Back to Archived Journals » Journal of Receptor, Ligand and Channel Research » Volume 7
BKCa channels as physiological regulators: a focused review
Authors Vetri F, Saha Roy Choudhury M, Pelligrino DA, Sundivakkam P
Received 22 July 2013
Accepted for publication 31 January 2014
Published 31 March 2014 Volume 2014:7 Pages 3—13
DOI https://doi.org/10.2147/JRLCR.S36065
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Francesco Vetri,1,* Moumita Saha Roy Choudhury,2 Dale A Pelligrino,1 Premanand Sundivakkam2,*
1Department of Anesthesiology, 2Department of Medicine, University of Illinois at Chicago, IL, USA
*These authors contributed equally to this work
Abstract: Large-conductance Ca2+- and voltage-gated big K+ (BKCa, MaxiK, or Slo1) channels are expressed in almost every cell of mammalian tissues and participate in a multitude of physiological processes such as vascular tone regulation, neuronal excitability, neurotransmitter release, neurovascular coupling, bladder tone regulation, urinary K+ excretion, and retinal circulation. BKCa channel is a tetramer of the pore-forming α-subunit encoded by a single gene, Slo. The BKCa-α-subunits are associated with the modulatory β-subunits, which contribute to the functional diversity of the channel. BKCa channels sense and regulate membrane voltage and intracellular Ca2+, which then modulates several cell signaling and metabolic pathways. This review focuses on the main physiologic roles of BKCa channels and the pathogenesis of diseases associated with their loss or malfunction. The mechanistic information highlighted in this review is aimed to enhance the understanding of the unique and diverse roles of BKCa channels in various physiological and pathophysiological phenomena.
Keywords: neurovascular coupling, large conductance calcium, Ca2+-activated potassium channels, BKCa channel physiology
Introduction
Calcium (Ca2+)-activated potassium channels (KCa) or the channels possessing large conductance (BKCa, MaxiK, KCa1.1) are mainly characterized by a high unitary conductance of ~100–300 pS.1 Unlike other subfamilies of KV, BKCa channels are both voltage- and Ca2+-regulated potassium channels. The native BKCa channel is formed by four pore-forming subunits (α) that are encoded by the Slo1 gene.1–3 Splicing of the Slo1 messenger (m)RNA has been shown to contribute to differences in the regulatory properties of the channel as a result of variability in the responses to steroids and the availability of the phosphorylation sites. Furthermore, studies have demonstrated the contributory role of different splice variants between tissues in voltage sensitivity of the channels.4 Importantly, the splice variation of an α-subunit may significantly alter the localization of BKCa channels to endoplasmic reticulum.2–7
BKCa channels belong to the family of voltage-gated potassium channels.5–7 However, BKCa channels are also known to be activated solely by a stimulus-evoked increase in intracellular Ca2+ concentrations ([Ca2+]i). Interestingly, the resultant large efflux of K+ ions through the activation or opening of BKCa channels repolarizes the membrane, closes the voltage-gated calcium channels (VGCC), and reduces Ca2+ influx into the cells.8–12 The properties of BKCa channels therefore integrate various cellular and molecular signaling events via modulation of membrane excitability and Ca2+ homeostasis.
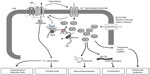
BKCa channels have also been outlined as negative feedback regulators of membrane potential and Ca2+ homeostasis in numerous physiological processes (Figure 1). These include modulating neurotransmitter release,8 neurovascular coupling,13 regulating vascular and respiratory tone,14,15 endocrine secretion,16,17 interspike interval and spike frequency adaptation,8 and urinary bladder tone.18 Given their relevance in essential physiological processes, these channels are encoded by a substantial number of genes in higher organisms. BKCa channels are implicated in several disease conditions including epilepsy,19 diabetes,20 Alzheimer’s disease,21 subarachnoid hemorrhage,22 neuromuscular abnormalities,23 motor impairment,24 hypertension,14,25 urinary incontinence,26 overactive urinary bladder,27,28 and noise-induced hearing loss.29 This review discusses the scientific know-how on BKCa channels serving as a key regulator in various physiologic and pathophysiologic processes.
BKCa channel structure and properties
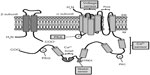
The native BKCa channel is composed of four α- and four β-subunits, present in a 1:1 ratio (Figure 2).30,31 The α-subunit contains seven putative transmembrane-spanning α helical segments and is accountable for the ion conduction, for selectivity, and for sensing the voltage alteration.32 The cytoplasmic carboxy tail contains two intrinsic high-affinity Ca2+ binding sites and phosphorylation sites and has been implicated in the direct gating of the channel. The β-subunit is composed of two transmembrane domains with a long extracellular linker, whereas the amino- and carboxy-terminals are located in the cytoplasm. BKCa-β-subunits have been shown to influence the Ca2+ sensitivity of channel gating33–36 and trafficking of channels to the plasma membrane.13,37,38 Evidence clearly shows that although BKCa channels exist in differing subunit stoichiometries, a full complement of four β1-subunits is crucial for the optimal effect of the channel.39 Differences in subunit stoichiometry, isoform expression, degree of phosphorylation, or the expression of splice variants may result in channels of varying voltage, Ca2+, and stress sensitivity and selectivity (Figure 2). Furthermore the spatial localization of BKCa channels has also been reported to influence its function within a given tissue. Interestingly, an efficient activation of BKCa channels (by locally produced Ca2+ transients or sparks) requires a close proximal arrangement between sarcoplasmic reticulum (SR) and plasma membrane (PM). It is interesting to note that this spatial arrangement is usually observed in smooth muscle and endothelial cells where the SR/endoplasmic reticulum (ER) is close to PM invaginations.40 In fact, the α-subunit of BKCa is known to contain two Caveolin (Cav) binding domains and is associated with Cav-1 and Cav-2 in endothelial cells.41 It is likely that the binding of BKCa channels to Cav-1/2 proteins or its localization in caveolae may facilitate its association with other signaling partners, either directly or indirectly, such as c-Src tyrosine kinase,42 nonselective cation channels, the Trp family of proteins,43 the G-protein-coupled receptor-mediated signaling cascade,44 and actin filaments,41,45 to mention a few.
