Back to Journals » Drug Design, Development and Therapy » Volume 14
Bergenin Exerts Hepatoprotective Effects by Inhibiting the Release of Inflammatory Factors, Apoptosis and Autophagy via the PPAR-γ Pathway
Authors Xiang S, Chen K , Xu L , Wang T, Guo C
Received 28 August 2019
Accepted for publication 6 January 2020
Published 13 January 2020 Volume 2020:14 Pages 129—143
DOI https://doi.org/10.2147/DDDT.S229063
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Cristiana Tanase
Shihao Xiang, 1,* Kan Chen, 2,* Ling Xu, 3 Ting Wang, 3 Chuanyong Guo 1, 2
1Medical College of Soochow University, Suzhou, 215006, People’s Republic of China; 2Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai 200072, People’s Republic of China; 3Department of Gastroenterology, Shanghai Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200336, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuanyong Guo Email [email protected]
Objective: Hepatic ischemia reperfusion (IR) limits the development of liver transplantation technology. The aim of this study was to explore the protective effects of Bergenin on hepatic IR, particularly the elimination of reactive oxygen species (ROS) and activation of the peroxisome proliferators activated receptor γ (PPAR-γ) pathway.
Methods: Initial experiments were performed to confirm the non-toxicity of Bergenin. Mice were randomly divided into sham, IR, and IR + Bergenin (10, 20 and 40 mg/kg) groups, and serum and tissue samples were obtained at 2, 8 and 24 h for detection of liver enzymes (ALT and AST), inflammatory factors (TNF-α, IL-6 and IL-1β), ROS, cell death markers (Bcl-2, Bax, Beclin-1 and LC3) and related important pathways (PPAR-γ, P38 MAPK, NF-κB p65 and JAK2/STAT1).
Results: Bergenin reduced the release of ROS, down-regulated inflammatory factors, and inhibited apoptosis and autophagy. Additionally, expression of PPAR-γ-related genes was increased and phosphorylation of P38 MAPK, NF-κB p65 and JAK2/STAT1-related proteins was decreased in Bergenin pre-treatment groups in a dose-dependent manner.
Conclusion: Bergenin exerts hepatic protection by eliminating ROS, affecting the release of inflammatory factors, and influencing apoptosis- and autophagy-related genes via the PPAR-γ pathway in this model of hepatic IR injury.
Keywords: hepatic ischemia reperfusion, Bergenin, reactive oxygen species, apoptosis, autophagy
Introduction
With economic and medical developments, more and more patients with end-stage liver disease are needing liver transplants.1,2 However, hepatic ischemia-reperfusion (IR) injury is an inevitable pathophysiological condition following this type of surgery. IR can cause acute inflammation, liver failure, multiple organ failure and even death.3,4 At present, there is no definite and effective intervention method, hence it is necessary to identify more economical and effective drugs for the prevention and treatment of symptoms suffered by liver transplant recipients.
The molecular mechanisms involved in liver IR injury are complex and diverse, involving the activation of Kupffer cells and neutrophils, the release of reactive oxygen species (ROS) and the production of inflammatory chemokines.5,6 Kupffer cells and neutrophils play a central regulatory role in the pathological mechanism. Activated Kupffer cells in the early stages of hepatic ischemia produce large quantities of ROS, which induces oxidative stress and stimulates inflammatory cascades, resulting in hepatocyte necrosis and apoptosis by promoting TNF-α, IL-6, IL-1β and other inflammatory factors.7 ROS can act as central metabolites that initiate activation of apoptosis-related signalling pathways.3,8 Normally, ROS are in a dynamic equilibrium, but during reperfusion, ROS released by neutrophils reach cytotoxic levels, resulting in the loss of protein function, which leads to rapid necrosis of hepatocytes.4 This also affects PPAR-γ in endothelial cells and hepatocytes, thereby activating sensitive transcription factors such as NF-κB p65 and JAK/STATs.
PPAR-γ is a nuclear transcription factor belonging to the nuclear hormone receptor superfamily and is expressed in liver, kidney, the nervous system and tumours.9 It can affect the expression of ROS and inflammatory factors by regulating macrophage activation.10,11 In recent years, the role of PPAR-γ in IR has been extensively studied, most intensely for myocardial IR.12,13 PPAR-γ and AMPK were identified as advantageous targets for myocardial IR therapy, and MicroRNA-370 can protect against myocardial IR injury in mice following sevoflurane anaesthetic preconditioning via the PPAR signalling pathway.14,15 Similar findings have also been reported for IR studies in kidney, intestine and brain.16–19 These studies indicate that PPAR-γ is down-regulated in IR, and agonists such as 15-deoxy-Δ12,14-prostaglandin J2 and rosiglitazone can alleviate IR-induced injury. Furthermore, ROS and inflammatory factors such as TNF-α, IL-6 and IL-1β can down-regulate PPAR-γ, the status of which is mainly influenced by the mitogen-activated protein kinase (MAPK) family pathway.20,21 PPAR-γ and activated retinoid X receptor α (RXR-α) form isomeric dimers that regulate the subsequent expression of NF-κB p65 and JAK/STATs, and further affect the regulation of inflammatory factors, apoptosis, and autophagy.22 Therefore, PPAR-γ is one of the key pathways.
