillation After Cardiac Surgery was proposed by the American College of Chest Physicians in 2005.29 Then, the updated recommendation for management of POAF appeared in 2016.30 Possible mechanisms may be the activation of a systemic proinflammatory state, myocardial irritation and heightened sympathetic tone. Although various functional drugs are recommended, it remains unclear whether these drugs have definitive effects. Thus, clinical research is critical for the development of new drugs.
DEX is a highly selective, new-generation alpha-2 adrenergic receptor agonist, and it was applied safely far beyond sedation. It also exhibits anxiolytic, analgesic and sympatholytic properties.31 By activating G-protein transmembrane alpha-2 receptors located within the brain, DEX can theoretically influence the transmission of sympathetic activity from the central nervous system to the peripheral nervous system and could play an antiarrhythmic role. This anti-epinephrine effect was already proven effective by Hayashi et al’s research.32 The activation of vagus nerve was also thought to be one of the mechanisms responsible for the antidysrhythmic effect several years later.33 Additionally, studies have referred to DEX’s multifunctional characteristics in cardiac surgery, which include reducing myocardial ischemia–reperfusion injury10,34 and inhibiting the inflammatory response.35,36 In conclusion, all of the above features seem to indicate that DEX has antiarrhythmic effects. However, AF prophylaxis is a controversial topic in different types of patients. Ai et al confirmed the ineffectiveness of DEX in lung cancer patients,37 but Liu et al’s study indicated a positive effect of DEX in the prevention of AF.15 Some related reviews have also strived to clarify the incidence of AF risk factors and drug prevention strategies.3,30
Our meta-analysis studied possible factors for clinical statistical heterogeneity and concluded that DEX exhibited no effective antiarrhythmic quality compared to control drugs for AF. Moreover, this study was graded to have low quality of evidence (Table 2). On the other hand, the subgroup analysis under CPB exhibited obvious heterogeneity (I2=69%), which was eliminated after sensitivity analysis by the removal of one study.15 Subsequently, consistent results were found. The heterogeneity from the removed study was likely due to the young age of the sample (~50 years) and the shortened clamp-close time (~50 minutes) of the enrolled patients, and thus it differed from other studies. It is known that increasing age and clamp-close time are risk factors for POAF,38–40 directly influencing the rate of AF and prognosis of patients. Another finding of this study was the 1.07 times increased incidence of AF in the DEX subgroup compared to the control subgroup if patients were accompanied with history of AF. However, the specific reason for this was not within the scope of this study and thus deserves further study.
Several limitations were found in our meta-analysis, which are as follows:
- Due to the shortage of RCTs and poor quality, an inconclusive conclusion may be drawn, especially since only one study of the control drug was enrolled, which can lead to an inability to quantitatively merge the results.
- The main outcome was found in only one included study; therefore, it is possible that the tests had inadequate power, and the detection of AF could have been missed.
- Many design differences between the studies made it difficult to reduce clinical heterogeneity, including variations in the timing of DEX administration, the duration of DEX infusion, the presence or lack of a loading dose and the infusion drug dosage.
Conclusion
From this meta-analysis, we conclude with low-quality evidence that DEX cannot decrease the incidence of AF compared to control drugs after cardiac surgery. Furthermore, DEX may increase the incidence of AF when patients have existing history of AF. However, cautious interpretation should be exercised due to the clinical heterogeneity. More RCTs and high-quality studies are warranted in the future.
Acknowledgment
We are grateful to all colleagues in our anesthesiology department for their generous support.
Disclosure
The authors report no conflicts of interest in this work.