The association of BKCa channels with specific membrane domains has also been implicated in the functional coupling of this channel to other ion channels, including nonselective cation channels, transient receptor potential and VGCC.46,47 Thus, the BKCa channel serves as a physiological regulator in association with other proteins. Proteomic analysis of BKCa channel binding partners in mouse cochlea revealed that 50% of the proteins have affiliations with K+ and Ca2+ channels, whereas almost 20% of the proteins are related to mitochondria,26 suggesting a potential role of BKCa channels in many aspects of cellular and molecular dynamics. Therefore, it is imperative to delineate the structure, localization, and function of these channels to develop new and effective treatment strategies.
BKCa channels regulation of vascular tone
Vascular tone in small arteries and arterioles is the major determinant of vascular resistance in response to several stimuli, including myogenic (pressure) components and vasoactive agents.14 Myogenic constriction is a characteristic of resistance blood vessels and plays an essential role in regulating microcirculation blood flow, providing the basal tone in resistance arteries. Increased myogenic constriction has been reported in several hypertensive models and is associated with vascular diseases.
The organs that have a higher vascular tone (eg, myocardium, skeletal muscle, skin, splanchnic circulation) exhibit large vasodilatory capacity, whereas those having relatively low vascular tone (eg, cerebral and renal circulation) have low capacity. A noticeable exception to this rule is cerebral vasospasm occurring after subarachnoid hemorrhage. The blood vessels, both arterial and venous, exhibit some degree of vascular smooth muscle contraction under basal conditions, which determines the tone, and hence the diameter, of the vessel.48 Baseline [Ca2+]i, vasoconstrictor-mediated increase in [Ca2+]i, and Ca2+ sensitivity significantly contribute to the contractile state of the blood vessel wall. These processes are orchestrated via different ion channels (K+, Cl−, and nonselective cation channels), which govern the membrane potential and affect the Ca2+ influx and VGCC activity. The Ca2+ flux that activates K+ channels (mainly BKCa) indirectly hyperpolarizes the membrane, promoting the closure of VGCC. The Ca entry occurring in the vicinity of ryanodine receptors on sub-plasma membrane endoplasmic reticulum is key because the Ca2+ sparks generated by the ryanodine receptor “event” are linked to plasma membrane BKCa opening and the extrusion of K+ (Figure1). Thus, BKCa channels serve as a counter-regulatory mechanism by reverting vasoconstriction, particularly in the intense myogenic constriction of resistance vessels exposed to high intraluminal pressures.14,15 Therefore, BKCa channels are the key regulators in protecting excessive vasoconstriction through a Ca2+-dependent relaxation mechanism.
The function of BKCa channels, especially in vascular smooth muscle cells, is finely tuned by its regulatory β1-subunit through enhancing the channel for its Ca2+ sensitivity. Using BKCa-β1−/− mice49 and insulin-resistant hypertensive rat models,50–52 studies have revealed an increase in arterial blood pressure and left ventricular hypertrophy. Interestingly, the lack of functional β1-subunit altered the coupling between Ca2+ signaling and membrane potential changes. Furthermore, pharmacological studies blocking BKCa channels have demonstrated an increase in the decaying conduction of a local depolarization, which basically represents the junctional and plasma membrane resistance.50 A decrease in expression of β1-subunit of the BKCa channel has also been reported in coronary artery aging in humans and rats.53,54
BKCa channels are also present in endothelial cells,14,41,55–58 where they contribute to hyperpolarization,59 participate in endothelial-dependent vasodilation,60 and improve endothelial dysfunction.61–63 Interestingly, studies specifically designed to target endothelial BKCa channels with luminal administration of the specific blocker iberiotoxin in arteries demonstrated the restoration of vasoconstrictor responsiveness and the normalization of the membrane potential to control levels,64 suggesting an involvement of endothelial BKCa channels in vessel reactivity. Furthermore, recent studies have shown a cholesterol-dependent activation of BKCa, suggesting the role of Cav-1 in the regulation of its activity.65 However, the exact mechanisms by which Cav-1 proteins or the caveolae invaginations may affect either the localization of BKCa channels to the plasma membrane or the downstream signaling molecules, such as nitric oxide synthase, are still not clear.
BKCa channels have also been involved in coronary artery vasodilation,66–69 mainly through the endothelium-mediated stimulation-dependent responses of coronary artery smooth muscle cells (CASMCs).70–74 The mediators, termed endothelium-derived hyperpolarizing factor, released from endothelial cells activate BKCa channels in CASMCs.75,76 Typically, substances that constrict coronary vessels inhibit BKCa channels in CASMCs, including angiotensin II,77,78 endothelin 1,75,79 and thromboxane A2.80 Inhibition of BKCa channels by these G-protein-coupled receptors may alter several of the downstream signaling cascade, mainly protein kinase C44, c-Src kinase,42 and so on. However, studies designed to explore the role of BKCa channels in ischemic81 and metabolic vasodilation82 showed no or little effect. Nevertheless, alterations in the activity of BKCa channels were demonstrated in several vascular pathologies, including diabetes,20,83,84 atherosclerosis and ischemia,85 hypertension,25,51,76 cardiac hypertrophy,15 and cardiomyopathy.