At present, there is no definite and effective method for the prevention and/or treatment of IR. Remote ischemic preconditioning has been proposed, but not yet demonstrated. Drug pre-treatment, which uses exogenous bioactive substances to reduce IR injury, has been applied in some clinical studies, and some low- and medium-molecular-weight natural products have been developed that are cheap to extract and have minimal side effects. For example, we previously showed that salidroside, astaxanthin, fucoidan and other compounds are effective against liver IR-induced injury.23–25 Bergenin is an isocoumarin compound first isolated from Brassica oleracea that has since been extracted from a wide variety of plant sources using simple and economical extraction processes. Bergenin has many biological activities including antioxidant, anti-inflammatory, anti-tumour, anti-virus, analgesia and cough relief properties.26–29 Intragastric administration at a dose of 3 g/kg has no toxic effects on the growth and development of mice, and no toxic manifestations in heart, lung, liver, kidney or gastrointestinal organs.30 Bergenin protects liver function against D-galactosamine-induced hepatotoxicity in rats.31 However, there is no definitive mechanistic research on the protective effects against hepatic IR.
In view of the important role of oxidative stress-related ROS production in liver IR injury, ROS clearance may be important for achieving therapeutic effects. To this end, we herein explored the strong antioxidant activity of Bergenin. The effects of Bergenin on PPAR-γ and its associated pathways were investigated, especially from the perspective of ROS clearance and interference with apoptosis and autophagy induced by IR. The findings provide a valuable theoretical basis for clinical application.
Materials and Methods
Reagents
Bergenin (CAS: 477-90-7, purity ≥98.0%) was purchased from Sigma-Aldrich (Cat. No. 80479, St. Louis, MO, USA) and stored at 4°C. It was dissolved in physiological saline for animal treatments. GW9662 (Cat. No. HY-16578, purity: 99.53%) was obtained from MedChemExpress (NJ, USA) and dissolved in DMSO. Primary antibodies used in experiments were Bcl-2 (Cat. No. 3498), Bax (Cat. No. 2772), Caspase-9 (Cat. No. 9508), P38 MAPK (Cat. No. 8690), p-P38 MAPK (Cat. No. 9216), PPAR-γ (Cat. No. 2443), NF-κB p65 (Cat. No. 8242), p-NF-κB p65 (Cat. No. 3033), STAT1 (Cat. No. 14994) and p-STAT1 (Cat. No. 9167) from Cell Signaling Technology, Danvers, MA, USA and LC3-I/II (Cat. No. 14600-1-AP), Beclin-1 (Cat. No. 11306-1-AP), P62 (Cat. No. 18420-1-AP), JAK2 (Cat. No. 17670-1-AP), p-JAK2 (Cat. No. 15234-1-AP), RXR-α (Cat. No. 21218-1-AP), and GAPDH (Cat. No. 60004-1-Ig) from Proteintech, Chicago, IL, USA. ROS Fluorescent Probe-DHE was purchased from Vigorous (Beijing, China). The PrimeScript RT Reagent Kit and SYBR Premix Ex Taq were obtained from TaKaRa Biotechnology (Cat. No. RR420A, Dalian, China). The terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) apoptosis assay kit was purchased from Roche (Cat. No. 11570013910, Basel, Switzerland). Oligonucleotide primers were synthesised by Generay (Shanghai, China). Enzyme linked immunosorbent assay (ELISA) kits for TNF-α, IL-6 and IL-1β were purchased from eBioscience (San Diego, CA, USA).
Animal Preparation
Male Balb/c mice (body weight 23 ± 2 g) were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). They were raised under a 12 h light-dark cycle at a constant temperature of 24 ± 2°C and given free access to food and water. All experimental protocols were performed according to the National Institutes of Health Guidelines and approved by the Animal Care and Use Committee of Shanghai Tongji University. Throughout the course of this study, all efforts were made to minimise the pain and suffering of animals.
We established a 70% liver warm IR model, as described previously.4 Firstly, all mice were fasted before surgery and given free access to water. Intraperitoneal injection was performed using 1.25% sodium pentobarbital (Nembutal, St. Louis, MO, USA), and surgery was performed after successful anaesthesia. After mouse abdomens were depilated and disinfected, a midline incision was performed to open the abdominal cavity, and the portal vein and hepatic artery between the left and middle lobes of the liver were carefully separated. Clamping with non-invasive blood vessel clamps showed that the liver lobe was obviously whitened, and the abdominal cavity was covered with gauze soaked in physiological saline and placed on a 37°C constant temperature heating pad for heat preservation treatment. After 60 min, the blood vessel clip was quickly removed, and the liver was recovered (bright red). The abdominal cavity was closed rearing was continued after awakening.
Experimental Design
In the preliminary study, 24 mice were divided randomly into four groups of six animals per group; Control, Sham, Sham+saline, and Sham+ Bergenin (40 mg/kg). In the formal study, 120 mice were randomly divided into five groups of 24 animals per group; Sham, IR, IR+Bergenin (10 mg/kg, IRB10), IR+Bergenin (20 mg/kg, IRB20), IR +Bergenin (40 mg/kg, IRB40). The Bergenin dose was chosen based on previous research.32–34 Sham groups underwent no action apart from opening and stitching of the abdominal cavity. Bergenin (dissolved in physiological saline) groups were given a corresponding dose (10, 20 or 40 mg/kg) of Bergenin by gavage at 3 days (once before the operation, once per day). Mice in the formal study were sacrificed at 2, 8 and 24 h after surgery (eight animals at each time point), and serum and liver tissues were collected for subsequent experiments.
Cell Culture and Viability
Normal hepatocyte LO2 cells were purchased from the Chinese Academy of Sciences Committee Type Culture Collection Cell Bank. Cell lines were cultured in RPMI-1640 medium and plated at a density of 2×105 cells/well in 96-well plates in 100 μL of medium per well. Cells were treated with Bergenin (3 mM) and GW9662 (10 μM) according to the experiment design for 60 min. Cells were then treated with hypoxia (3% O2, 5% CO2, and 92% N2) for 24 h and reoxygenated (5% CO2, 95% air) for 2 h. Cell viability was evaluated by CCK-8 assay. All experiments were repeated five times.