References
Worden JC, Asare K. Postoperative atrial fibrillation: role of inflammatory biomarkers and use of colchicine for its prevention. Pharmacotherapy. 2014;34(11):1167–1173. | ||
Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336(20):1429–1434. | ||
Banach M, Kourliouros A, Reinhart KM, et al. Postoperative atrial fibrillation – What do we really know? Curr Vasc Pharmacol. 2010;8(4):553–572. | ||
Lin YY, He B, Chen J, Wang ZN. Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? a meta-analysis. Crit Care. 2012;16(5):R169. | ||
Karaman Y, Abud B, Tekgul ZT, Cakmak M, Yildiz M, Gonullu M. Effects of dexmedetomidine and propofol on sedation in patients after coronary artery bypass graft surgery in a fast-track recovery room setting. J Anesth. 2015;29(4):522–528. | ||
Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576–584. | ||
Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery. Anesthesiology. 2016;124(2):362–368. | ||
Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial. Anesthesiology. 2009;111(5):1075–1084. | ||
Göksedef D, Balkanay OO, Ömeroğlu SN, et al. The effects of dexmedetomidine infusion on renal functions after coronary artery bypass graft surgery: a randomized, double-blind, placebo-controlled study. Turk J Thorac Cardiovasc Surg. 2013;21(3):594–602. | ||
Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med. 2013;6(2):497–502. | ||
Cao FF, Zhang HT, Feng X. Role of dexmedetomidine in the perioperative period of patients undergoing coronary artery bypass graft surgery: a meta-analysis. Med J Chin People’s Liberation Army. 2014;39(12):981–986. | ||
Constantin JM, Momon A, Mantz J, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: a meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med. 2016;35(1):7–15. | ||
Ettema RG, Van Koeven H, Peelen LM, Kalkman CJ, Schuurmans MJ. Preadmission interventions to prevent postoperative complications in older cardiac surgery patients: a systematic review. Int J Nurs Stud. 2014;51(2):251–260. | ||
Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: from second- to first-line sedative agents in the critical care setting? J Intensive Care Med. 2012;27(4):219–237. | ||
Liu X, Zhang K, Wang W, Xie G, Fang X. Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: a randomized controlled trial. Crit Care. 2016;20(1):298. | ||
Corbett SM, Rebuck JA, Greene CM, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. 2005;33(5):940–945. | ||
Liu X, Zhang K, Wang W, et al. Dexmedetomidine versus propofol sedation improves sublingual microcirculation after cardiac surgery: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2017;30(6):1509–1515. | ||
Turan A, Bashour CA, You J, et al. Dexmedetomidine sedation after cardiac surgery decreases atrial arrhythmias. J Clin Anesth. 2014;26(8):634–642. | ||
Narisawa A, Nakane M, Kano T, et al. Dexmedetomidine sedation during the nighttime reduced the incidence of postoperative atrial fibrillation in cardiovascular surgery patients after tracheal extubation. J Intensive Care. 2015;3(1):26. | ||
Higgins JP, Altman DG, Gotzsche PC, et al; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
The Nordic Cochrane Centre TCC. Review Manager (Rev Man). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. Anaesthesiol Intensive Ther. 2015;47(3):263–264. | ||
Heintz KM, Hollenberg SM. Perioperative cardiac issues: postoperative arrhythmias. Surg Clin North Am. 2005;85(6):1103–1114, viii. | ||
Kalisnik JM, Avbelj V, Trobec R, et al. Assessment of cardiac autonomic regulation and ventricular repolarization after off-pump coronary artery bypass grafting. Heart Surg Forum. 2006;9(3):E661–E667. | ||
Yadava M, Hughey AB, Crawford TC. Postoperative atrial fibrillation: incidence, mechanisms, and clinical correlates. Heart Fail Clin. 2016;12(2):299–308. | ||
Lomivorotov VV, Efremov SM, Pokushalov EA, Karaskov AM. New-onset atrial fibrillation after cardiac surgery: pathophysiology, prophylaxis, and treatment. J Cardiothorac Vasc Anesth. 2016;30(1):200–216. | ||
Zakkar M, Ascione R, James AF, Angelini GD, Suleiman MS. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther. 2015;154:13–20. | ||
Raiten J, Patel PA, Gutsche J. Management of postoperative atrial fibrillation in cardiac surgery patients. Semin Cardiothorac Vasc Anesth. 2015;19(2):122–129. | ||
Banach M, Goch A, Misztal M, et al. Relation between postoperative mortality and atrial fibrillation before surgical revascularization-3-year follow-up. Thorac Cardiovasc Surg. 2008;56(1):20–23. | ||
Fleisher LA, Bass EB, McKeown P; American College of Chest Physicians. Methodological approach: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128(2 Suppl):17S–23S. | ||
Ha AC, Mazer CD, Verma S, Yanagawa B, Verma A. Management of postoperative atrial fibrillation after cardiac surgery. Curr Opin Cardiol. 2016;31(2):183–190. | ||
Nguyen V, Tiemann D, Park E, Salehi A. Alpha-2 Agonists. Anesthesiol Clin. 2017;35(2):233–245. | ||
Hayashi Y, Sumikawa K, Maze M, et al. Dexmedetomidine prevents epinephrine-induced arrhythmias through stimulation of central alpha 2 adrenoceptors in halothane-anesthetized dogs. Anesthesiology. 1991;75(1):113–117. | ||
Kamibayashi T, Hayashi Y, Mammoto T, Yamatodani A, Sumikawa K, Yoshiya I. Role of the vagus nerve in the antidysrhythmic effect of dexmedetomidine on halothane/epinephrine dysrhythmias in dogs. Anesthesiology. 1995;83(5):992–999. | ||
Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;128(16):e339–e340. | ||
Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37(5):1763–1770. | ||
Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69(7):693–700. | ||
Ai D, Xu G, Feng L, et al. Dexmedetomidine does not reduce atrial fibrillation after lung cancer surgery. J Cardiothorac Vasc Anesth. 2015;29(2):396–401. | ||
Tadic M, Ivanovic B, Zivkovic N. Predictors of atrial fibrillation following coronary artery bypass surgery. Med Sci Monit. 2011;17(1):CR48–CR55. | ||
Koletsis EN, Prokakis C, Crockett JR, et al. Prognostic factors of atrial fibrillation following elective coronary artery bypass grafting: the impact of quantified intraoperative myocardial ischemia. J Cardiothorac Surg. 2011;6:127. | ||
Banach M, Rysz J, Drozdz JA, et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006;70(4):438–441. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