BKCa channels in neuronal excitability and neurotransmitter release
BKCa channels are ubiquitously expressed in the central nervous system, and their expression is highly variable within different brain regions. BKCa channels play an important role in regulating neurotransmitter release at central nervous system nerve terminals, controlling action potential duration, firing frequency, and spike frequency adaptation, resulting in fast after-hyperpolarization.8 Studies intended to explore the regional distribution and the level of expression have revealed that BKCa channels are preferentially located at the axon terminals11,86 and dendrites.87,88 In neurons, the main functions of BKCa channels are to generate the fast and prolonged after-hyperpolarization (lasting from hundreds of milliseconds to seconds) after an action potential. Prominently, the generation of after-hyperpolarization contributes significantly to the maintenance of the shape and duration of the action potential.89
BKCa channels are primarily activated in response to elevations in [Ca2+]i through the opening of voltage-dependent87 or neurotransmitter-gated90 Ca2+ channels, as well as by release of Ca2+ stores.91 The activation of BKCa channels by rise in [Ca2+]i shifts the activation voltage concentration dependently into a physiological range by limiting the depolarization-induced bursting activity. In contrast, in Purkinje cells, which lack BKCa channels, the net result is a less-negative resting membrane potential and decreased amplitude of the after-hyperpolarization.92
Several studies point to a possible functional coupling between BKCa channels and voltage-gated Ca2+ channels in the central nervous system. It is clear that in several types of neurons, BKCa channels are physically associated with voltage-gated Ca2+ channels and that this complex invariably provides a mechanism by which micromolar concentrations of [Ca2+]i (calcium sparks) are delivered to BKCa channels and tightly control their activity without affecting other Ca2+-dependent signaling processes. Moreover, the characteristics of BKCa channels are largely determined by the specific subunit of voltage-gated Ca2+ channels to which they are associated and adapt BKCa channel function to the requirement of particular neurons or neuronal subcompartments. Interestingly, blocking voltage-gated Ca2+ channels correspondingly inhibits BKCa channels, as observed in conditions in which extracellular Ca2+ was removed. In lieu of voltage-gated Ca2+ channels bound to BKCa, these channels have also shown to be operated by more distant Ca2+ sources or by a global increase in [Ca2+]i. This functionality of BKCa channels has originated the term free BKCa. Free BKCa channels are well demonstrated in chromaffin cells93,94 and in CA3 pyramidal cells,11 where submillimolar concentrations of ethylene glycol tetraacetic acid inhibit the activity of BKCa channels. The free BKCa channels are believed to serve as an emergency brake in situations where extraordinarily large Ca2+ transients lead to cellular damage or apoptosis.11
Given their role in controlling neuronal excitability, BKCa channels have been increasingly implicated in several neurological disorders, including epilepsy, cerebellar ataxia, and paroxysmal movement disorders.95–97 In epilepsy, studies have indicated a missense mutation (D434G) in the α-subunit BKCa gene, which is characterized by an increase in the BKCa channel’s sensitivity to Ca2+ and increased membrane currents, resulting in a gain-of-function effect.98,99 Furthermore, this mutation, also observed in the pathophysiology of idiopathic absence epilepsy, confers specific changes in the regulatory properties of the BKCa channel subunits. However, a loss-of-function BKCa channel phenotype was demonstrated to be associated with temporal lobe epilepsy, where a polymorphism in the BKCa-β4-subunit was revealed.101 Moreover, loss-of-function BKCa channel has been implicated in tonic-clonic seizures and alcohol withdrawal seizures. Thus, both loss-of-function and gain-of-function BKCa channels might serve as molecular targets for drugs to suppress certain seizure phenotypes, including temporal lobe seizures and absence seizures, respectively.
BKCa channels in mitochondria
Channel activity similar to that of plasma membrane BKCa channels have been reported in the inner membrane of mitochondria (mitoBKCa). The mitoBKCa was initially found in the glioma cells102 and later in cardiac myocyte103 and rat brain neurons.100 Several observations confirmed the existence of BKCa channel β4-subunit in the inner membrane of neuronal mitochondria.104 The changes in the cytosolic Ca2+ concentration greatly affect neuronal cell metabolism via modulating mitochondrial response. Skalska et al105 have clearly demonstrated a Ca2+-induced dissipation of mitochondrial membrane potential, an underlying process for mitochondrial respiration, metabolism, and viability. Thus, the studies delineating the presence of mitoBKCa in neurons, its contribution to mitochondrial Ca2+ signaling, and mitochondrial membrane potential changes support the neuroprotective role of mitoBKCa in specific brain structure.
BKCa channels in neurovascular coupling
Neuronal activity is thought to communicate to arterioles in the brain to promote an adequate blood supply. This phenomenon is known as neurovascular coupling and employs multiple mechanisms, including, but not limited to, purinergic signaling, cytochrome P450 products, cyclooxygenase products, and K+. One of these mechanisms is through astrocytic Ca2+ signaling to cause local vasodilation in the activated brain area. In particular, K+ released from astrocytic end feet via BKCa channels is thought to interact with K+ inward rectifier channels on pial arteriolar smooth muscle cells, inducing hyperpolarization and relaxation.13,106
Paradoxically, this communication may cause vasoconstriction in some cases. Modest increases in Ca2+ induce dilation, whereas larger increases switch dilation to constriction.107 BKCa channels in astrocytic end feet are believed to mediate the majority of the dilation and the entire vasoconstriction, implicating local extracellular K+ as a vasoactive signal for both dilation and constriction. Therefore, BKCa channels at the astrocytic end foot are able to determine both arteriolar dilation and constriction based on the [Ca2+]i changes.