Biochemical Assays and Histopathological Evaluation
Serum was collected by centrifuging at 4000 rpm for 10 min and stored at −80°C. Serum liver enzymes (ALT and AST) were analysed using an automatic biochemical analyser (Olympus AU1000, Tokyo, Japan). Inflammatory factors were assessed by ELISA kits according to manufacturer’s protocols. Liver tissue was fixed with 4% paraformaldehyde for at least 24 h, embedded in paraffin, and cut into 5 μm thick sections that were preserved at room temperature. Some sections were stained with hematoxylin-eosin (HE) stain to show pathological changes. Nuclei were stained purple blue, and the cytoplasm appeared red under a light microscope (Leica, Wetzlar, Germany).
Liver Tissue ROS Assay
Dihydroethidine (DHE) enters cells through living cell membranes and is oxidised by ROS to form red fluorescent ethidine oxide, which can be used to determine quantify ROS content in cells and measure changes. Liver tissue was washed with saline and dehydrated overnight at 4°C with 30% sucrose solution. Tissues were prepared with optimum cutting temperature compound (OCT) and frozen sections were stored at −20°C. After washing, 10 μM DHE solution was dripped onto sections and samples were incubated at room temperature for 30 min. Red areas of tissues were observed under a light microscope after washing.
SYBR Green Real-Time PCR
Total RNA from liver tissues was extracted with TRIzol, purified, and quantitatively analysed. RNA was reverse-transcribed into cDNA and stored at −20°C. SYBR Green quantitative real-time PCR was performed to detect the expression of target genes using a 7900HT fast real-time PCR system (Applied Biosystems, New York, NY, USA) using appropriate proportions of primers and SYBR Green reagent. Differences in gene expression were calculated according to dissolution curves and reference gene expression. Sequences of primers used in experiments are shown in Table 1.
 |
Table 1 Nucleotide Sequences of Primers Used for qRT-PCR |
Western Blotting Analysis
Fresh liver tissue was ground into a slurry with liquid nitrogen and radioimmunoprecipitation assay (RIPA) lysis buffer, centrifuged, quantified with bicinchoninic acid (BCA), mixed with loading buffer and stored at −20°C. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE; 8–12.5% gels) was subsequently performed using samples with the same protein content at 80 V for concentration and 120 V for separation. Separated proteins were transferred to 0.22 μm polyvinylidene fluoride (PVDF) membranes, blocked with 5% bovine serum albumin (BSA), and incubated with the corresponding primary antibodies overnight at 4°C. On the second day, phosphate buffer solution containing 0.1% Tween 20 (PBST) was used to remove primary antibodies and membranes were incubated with fluorescent secondary antibody (anti-rabbit or anti-mouse IgG) for 1 h at room temperature. The strength of protein signals was measured using an Odyssey two-colour infrared laser imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Immunohistochemistry
Paraffin-embedded slices were dried in an oven at 67°C for 2 h. After dewaxing with alcohol and xylene, slices were rinsed with phosphate-buffered saline (PBS) three times, and antigen retrieval was performed citrate buffer(pH 6). After microwave treatment for 10 min on medium power, removal of water and cooling at room temperature, samples were rinsed with PBS. Each slice was then incubated at room temperature for 10 min with one drop of 3% hydrogen peroxide to block the activity of endogenous peroxidase. After washing, one drop of the corresponding primary antibody was added and incubated at room temperature for 2 h. After rinsing in PBS again, polymer reinforcing agent was added and incubated at room temperature for 20 min. After incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies, diaminobenzidine (DAB) was used to visualise granular brown substances. Finally, samples were sealed with neutral gum and imaged using a microscope equipped with a digital camera (Leica). Image Pro Plus Software 6.0 (Media Cybernetics, Silver Spring, MD, USA) was used to measure the integrated optical densities (IOD) of sections and analyse differences.
TdT-Mediated dUTP Nick End Labelling (TUNEL) Staining
Paraffin sections were washed twice with xylene and once with gradient ethanol. Proteinase K working solution was used to digest tissues for 15–30 min at 37°C. The TUNEL assay reaction mixture was prepared according to the manufacturer’s instructions and used to treat the corresponding tissues at room temperature for 20 min. After drying slides, samples were sealed with neutral gum and a drop of PBS or glycerol was added to observe apoptotic cells. The specific observation and analysis methods were performed as described above for immunohistochemistry experiments.
Transmission Electron Microscopy (TEM)
Flushed liver tissue was perfused with glutaraldehyde buffer (3%) containing 0.2 mM cacodylate. Samples were then post-fixed in 1% osmium tetroxide (OsO4) for 1 h and sections were viewed by TEM using a JEM1230 instrument (JEOL, Japan) to identify autophagosomes and for further analysis.
Statistical Analysis
Experimental data are presented as mean± standard deviation (SD) and all statistical analyses were performed using SPSS v25.0 software (IBM Corp., Armonk, NY, USA). Student’s t-tests and one-way analysis of variance (ANOVA) with the Student-Newman-Keuls method followed by Tukey’s tests were applied when F was significant, according to the characteristics of the data. In all cases, p <0.05 was considered statistically significant. Histograms were generated using GraphPad Prism Software v7.0 for Windows (GraphPad, San Diego, USA).