Interestingly, BKCa channel dysfunction has been recently associated with pathophysiologic changes occurring during type 1 diabetes mellitus.20 In particular, a significant decrease in the pial arteriolar dilations evoked by somatosensory activation, via sciatic nerve stimulation, was found in streptozotocin-treated diabetic rats. This depressed neurovascular coupling response is likely linked to PKC-mediated changes in BKCa and K+ inward rectifier channel activity, as normal dilating responses of pial arterioles to sciatic nerve stimulation and applications of K+-channel openers were readily restored by acute PKC inhibition. Interestingly, in a model of type 2 diabetes mellitus, whole-cell currents of BKCa channels were significantly decreased in cerebral artery smooth muscle cells, compared with control, and the sensitivities of BKCa channels to voltage, paxilline, and NS1619 were all diminished in diabetic rats.108
BKCa channels in regulating retinal circulation
Vertebrate retinas share the same fundamental neuronal organization, comprising various cell classes such as photoreceptors, bipolar cells, amacrine cells, and ganglion cells. The retina receives oxygen and nutrients diffused from the choriocapillaries to the rods, cones, and nerve layers in the inner retina. Therefore, to preserve the delicate balance between the flow of blood and the needs of the retinal nerve layers, the vasculature of the retina is designed to maximize the control of capillary perfusion.
Several lines of evidence have demonstrated the existence and functional role of BKCa channels in rod signaling.109 In addition, BKCa channels have been shown to be located at the synaptic terminal, contributing to the amplification of glutamate release at the rod photoreceptor synapse.110 The signaling of BKCa channels in the cone pathway is poorly studied compared with the rod pathways. Work by Yagi and Macleish111 has hinted at the absence of BKCa channels in the cones of the primate retina. However, blocking BKCa channels induced a reduction in light-evoked input from bipolar cells and amacrine cells to ganglions in mouse retina,112 thus suggesting a possible existence of BKCa channels in the cone pathway in rodents. Furthermore, genetic deletion of BKCa channels has been shown to affect the photoreceptor and bipolar cell responses in mouse retina.
Moreover, recent studies have shown the contributions of BKCa channels in the regulation of retinal blood flow via the action of several vasodilators in endothelium and vascular smooth muscle.113 Administration of a BKCa channel opener (BMS-191011) to male Wistar rats specifically improved retinal circulation without affecting cardiovascular functions.114 Studies in diabetic retinal models demonstrate a decreased Ca2+ sensitivity of BKCa channels and an uncoupling of BKCa channel activation from Ca2+ release in diabetic retinal vascular smooth muscle cells.115 The drastic reduction in spontaneous Ca2+ sparks results in delayed activation of BKCa channel-mediated K+ outward currents, an underlying process for arteriolar vasoconstriction, as commonly observed in retinal diseases, mainly diabetic retinopathy.115–117 Hitherto, studies delineating the roles of BKCa channels in retinal circulation and physiology have been unclear. More detailed investigations are warranted to enhance the understanding of the significance of BKCa channels in retinal circulation physiology.
BKCa channels in the urinary system
Maintenance of K+ concentration within the physiological range is vital for various cellular functions, including cell volume regulation and regulation of membrane electrical properties. The kidney is the primary site where balancing K+ concentration and K+ secretion in the distal convoluted tubules of nephron is critical for determining the amount of K+ excretion. Several segments of the distal convoluted tubules of nephron have been shown to express BKCa and renal outer medullary K+ channels. Although renal outer medullary K+ channels are considered the primary channels involved in K+ secretion because of their open probability, BKCa channels are suggested to contribute to the volume regulation in the distal convoluted tubules of nephron.
BKCa channels have been reported in a variety of renal cell types, including urinary bladder smooth muscle cells,118 afferent arterioles,119 glomerular mesangial cells,120,121 and visceral epithelial cells (podocytes) in the Bowman’s capsule.3 BKCa channels have been demonstrated to be negative feedback, counteracting agonist-induced contraction, mainly in mesangial cells.120,121 BKCa channels act as a conduit of K+ secretion in the distal convoluted tubules, medullary, and cortical thick ascending limbs,122 distal connecting tubules,123 and cortical collecting ducts.124 Investigations on BKCa-β1−/−-subunit knockout models failed to demonstrate an increase in flow-mediated K+ secretion, whereas BKCa-α1−/− mice showed diminished capacity to secrete K+,125 suggesting the significance of the β1-subunit in maintaining a proper renal kaliuretic function via regulating flow-mediated K+ secretion.19,126 This may also be explained by the activation of BKCa channel activation in response to cyclic guanosine monophosphate and nitric oxide synthase through the protein kinase G (PKG) pathway. Activation of BKCa by PKG via its β1-subunit127 synergistically increases BKCa currents under conditions of increased flow via enhancing the Ca2+ sensitivity of the channel.35
In addition to mediating flow-induced K+ secretion, BKCa channels in the distal nephron have been demonstrated to respond to arginine vasopressin via the PLC/Ca2+/PKC signaling pathway. Furthermore, BKCa channels have also been shown to play a role in the renal response to aldosterone and/or a high-K+ diet. Studies using iberiotoxin, a specific BKCa channel blocker, confirmed the inhibition of renal K+ secretion associated with a high-K+ diet.128 Interestingly, a study by Najjar et al129 using the isolated, perfused cortical collecting duct from rabbits administered a high-K+ diet showed an increase in the expression of BKCa channels in the apical membrane. However, it is not clear whether an accelerated K+ secretion on high K+ diet is a result of an effect of aldosterone on BKCa channel activity or its localization to the cell membrane.
BKCa channels have also been shown to play an important role in regulating urinary bladder smooth muscle function, which is associated with urinary frequency and overactive bladder. Overactive bladder is a common pathologic condition resulting from the alteration of detrusor muscle excitability linked to several myogenic and neurological factors.130 BKCa channels are predominantly involved in the relaxation of bladder smooth muscle.131 Therefore, decreased expression of BKCa channels, mainly in the bladder outlet, may result in alteration of sensory afferent activity leading to enhanced detrusor tone during urine storage.130
Physiological regulators of BKCa channels
Studies in recent years have identified an enormous list of regulatory physiological mechanisms, which may serve as potential drug targets for interfering with BKCa channel activity. Mechanisms modulating channel function include subunit composition,132 phosphorylation,132 palmitoylation,133 and alternative splicing.134,135 However, channel function may be affected at different levels, such as protein synthesis, cellular localization, and trafficking. Furthermore, several upstream signaling molecules participating in orchestrating the above mentioned regulatory mechanisms are the object of research. In general, any mechanisms that alter the presence or function of BKCa channels in the plasma membrane may profoundly influence the magnitude of whole-cell BKCa channel currents and, consequently, cell and tissue physiology.
In addition, investigations aimed to understand the molecular aspects of BKCa channel activity have revealed various target sites of the channel protein. Several allosteric inhibitors were identified and developed to inhibit its activity and functions. Few of the inhibitors, namely, tetraethylammonium, the peptide inhibitors charybdotoxin and iberiotoxin, and the fungal alkaloids paxilline and lolitrem B are widely used in both in vitro and in vivo models. Among these, iberiotoxin is the best characterized inhibitor of BKCa channel activity.64,128,136 However, iberiotoxin was identified to have several limitations on its use in whole-animal experiments because of its low-activity against channels containing the β4-subunit,137,138 as well as its impermeable nature across the cell membrane. The membrane-permeable fungal alkaloid paxilline has become widely used as a BKCa channel inhibitor in molecular physiology because of its ability to block BKCa channels complexes with β4-subunits.139 More recently, however, another fungal alkaloid, lolitrem B, has been shown to be five times more potent at inhibiting BK channels in comparison with paxilline.140,141 Seven lolitrem compounds have also been shown to be BK channel inhibitors.142 Lolitrem B is the causative agent of ryegrass staggers, a nervous disorder of animals that graze perennial ryegrass infected with the endophytic fungi Neotyphodium lolii. Using a mouse model of ryegrass staggers, it has been shown that lolitrem B produces ataxia and tremors by inhibiting BK channels.141 In addition to lolitrem B, this endophyte-grass symbiosis also produces other structurally related lolitrem analogues in which only minor structural changes have a dramatic effect on tremorgenicity.143–145
Summary
This review highlights the potential roles of BKCa channels in regulating various physiological processes. Furthermore, the functional versatility of BKCa channels conferred by the assembly of auxiliary subunits and alternative splicing of the pore-forming subunits has been addressed. The information provided in this review strongly suggests that the BKCa channel, its subunits, and its associated proteins are promising targets for the regulation of various biological and physiological processes, and hence for the treatment of several diseases. This review addressed how the understanding of BKCa channel-mediated mechanisms can be used therapeutically to treat or prevent several pathologies. More studies to understand the allosteric modulations of these channels or upstream mediators, which may result in both gain- and loss-of-function, will likely result in clinically relevant compounds. In addition, the identification of BKCa channel subunit variants and their unique contribution to physiological processes is crucial to selectively target pathophysiological cascades.
Disclosure
The authors report no conflicts of interest in this work.
References
Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981;291(5815):497–500. | |
Chen L, Jeffries O, Rowe IC, et al. Membrane trafficking of large conductance calcium-activated potassium channels is regulated by alternative splicing of a transplantable, acidic trafficking motif in the RCK1-RCK2 linker. J Biol Chem. 2010;285(30):23265–23275. | |
Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience. 2007;146(4):1652–1661. | |
Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett. 2007;581(5):1000–1008. | |
Zarei MM, Eghbali M, Alioua A, et al. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci U S A. 2004;101(27):10072–10077. | |
Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276(19):16232–16239. | |
Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A. 2013;110(26):10836–10841. | |
Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 2003;9(3):181–194. | |
Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389: 187–203. | |
Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521 Pt 1:135–146. | |
Hu H, Shao LR, Chavoshy S, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21(24):9585–9597. | |
Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580(Pt.3):859–882. | |
Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. | |
Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010;584(10):2033–2042. | |
Kim N, Chung J, Kim E, Han J. Changes in the Ca2+-activated K+ channels of the coronary artery during left ventricular hypertrophy. Circ Res. 2003;93(6):541–547. | |