Results
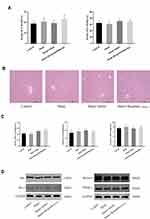
Bergenin Has No Significant Side Effects on Liver or Other Major Organs
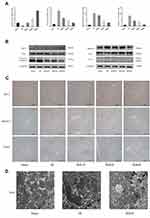
The non-toxicity of Bergenin was firstly validated. Mice were randomly divided into treatment and sham groups and given 40 mg/kg Bergenin (or the same volume of saline for sham groups) for 3 days. The results showed revealed no significant differences in ALT and AST between Bergenin and normal groups, and the other three groups had almost the same levels of these liver enzymes (Figure 1A). Furthermore, HE staining of liver was performed to investigate pathological morphology, and all samples displayed microcytic fission disorder, which may be related to drug metabolism in vivo (Figure 1B). Indeed, there were no significant differences in the levels of TNF-α, IL-6 and IL-1β released in serum, or in apoptosis- and autophagy-related proteins Bcl-2, Bax, Beclin-1 and key pathways related to PPAR-γ in liver tissues (Figure 1C and D). The above results indicate that Bergenin had no obvious side effects on the body.
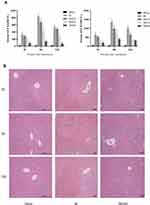
Bergenin Alleviates Liver Function Injury Induced by Ischemia-Reperfusion
The rapid increase in ALT and AST is an important marker of acute liver injury, and the severity of liver IR injury is closely correlated with time. We selected 2, 8 and 24 h as time points according to previous studies.24,25,35 The results showed that ALT and AST were increased at 2 h after reperfusion and peaked at 8 h, then declined at 24h. Moreover, at all three time points, levels of these liver enzymes in Bergenin treatment groups were decreased significantly in a dose-dependent manner, which indicates that Bergenin had an obvious protective effect on liver function (Figure 2A). To further validate the above results, we evaluated pathological changes in liver tissue, and the IR group displayed disordered morphological cell arrangement and damaged tissue structure, and these features worsened over time. However, the area of necrotic liver tissue was significantly reduced after drug pre-treatment in the IRB40 group (Figure 2B). The above results indicate that Bergenin can reduce cell necrosis caused by hepatic IR, and the higher the dose, the better the effect.
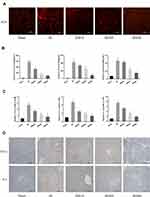
Bergenin Can Effectively Eliminate ROS and Inhibit the Release of Inflammatory Factors
The release of ROS and inflammatory factors (TNF-α, IL-6 and IL-1β) following macrophage activation induced by ischemia is an important link in IR injury with key significance in aggravating microcirculation disorders in liver. Therefore, we chose the 8h time point characterised by the most severe injuries for further exploration. It was found that the ROS content (red fluorescence) was increased significantly in the IR group, but decreased in drug treatment groups (Figure 3A). Furthermore, the effect of a high dose was more pronounced than a low dose. We also explored inflammatory factors in terms of serum levels, gene transcription and protein expression. ELISA and PCR data showed that pre-treatment with Bergenin significantly inhibited the release of inflammatory factors at each time point (Figure 3B and C). In order to visualise morphological changes, we performed immunohistochemical staining of liver slices, and the appearance of brown granules confirmed expression of TNF-α and IL-6. Consistent with the observed changes in serology and gene transcription, inflammatory factors were elevated in the IR group but decreased significantly after Bergenin treatment (Figure 3D). These results suggest that Bergenin can eliminate the production of ROS and inflammatory factors induced by liver IR.
Bergenin Activates PPAR-γ and Inhibits Phosphorylation of Factors in Related Pathways
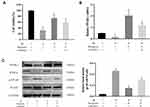
PPAR-γ is an important pathway in hepatic IR. It plays a crucial role in regulating cell growth, differentiation and adaptation to environmental stress. We screened key pathways related to ROS and inflammatory factors, such as P38 MAPK, NF-κB p65 and JAK2/STAT1, according to the proposed IR mechanism, in order to explore the hepatoprotective effects of Bergenin. Firstly, we measured the expression of PPAR-γ in liver. The results showed that PPAR-γ was significantly increased in Bergenin-treated liver tissues at both mRNA and protein levels, and RXR-α was up-regulated synchronously, but inhibited in the IR group (Figure 4A). Furthermore, the upstream P38 MAPK is also very sensitive to ROS generation in the IR group. We therefore measured phosphorylation of P38 MAPK in different groups, and phosphorylation was clearly inhibited by drug treatment (Figure 4B). We also analysed the phosphorylation ratio of P38 MAPK, and the results were consistent with the observed changes in ROS (Figure 4C). Meanwhile, PPAR-γ regulates a variety of downstream genes, and NF-κB p65 and JAK2/STAT1 are closely related to oxidative stress and inflammation. We therefore evaluated protein phosphorylation and tissue expression levels, and the results were consistent with the inflammatory factor release levels described above (Figure 4D). Therefore, we believe that Bergenin activates PPAR-γ by clearing ROS and regulating the P38 MAPK pathway, which subsequently regulates downstream pathways related to inflammatory factor release.
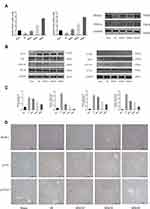
Bergenin Can Effectively Inhibit Liver IR-Induced Apoptosis and Autophagy
Apoptosis and autophagy are important death modes besides cell necrosis, and the interaction between apoptosis and autophagy plays an important role in IR injury. Therefore, we assessed the known markers Bcl-2, Bax, and caspases (for apoptosis) and Beclin-1, LC3-II and P62 (for autophagy) to evaluate programmed cell death. The results of PCR and Western blotting showed that Bergenin promoted the expression of anti-apoptotic protein Bcl-2, consistent with the expression of PPAR-γ. At the same time, it inhibited the expression of Bax and caused an imbalance in the Bcl-2/Bax ratio (Figure 5A and B), which is important for apoptosis in vivo. The corresponding mitochondrial apoptotic proteins caspase-9 were clearly decreased (Figure 5B). Similarly, the increase in Beclin-1 and LC3-II and the decrease in P62 in the IR group indicate that autophagy was increased significantly, while Bergenin inhibited the occurrence of autophagy. The above results were verified by immunohistochemical staining and shown to be reliable (Figure 5C).