Wu SN, Ho LL, Li HF, Chiang HT. Regulation of Ca(2+)-activated K+ currents by ciglitazone in rat pituitary GH3 cells. J Investig Med. 2000;48(4):259–269. | |
Matzkin ME, Lauf S, Spinnler K, et al. The Ca2+-activated, large conductance K+-channel (BKCa) is a player in the LH/hCG signaling cascade in testicular Leydig cells. Mol Cell Endocrinol. 2013;367(1–2):41–49. | |
Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-beta1-/- mice. Am J Physiol Renal Physiol. 2003;284(6):F1274–F1279. | |
Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9(4):413–424. | |
Vetri F, Xu H, Paisansathan C, Pelligrino DA. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am J Physiol Heart Circ Physiol. 2012;302(6):H1274–H1284. | |
Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience. 2009;159(3):1055–1069. | |
Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci U S A. 2012;109(21):E1387–E1395. | |
Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79(4):1317–1372. | |
Cheney JA, Weisser JD, Bareyre FM, et al. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J Cereb Blood Flow Metab. 2001;21(4):396–403. | |
Yang Y, Li PY, Cheng J, et al. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61(2):519–525. | |
Lawson K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000;57(3):838–845. | |
Aydin M, Wang HZ, Zhang X, et al. Large-conductance calcium-activated potassium channel activity, as determined by whole-cell patch clamp recording, is decreased in urinary bladder smooth muscle cells from male rats with partial urethral obstruction. BJU Int. 2012;110(8 Pt B):E402–E408. | |
Li L, Jiang C, Song B, Yan J, Pan J. Altered expression of calcium-activated K and Cl channels in detrusor overactivity of rats with partial bladder outlet obstruction. BJU Int. 2008;101(12):1588–1594. | |
Rüttiger L, Sausbier M, Zimmermann U, et al. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A. 2004;101(35):12922–12927. | |
Knaus HG, Eberhart A, Glossmann H, Munujos P, Kaczorowski GJ, Garcia ML. Pharmacology and structure of high conductance calcium-activated potassium channels. Cell Signal. 1994;6(8):861–870. | |
Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502(Pt 3):545–557. | |
Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1–S4 voltage sensor of BK channels. J Gen Physiol. 2006;127(3):309–328. | |
McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14(3):645–650. | |
Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol. 2005;126(4):393–412. | |
Brenner R, Peréz GJ, Bonev AD, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. | |
Sweet TB, Cox DH. Measurements of the BKCa channel’s high-affinity Ca2+ binding constants: effects of membrane voltage. J Gen Physiol. 2008;132(5):491–505. | |
Toro B, Cox N, Wilson RJ, et al. KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience. 2006;142(3):661–669. | |
Zarei MM, Song M, Wilson RJ, et al. Endocytic trafficking signals in KCNMB2 regulate surface expression of a large conductance voltage and Ca(2+)-activated K+ channel. Neuroscience. 2007;147(1):80–89. | |
Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci. 2002;22(5):1550–1561. | |
McCarron JG, Chalmers S, Bradley KN, MacMillan D, Muir TC. Ca2+ microdomains in smooth muscle. Cell Calcium. 2006;40(5–6):461–493. | |
Wang XL, Ye D, Peterson TE, et al. Caveolae targeting and regulation of large conductance Ca(2+)-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280(12):11656–11664. | |
Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci U S A. 2002;99(22):14560–14565. | |
Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296(3):C403–C413. | |
Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1993;190(1):263–269. | |
Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289(1):C49–C57. | |
Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. | |
Kwan HY, Shen B, Ma X, et al. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104(5):670–678. | |
Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256(5056):532–525. | |
Plüger S, Faulhaber J, Fürstenau M, et al. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87(11):E53–E60. | |
Dimitropoulou C, Han G, Miller AW, et al. Potassium (BK(Ca)) currents are reduced in microvascular smooth muscle cells from insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2002;282(3):H908–H917. | |
Zhao Q, Wang L, Yang W, et al. Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics. 2008;18(6):459–466. | |
Howitt L, Sandow SL, Grayson TH, Ellis ZE, Morris MJ, Murphy TV. Differential effects of diet-induced obesity on BKCa {beta}1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol. 2011;301(1):H29–H40. | |
Hald BO, Jacobsen JC, Braunstein TH, et al. BKCa and KV channels limit conducted vasomotor responses in rat mesenteric terminal arterioles. Pflugers Arch. 2012;463(2):279–295. | |
Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559(Pt 3):849–862. | |
Faehling M, Koch ED, Raithel J, Trischler G, Waltenberger J. Vascular endothelial growth factor-A activates Ca2+-activated K+ channels in human endothelial cells in culture. Int J Biochem Cell Biol. 2001;33(4):337–346. | |
Kuhlmann CR, Wu Y, Li F, et al. bFGF activates endothelial Ca2+-activated K+ channels involving G-proteins and tyrosine kinases. Vascul Pharmacol. 2004;41(6):181–186. | |
Wrzosek A. Endothelium as target for large-conductance calcium-activated potassium channel openers. Acta Biochim Pol. 2009;56(3):393–404. | |
Wrzosek A, Łukasiak A, Gwóźdź P, et al. Large-conductance K+ channel opener CGS7184 as a regulator of endothelial cell function. Eur J Pharmacol. 2009;602(1):105–111. | |
Simon A, Harrington EO, Liu GX, Koren G, Choudhary G. Mechanism of C-type natriuretic peptide-induced endothelial cell hyperpolarization. Am J Physiol Lung Cell Mol Physiol. 2009;296(2):L248–L256. | |
Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda). 2006;21:69–78. | |
Félétou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156(4):545–562. | |
Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol. 2006;291(3):H985–H1002. | |
Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–1225. | |
Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol. 2010;299(5):H1439–H1450. | |
Riddle MA, Hughes JM, Walker BR. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am J Physiol Cell Physiol. 2011;301(6):C1404–C1414. | |
Node K, Kitakaze M, Kosaka H, Minamino T, Hori M. Bradykinin mediation of Ca(2+)-activated K+ channels regulates coronary blood flow in ischemic myocardium. Circulation. 1997;95(6):1560–1567. | |
Node K, Kitakaze M, Kosaka H, et al. Roles of NO and Ca2+-activated K+ channels in coronary vasodilation induced by 17beta-estradiol in ischemic heart failure. FASEB J. 1997;11(10):793–799. | |
Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17beta-estradiol: role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96(6):1953–1963. | |
Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation. 1999;99(24):3132–3138. | |
Bychkov R, Gollasch M, Steinke T, Ried C, Luft FC, Haller H. Calcium-activated potassium channels and nitrate-induced vasodilation in human coronary arteries. J Pharmacol Exp Ther. 1998;285(1):293–298. | |
Khan SA, Higdon NR, Meisheri KD. Coronary vasorelaxation by nitroglycerin: involvement of plasmalemmal calcium-activated K+ channels and intracellular Ca++ stores. J Pharmacol Exp Ther. 1998;284(3):838–846. | |
Price JM, Hellermann A. Inhibition of cGMP mediated relaxation in small rat coronary arteries by block of CA++ activated K+ channels. Life Sci. 1997;61(12):1185–1192. | |
Pataricza J, Toth GK, Penke B, Hohn J, Papp JG. Effect of selective inhibition of potassium channels on vasorelaxing response to cromakalim, nitroglycerin and nitric oxide of canine coronary arteries. J Pharm Pharmacol. 1995;47(11):921–925. | |
Weston AH, Félétou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005;145(6):775–784. | |
Wellman GC, Brayden JE, Nelson MT. A proposed mechanism for the cardioprotective effect of oestrogen in women: enhanced endothelial nitric oxide release decreases coronary artery reactivity. Clin Exp Pharmacol Physiol. 1996;23(3):260–266. | |
Li PL, Zou AP, Campbell WB. Regulation of potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29(1 Pt 2):262–267. | |
Minami K, Hirata Y, Tokumura A, Nakaya Y, Fukuzawa K. Protein kinase C-independent inhibition of the Ca(2+)-activated K+ channel by angiotensin II and endothelin-1. Biochem Pharmacol. 1995;49(8):1051–1056. | |
Toro L, Amador M, Stefani E. ANG II inhibits calcium-activated potassium channels from coronary smooth muscle in lipid bilayers. Am J Physiol. 1990;258(3 Pt 2):H912–H915. | |
Hu SL, Kim HS, Jeng AY. Dual action of endothelin-1 on the Ca2(+)-activated K+ channel in smooth muscle cells of porcine coronary artery. Eur J Pharmacol. 1991;194(1):31–36. | |
Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992;262(3 Pt 1):C708–C713. | |
Borbouse L, Dick GM, Payne GA, et al. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2010;298(4):H1182–H1189. | |
Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006;291(5):H2090–H2097. | |
Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol. 2008;295(1):F171–F178. | |
Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol. 2002;38(1):43–49. | |
Wiecha J, Schläger B, Voisard R, Hannekum A, Mattfeldt T, Hombach V. Ca(2+)-activated K+ channels in human smooth muscle cells of coronary atherosclerotic plaques and coronary media segments. Basic Res Cardiol. 1997;92(4):233–239. | |
Knaus HG, Schwarzer C, Koch RO, et al. Distribution of high-conductance Ca(2+)-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16(3):955–963. | |
Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24(40):8818–8822. | |
Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24(14):3511–3521. | |
Bond CT, Herson PS, Strassmaier T, et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24(23):5301–5306. | |
Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca(2+)-activated K+ channels. Neuron. 2001;31(6):1027–1034. | |
Chavis P, Ango F, Michel JM, Bockaert J, Fagni L. Modulation of big K+ channel activity by ryanodine receptors and L-type Ca2+ channels in neurons. Eur J Neurosci. 1998;10(7):2322–2327. | |
Sausbier M, Hu H, Arntz C, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101(25):9474–9478. | |
Prakriya M, Lingle CJ. Activation of BK channels in rat chromaffin cells requires summation of Ca(2+) influx from multiple Ca(2+) channels. J Neurophysiol. 2000;84(3):1123–1135. | |
Prakriya M, Solaro CR, Lingle CJ. [Ca2+]i elevations detected by BK channels during Ca2+ influx and muscarine-mediated release of Ca2+ from intracellular stores in rat chromaffin cells. J Neurosci. 1996;16(14):4344–4359. | |
Eunson LH, Rea R, Zuberi SM, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48(4):647–656. | |
Du W, Bautista JF, Yang H, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37(7):733–738. | |
Turnbull J, Lohi H, Kearney JA, et al. Sacred disease secrets revealed: the genetics of human epilepsy. Hum Mol Genet. 2005;14 Spec No. 2: 2491–2500. | |
Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci U S A. 1999;96(7):4137–4142. | |
Wang B, Rothberg BS, Brenner R. Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J Gen Physiol. 2009;133(3):283–294. | |
Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139(4):1249–1261. | |
Cavalleri GL, Weale ME, Shianna KV, et al. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case-control study. Lancet Neurol. 2007;6(11):970–980. | |
Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257(2):549–554. | |
Xu W, Liu Y, Wang S, et al. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298(5595):1029–1033. | |