TUNEL staining and electron microscopy are important methods for probing apoptosis and autophagy. Compared with the sham group, autophagic vacuoles were increased significantly in the IR group, but autophagosomes were not easily detected after drug treatment. Meanwhile, TUNEL staining also revealed that apoptotic cells were increased in the IR group and decreased in the drug treatment groups (Figure 5D). In conclusion, these results suggest that Bergenin not only reduces cell necrosis, but also inhibits cell apoptosis and autophagy.
Bergenin Activates PPAR-γ and Inhibits Phosphorylation of NF-κB P65 in vitro
In order to further verify the mechanism, we used normal hepatocyte (LO2) cells for hypoxia treatment to simulate the injury mechanism in vivo. Before this, Bergenin and PPAR-γ inhibitor GW9662 were added to cells for pre-treatment. The results showed that Bergenin could effectively protect against cell death caused by hypoxia, and the proportion of dead cells was increased again after inhibiting PPAR-γ activation (Figure 6A). By contrast, PCR and Western blotting results showed that Bergenin may also play a role in hepatocyte protection by activating PPAR-γ and inhibiting NF-κB p65 in vitro (Figure 6B and C).
Discussion
Liver IR injury is an important factor restricting the development of liver transplantation in patients with end-stage liver disease, and reducing the extent of IR injury could represent a breakthrough. Bergenin is a natural secondary metabolite extracted from the rhizomes, bark and leaves of many plant families and genera. Its pharmacological activities are diverse, and is has been included in the Pharmacopoeia as an antitussive drug, but it is also useful for antioxidation treatment.
Non-toxicity of drugs is a precondition for their application. Herein, we first used a high dose of Bergenin (40 mg/kg) to explore potential changes in liver function and the pathological performance of organs in mice, and found that this dose had no significant effects in vivo. Thereafter, we established a transient IR model and performed pre-treatment with various doses of Bergenin before the model was established. A significant increase in liver enzymes indicated that liver IR caused extensive hepatocyte necrosis, since ALT and AST were released into the blood in large quantities. By contrast, in the drug treatment groups, liver enzyme levels displayed a steady decline with increasing drug dosage. HE staining showed that the necrotic area was consistent with the levels of hepatic enzymes, which indicates that Bergenin played a significant role in liver protection. This is consistent with the results of previous studies.30
When cells are stimulated by physical, chemical or biological factors, excessive quantities of ROS can be produced and this stress is important for adaptation. Studies have shown that ROS in liver IR are produced by Kupffer cells and neutrophils. ROS activate a series of pathways including the MAPK signalling pathway, and induce the release of TNF-α, IL-6 and IL-1β, which further aggravate cell damage.24 Gao and colleagues demonstrated that Bergenin decreases levels of NO, TNF-α, IL-6 and IL-1β, which are increased in lipopolysaccharide (LPS)-induced mouse mastitis.27 Meanwhile, Bergenin is known to ameliorate diabetic nephropathy in rats by suppressing renal inflammation.36 Thus, we investigated ROS and inflammatory factors related to this process using molecular biological methods, and the results also confirmed that Bergenin can effectively eliminate oxidative stress products produced during IR, and reduce the production of inflammatory factors. This may be related to the formation of beta-conformational carbohydrates from 4-methylated gallic acid and glucose, consistent with previous studies on IR, but the pathway through which ROS plays a protective role in hepatocytes has not been clearly elucidated.
Studying PPAR-γ and its receptors is important for understanding IR.13 PPAR-γ controls the metabolism of many cells and it regulates upstream MAPK family members, while downstream factors mediate multiple pathways such as NF-κB p65, JAK2/STAT1 and NFAT pathways.37,38 MAPK is an important transmitter of signals from the cell surface to the nucleus that can be activated by various extracellular stimuli such as cytokines, neurotransmitters, hormones, cell stress and cell adhesion.39,40 NF-κB p65 and JAK2/STAT1 signalling pathways are involved in inflammation through complex molecular regulation. In a previous study, our team demonstrated that 15-Deoxy-△-12,14-Prostaglandin J2 (15d-PGJ2) can improve liver function by activating PPAR-γ to inhibit NF-κB p65, and the role and mechanism of the PPAR-γ natural ligand activator-15d-PGJ2 in liver has since reviewed.41–43 Herein, we carried out molecular biological studies on PPAR-γ as well as upstream and downstream pathways. Firstly, we found that activation and expression of PPAR-γ in IR were significantly inhibited, whereas PPAR-γ was activated in drug treatment groups, corresponding to improved liver function. By contrast, increased phosphorylation of P38 MAPK in the IR group was decreased following Bergenin pre-treatment, consistent with the observed changes in ROS. In addition, down-regulation of phosphorylated NF-κB p65 and JAK2/STAT1 by PPAR-γ in drug treatment groups may affect the release of inflammatory factors, leading to cell death. Thus, we demonstrated that the release of inflammatory factors such as TNF-α, IL-6 and IL-1β was reduced by Bergenin. The mechanism may involve activation of PPAR-γ induced by inhibiting P38 MAPK phosphorylation, thereby weakening the ability to induce hepatocyte death via NF-κB p65 and JAK2/STAT1.