Piwonska M, Wilczek E, Szewczyk A, Wilczynski GM. Differential distribution of Ca2+-activated potassium channel beta4 subunit in rat brain: immunolocalization in neuronal mitochondria. Neuroscience. 2008;153(2):446–460. | |
Skalska J, Bednarczyk P, Piwońska M, et al. Calcium ions regulate K+ uptake into brain mitochondria: the evidence for a novel potassium channel. Int J Mol Sci. 2009;10(3):1104–1120. | |
Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol. 2010;299(6):H2009–H2017. | |
Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107(8):3811–3816. | |
Wang Y, Zhang HT, Su XL, et al. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res. 2010;7(2):75–84. | |
Pelucchi B, Grimaldi A, Moriondo A. Vertebrate rod photoreceptors express both BK and IK calcium-activated potassium channels, but only BK channels are involved in receptor potential regulation. J Neurosci Res. 2008;86(1):194–201. | |
Xu JW, Slaughter MM. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci. 2005;25(33):7660–7668. | |
Yagi T, Macleish PR. Ionic conductances of monkey solitary cone inner segments. J Neurophysiol. 1994;71(2):656–665. | |
Nemargut JP, Zhu J, Savoie BT, Wang GY. Differential effects of charybdotoxin on the activity of retinal ganglion cells in the dark- and light-adapted mouse retina. Vision Res. 2009;49(3):388–397. | |
Nelson MT, Cheng H, Rubart M, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. | |
McGahon MK, Dash DP, Arora A, et al. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100(5):703–711. | |
Mori A, Suzuki S, Sakamoto K, Nakahara T, Ishii K. BMS-191011, an opener of large-conductance Ca2+-activated potassium channels, dilates rat retinal arterioles in vivo. Biol Pharm Bull. 2011;34(1):150–152. | |
Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278(2):C235–C256. | |
Jaggar JH, Nelson MT. Differential regulation of Ca(2+) sparks and Ca(2+) waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279(5):C1528–C15239. | |
Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R60–R68. | |
Fallet RW, Bast JP, Fujiwara K, Ishii N, Sansom SC, Carmines PK. Influence of Ca(2+)-activated K(+) channels on rat renal arteriolar responses to depolarizing agonists. Am J Physiol Renal Physiol. 2001;280(4):F583–F591. | |
Stockand JD, Sansom SC. Large Ca(2+)-activated K+ channels responsive to angiotensin II in cultured human mesangial cells. Am J Physiol. 1994;267(4 Pt 1):C1080–C1086. | |
Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78(3):723–744. | |
Guggino SE, Guggino WB, Green N, Sacktor B. Ca2+-activated K+ channels in cultured medullary thick ascending limb cells. Am J Physiol. 1987;252(2 Pt 1):C121–C127. | |
Belfodil R, Barrière H, Rubera I, et al. CFTR-dependent and -independent swelling-activated K+ currents in primary cultures of mouse nephron. Am J Physiol Renal Physiol. 2003;284(4):F812–F828. | |
Stoner LC, Morley GE. Effect of basolateral or apical hyposmolarity on apical maxi K channels of everted rat collecting tubule. Am J Physiol. 1995;268(4 Pt 2):F569–F580. | |
Rieg T, Vallon V, Sausbier M, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72(5):566–573. | |
Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-{beta}1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol. 2005;288(4):F846–F854. | |
Kudlacek PE, Pluznick JL, Ma R, Padanilam B, Sansom SC. Role of hbeta1 in activation of human mesangial BK channels by cGMP kinase. Am J Physiol Renal Physiol. 2003;285(2):F289–F294. | |
Bailey MA, Cantone A, Yan Q, et al. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70(1):51–59. | |
Najjar F, Zhou H, Morimoto T, et al. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2005;289(4):F922–F932. | |
Wein AJ, Rackley RR. Overactive bladder: a better understanding of pathophysiology, diagnosis and management. J Urol. 2006;175(3 Pt 2):S5–S10. | |
Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288(6):C1255–C1263. | |
Shruti S, Urban-Ciecko J, Fitzpatrick JA, Brenner R, Bruchez MP, Barth AL. The brain-specific Beta4 subunit downregulates BK channel cell surface expression. PLoS One. 2012;7(3):e33429. | |
Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22(10):505–512. | |
Shipston MJ. Ion channel regulation by protein palmitoylation. J Biol Chem. 2011;286(11):8709–8716. | |
Shipston MJ. Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol. 2001;11(9):353–358. | |
Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca(2+)-activated K+ channel. Biophys J. 1992;63(2):583–590. | |
Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474(1):99–106. | |
Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97(10):5562–5567. | |
Hu H, Shao LR, Chavoshy S, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21(24):9585–9597. | |
Dalziel JE, Finch SC, Dunlop J. The fungal neurotoxin lolitrem B inhibits the function of human large conductance calcium-activated potassium channels. Toxicol Lett. 2005;155(3):421–426. | |
Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE. The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J Pharmacol Exp Ther. 2008;327(3):657–664. | |
Imlach WL, Finch SC, Dunlop J, Dalziel JE. Structural determinants of lolitrems for inhibition of BK large conductance Ca2+-activated K+ channels. Eur J Pharmacol. 2009;605(1–3):36–45. | |
Miles CO, Munday SC, Wilkins AL, Ede RM, Towers NR. Large-scale isolation of lolitrem B and structure determination of lolitrem E. J Agric Food Chem. 1994;42:1488–1492. | |
Munday-Finch SC, Miles CO, Wilkins AL, Hawkes AD. Isolation and structure elucidation of lolitrem A, a tremorgenic mycotoxin from perennial ryegrass infected with Acremonium lolii. J Agric Food Chem. 1995;43:1283–1288. | |
Munday-Finch SC, Wilkins AL, Miles CO, Ede RM, Thomson RA. Structure elucidation of lolitrem F, a naturally occurring stereoisomer of the tremorgenic mycotoxin lolitrem B, isolated from Lolium perenne infected with Acremonium lolii. J Agric Food Chem. 1996;44: 2782–2788. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.