It is generally believed that cell death may involve necrosis, apoptosis and autophagy, and the balance between apoptosis and autophagy plays an important role in cell survival.44,45 Besides ROS-induced cell necrosis, apoptosis and autophagy are also important in cell death in IR. Apoptosis is related to the severity of ischemia and the time of reperfusion.46,47 Effective inhibition of apoptosis can protect the liver from IR injury.35 Autophagy is a ubiquitous life process in eukaryotic cells. Under short-term hypoxia and starvation conditions, autophagy is activated and plays a dual regulatory role.48 Epicatechin gallate can protect HBMVECs from IR injury by ameliorating apoptosis and autophagy, but Alliin alleviates myocardial IR injury by promoting autophagy.49,50 Initial autophagy is an important defence mechanism to remove damaged cell structures and aging organelles from degraded cells. However, excessive autophagy and apoptosis in the latter stages cause hepatocyte death and aggravate damage of hepatocytes. Therefore, if a compound can effectively inhibit apoptosis and autophagy, it may protect the liver to some extent. Studies have proved that PPAR-γ has an important relationship with Bcl-2, which can directly up-regulate Bcl-2 and down-regulate Bax to play an effective anti-apoptotic role.51–53 Free Bcl-2 has a BH3 domain that can bind to Beclin-1, a specific marker of autophagy, thereby affecting the occurrence of autophagy.54 Similarly, our present results showed that Bax, Bcelin-1 and LC3-II were increased significantly in liver IR while Bcl-2 was decreased. However, after drug treatment, these indices were reversed in a dose-dependent manner.
Combining the above theories and results, Bergenin appears to mediate the activation of PPAR-γ by eliminating ROS-induced phosphorylation of P38 MAPK. PPAR-γ competes with and recruits CBP and p300, limiting their ability to inhibit entry of NF-κB p65 into the nucleus as well as JAK2/STAT1 phosphorylation, thereby blocking the release of TNF-α, IL-6 and IL-1β, causing hepatocyte injury. Additionally, PPAR-γ activation disrupts the Bcl-2/Bax ratio and decreases formation of the Bax/Bax heterodimer, which affects MPTP opening and prevents the release of cytochrome C from mitochondria. Finally, reduced cytochrome C release into the cytoplasm attenuates hepatocyte apoptosis and is accompanied by a decrease in cleaved caspase-9. On the other hand, up-regulation of Bcl-2 results in greater binding to free active Beclin-1, which mediates the conversion of LC3-I to LC3-II. Reducing Beclin-1 activity renders it unable to bind to P62 carrying LC3-II, which reduces cell autophagy. This could explain the protective effect of Bergenin on liver function from three aspects; necrosis, apoptosis and autophagy (Figure 7). Due to the complexity of natural products and their mechanisms, the exact targets and mechanisms need to be further explored and verified.
In conclusion, the antioxidant effect of Bergenin plays an important role in IR, mainly by eliminating ROS, mediating the release of inflammatory factors, and influencing apoptosis- and autophagy-related genes via the PPAR-γ pathway. Bergenin exhibits potent pharmacological activity, and it can protect many organs through multiple mechanisms simultaneously. Therefore, it is of great clinical value for the prevention and treatment of IR in liver transplantation. In-depth research is needed to develop derivatives with higher activity and lower toxicity.
Funding
This work was supported by the Science and Technology Committee of Shanghai (grant No. 16411972700), and the Science and Technology Committee of Changning District, Shanghai (grant No. CNKW2017Y05).
Disclosure
The authors report no conflicts of interest in the present study.
References
1. Santopaolo F, Lenci I, Bosa A, Angelico M, Milana M, Baiocchi L. Domino liver transplantation: where are we now? Rev Recent Clin Trials. 2019;14:183–188. doi:10.2174/1574887114666190320123824
2. Hecht EM, Kambadakone A, Griesemer AD, et al. Living donor liver transplantation: overview, imaging technique, and diagnostic considerations. AJR Am J Roentgenol. 2019;1–11.
3. Marshall K, Jin J, Atkinson C, et al. Natural immunoglobulin M initiates an inflammatory response important for both hepatic ischemia reperfusion injury and regeneration in mice. Hepatology. 2017;67:721–735.
4. Abe Y, Hines IN, Zibari G, et al. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med. 2009;46(1):1–7. doi:10.1016/j.freeradbiomed.2008.09.029
5. Kurokawa T, Nonami T, Harada A, Nakao A, Takagi H. Mechanism and prevention of ischemia-reperfusion injury of the liver. Semin Surg Oncol. 1996;12(3):179–182. doi:10.1002/(ISSN)1098-2388
6. Takenaka H. Genesis and mechanism of ischemia-reperfusion injuries. Seikagaku. 2000;72(12):1433–1436.
7. Li H, Xia Z, Chen Y, Qi D, Zheng H. Mechanism and therapies of oxidative stress-mediated cell death in ischemia reperfusion injury. Oxid Med Cell Longev. 2018;2018:2910643.
8. Kang KJ. Mechanism of hepatic ischemia/reperfusion injury and protection against reperfusion injury. Transplant Proc. 2002;34(7):2659–2661. doi:10.1016/S0041-1345(02)03465-6
9. Kinarivala N, Suh JH, Botros M, Webb P, Trippier PC. Pharmacophore elucidation of phosphoiodyn A - potent and selective peroxisome proliferator-activated receptor beta/delta agonists with neuroprotective activity. Bioorg Med Chem Lett. 2016;26(8):1889–1893. doi:10.1016/j.bmcl.2016.03.028
10. Zolezzi JM, Silva-Alvarez C, Ordenes D, et al. Peroxisome proliferator-activated receptor (PPAR) gamma and PPARalpha agonists modulate mitochondrial fusion-fission dynamics: relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS One. 2013;8(5):e64019. doi:10.1371/journal.pone.0064019
11. Xia P, Pan Y, Zhang F, et al. Pioglitazone confers neuroprotection against ischemia-induced pyroptosis due to its inhibitory effects on HMGB-1/RAGE and Rac1/ROS pathway by activating PPAR. Cell Physiol Biochem. 2018;45(6):2351–2368. doi:10.1159/000488183
12. Linares I, Farrokhi K, Echeverri J, et al. PPAR-gamma activation is associated with reduced liver ischemia-reperfusion injury and altered tissue-resident macrophages polarization in a mouse model. PLoS One. 2018;13(4):e0195212. doi:10.1371/journal.pone.0195212
13. Elias-Miro M, Jimenez-Castro MB, Mendes-Braz M, Casillas-Ramirez A, Peralta C. The current knowledge of the role of PPAR in hepatic ischemia-reperfusion injury. PPAR Res. 2012;2012:802384. doi:10.1155/2012/802384
14. Morrison A, Li J. PPAR-gamma and AMPK–advantageous targets for myocardial ischemia/reperfusion therapy. Biochem Pharmacol. 2011;82(3):195–200. doi:10.1016/j.bcp.2011.04.004
15. Zhao YB, Zhao J, Zhang LJ, et al. MicroRNA-370 protects against myocardial ischemia/reperfusion injury in mice following sevoflurane anesthetic preconditioning through PLIN5-dependent PPAR signaling pathway. Biomed Pharmacother. 2019;113:108697. doi:10.1016/j.biopha.2019.108697
16. Singh AP, Singh N, Pathak D, Bedi PMS. Estradiol attenuates ischemia reperfusion-induced acute kidney injury through PPAR-gamma stimulated eNOS activation in rats. Mol Cell Biochem. 2019;453(1–2):1–9. doi:10.1007/s11010-018-3427-4
17. Wu XJ, Sun XH, Wang SW, Chen JL, Bi YH, Jiang DX. Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPAR gamma. Eur Rev Med Pharmacol Sci. 2018;22(17):5688–5696. doi:10.26355/eurrev_201809_15836
18. Al Rouq F, El Eter E. PPAR-gamma activator induces neuroprotection in hypercholesterolemic rats subjected to global cerebral ischemia/reperfusion injury: in vivo and in vitro inhibition of oxidative stress. Exp Gerontol. 2014;51:1–7. doi:10.1016/j.exger.2013.12.008
19. El-Sayyad SM, Soubh AA, Awad AS, El-Abhar HS. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: involvement of PPAR-gamma, GSK-3beta and Wnt/beta-catenin pathway. Eur J Pharmacol. 2017;809:80–86. doi:10.1016/j.ejphar.2017.05.021
20. Mo W, Wang C, Li J, et al. Fucosterol protects against concanavalin A-induced acute liver injury: focus on P38 MAPK/NF-kappaB pathway activity. Gastroenterol Res Pract. 2018;2018:2824139. doi:10.1155/2018/2824139
21. Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71(3):388–400.
22. Degrelle SA, Shoaito H, Fournier T. New transcriptional reporters to quantify and monitor PPARgamma activity. PPAR Res. 2017;2017:6139107. doi:10.1155/2017/6139107
23. Feng J, Zhang Q, Mo W, et al. Salidroside pretreatment attenuates apoptosis and autophagy during hepatic ischemia-reperfusion injury by inhibiting the mitogen-activated protein kinase pathway in mice. Drug Des Devel Ther. 2017;11:1989–2006. doi:10.2147/DDDT.S136792
24. Li J, Wang F, Xia Y, et al. Astaxanthin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy via the ROS/MAPK pathway in mice. Mar Drugs. 2015;13(6):3368–3387. doi:10.3390/md13063368
25. Li J, Zhang Q, Li S, et al. The natural product fucoidan ameliorates hepatic ischemia-reperfusion injury in mice. Biomed Pharmacother. 2017;94:687–696. doi:10.1016/j.biopha.2017.07.109
26. Kim HS, Lim HK, Chung MW, Kim YC. Antihepatotoxic activity of bergenin, the major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated hepatocytes. J Ethnopharmacol. 2000;69(1):79–83. doi:10.1016/S0378-8741(99)00137-3
27. Gao XJ, Guo MY, Zhang ZC, Wang TC, Cao YG, Zhang NS. Bergenin plays an anti-inflammatory role via the modulation of MAPK and NF-kappaB signaling pathways in a mouse model of LPS-induced mastitis. Inflammation. 2015;38(3):1142–1150. doi:10.1007/s10753-014-0079-8
28. Khan H, Amin H, Ullah A, et al. Antioxidant and antiplasmodial activities of bergenin and 11-O-galloylbergenin isolated from Mallotus philippensis. Oxid Med Cell Longev. 2016;2016:1051925. doi:10.1155/2016/1051925
29. Yang S, Yu Z, Wang L, et al. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J Ethnopharmacol. 2017;200:147–155. doi:10.1016/j.jep.2017.02.013
30. Roy VK, Chenkual L, Gurusubramanian G. Protection of testis through antioxidant action of Mallotus roxburghianus in alloxan-induced diabetic rat model. J Ethnopharmacol. 2015;176:268–280. doi:10.1016/j.jep.2015.11.006
31. Lim HK, Kim HS, Choi HS, Choi J, Kim SH, Chang MJ. Effects of bergenin, the major constituent of Mallotus japonicus against D-galactosamine-induced hepatotoxicity in rats. Pharmacology. 2001;63(2):71–75. doi:10.1159/000056115
32. Kaur R, Kaur S. Evaluation of in vitro and in vivo antileishmanial potential of bergenin rich Bergenia ligulata (Wall.) Engl. root extract against visceral leishmaniasis in inbred BALB/c mice through immunomodulation. J Tradit Complement Med. 2018;8(1):251–260.
33. Wang K, Li YF, Lv Q, Li XM, Dai Y, Wei ZF. Bergenin, acting as an agonist of PPARgamma, ameliorates experimental colitis in mice through improving expression of SIRT1, and therefore inhibiting NF-kappaB-mediated macrophage activation. Front Pharmacol. 2017;8:981.
34. Yun J, Lee Y, Yun K, Oh S. Bergenin decreases the morphine-induced physical dependence via antioxidative activity in mice. Arch Pharm Res. 2015;38(6):1248–1254. doi:10.1007/s12272-014-0534-y
35. Yu Q, Wu L, Liu T, et al. Protective effects of levo-tetrahydropalmatine on hepatic ischemia/reperfusion injury are mediated by inhibition of the ERK/NF-kappaB pathway. Int Immunopharmacol. 2019;70:435–445. doi:10.1016/j.intimp.2019.02.024
36. Yang J, Kan M, Wu GY. Bergenin ameliorates diabetic nephropathy in rats via suppressing renal inflammation and TGF-beta1-Smads pathway. Immunopharmacol Immunotoxicol. 2016;38(2):145–152. doi:10.3109/08923973.2016.1142560
37. Fanale D, Amodeo V, Caruso S. The interplay between metabolism, PPAR signaling pathway, and cancer. PPAR Res. 2017;2017:1830626. doi:10.1155/2017/1830626
38. Harnchoowong S, Suchonwanit P. PPAR-gamma agonists and their role in primary cicatricial alopecia. PPAR Res. 2017;2017:2501248. doi:10.1155/2017/2501248
39. Zhang G, He J, Ye X, et al. beta-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019;10(4):255. doi:10.1038/s41419-019-1492-6
40. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi:10.1155/2011/792639
41. Chen K, Li J, Wang J, et al. 15-Deoxy- gamma 12,14-prostaglandin J2 reduces liver impairment in a model of ConA-induced acute hepatic inflammation by activation of PPAR gamma and reduction in NF- kappa B activity. PPAR Res. 2014;2014:215631.
42. Chen K, Li JJ, Li SN, et al. 15-Deoxy-Delta(12,14)-prostaglandin J2 alleviates hepatic ischemia-reperfusion injury in mice via inducing antioxidant response and inhibiting apoptosis and autophagy. Acta Pharmacol Sin. 2017;38(5):672–687. doi:10.1038/aps.2016.108
43. Li J, Guo C, Wu J. 15-Deoxy–(12,14)-Prostaglandin J2 (15d-PGJ2), an endogenous ligand of PPAR-gamma: function and mechanism. PPAR Res. 2019;2019:7242030. doi:10.1155/2019/7242030
44. Makoukji J, Saadeh F, Mansour KA, et al. Flupirtine derivatives as potential treatment for the neuronal ceroid lipofuscinoses. Ann Clin Transl Neurol. 2018;5(9):1089–1103. doi:10.1002/acn3.2018.5.issue-9
45. Kinarivala N, Patel R, Boustany RM, Al-Ahmad A, Trippier PC. Discovery of aromatic carbamates that confer neuroprotective activity by enhancing autophagy and inducing the anti-apoptotic protein B-Cell lymphoma 2 (Bcl-2). J Med Chem. 2017;60(23):9739–9756. doi:10.1021/acs.jmedchem.7b01199
46. Tao L, Huang K, Wang J, et al. Retinol palmitate protects against myocardial ischemia/reperfusion injury via reducing oxidative stress and inhibiting apoptosis. Am J Transl Res. 2019;11(3):1510–1520.
47. Chen L, Zhang D, Yu L, Dong H. Targeting MIAT reduces apoptosis of cardiomyocytes after ischemia/reperfusion injury. Bioengineered. 2019;10:121–132. doi:10.1080/21655979.2019.1605812
48. Aghaei M, Motallebnezhad M, Ghorghanlu S, et al. Targeting autophagy in cardiac ischemia/reperfusion injury: a novel therapeutic strategy. J Cell Physiol. 2019;234:16768–16778. doi:10.1002/jcp.28345
49. Fu B, Zeng Q, Zhang Z, et al. Epicatechin gallate protects HBMVECs from ischemia/reperfusion injury through ameliorating apoptosis and autophagy and promoting neovascularization. Oxid Med Cell Longev. 2019;2019:7824684. doi:10.1155/2019/7824684
50. Zhao R, Xie E, Yang X, Gong B. Alliin alleviates myocardial ischemia-reperfusion injury by promoting autophagy. Biochem Biophys Res Commun. 2019;512:236–243.
51. Ren Y, Sun C, Sun Y, et al. PPAR gamma protects cardiomyocytes against oxidative stress and apoptosis via Bcl-2 upregulation. Vascul Pharmacol. 2009;51(2–3):169–174. doi:10.1016/j.vph.2009.06.004
52. Yang J. PPAR-gamma silencing inhibits the apoptosis of A549 cells by upregulating Bcl-2. Zhongguo Fei Ai Za Zhi. 2013;16(3):125–130. doi:10.3779/j.issn.1009-3419.2013.03.02
53. Nie H, Xue X, Li J, et al. Nitro-oleic acid attenuates OGD/R-triggered apoptosis in renal tubular cells via inhibition of Bax mitochondrial translocation in a PPAR-gamma-dependent manner. Cell Physiol Biochem. 2015;35(3):1201–1218. doi:10.1159/000373944
54. Chiang WC, Wei Y, Kuo YC, et al. High-throughput screens to identify autophagy inducers that function by disrupting beclin 1/Bcl-2 binding. ACS Chem Biol. 2018;13(8):2247–2260. doi:10.1021/acschembio.8b00421
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.