Back to Journals » Drug Design, Development and Therapy » Volume 9
Arsenic sulfide inhibits cell migration and invasion of gastric cancer in vitro and in vivo
Authors Zhang L, Kim S, Ding W, Tong Y, Zhang X, Pan M, Chen S
Received 3 June 2015
Accepted for publication 4 August 2015
Published 9 October 2015 Volume 2015:9 Pages 5579—5590
DOI https://doi.org/10.2147/DDDT.S89805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Lian Zhang,1 Sungkyoung Kim,1 Wenping Ding,1 Yingying Tong,1 Xiuli Zhang,1 Minggui Pan,2 Siyu Chen1
1Department of Oncology, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Oncology and Hematology, Kaiser Permanente Medical Center, Santa Clara, CA, USA
Background: We previously showed that arsenic sulfide (As4S4) induced cell cycle arrest and apoptosis in several human solid tumor cell lines, including those of gastric cancer. In this study, we investigated the effect of As4S4 on the migration and invasion of gastric cancer cells both in vitro and in vivo.
Methods: The human gastric cancer cell lines AGS and MGC803 were selected as in vitro models. Wound-healing migration assay and Transwell invasion assay were carried out to determine the effects of As4S4 on cell migration and invasion. The expressions of E-cadherin, β-catenin, Sp1, KLF4, and VEGF were measured by Western blotting analysis. The activities of matrix metalloproteinase (MMP)-2 and MMP-9 in MGC803 cells were demonstrated by zymography assay. A mouse xenograft model was established by inoculation with MGC803 cells, then intraperitoneal injected with As4S4 for 3 weeks and monitored for body weight and tumor changes. Finally, the inhibition rate of tumor growth was calculated, and the expression of proteins and genes associated with tumor invasion and metastasis in tumor tissues were measured by immunohistochemistry, Western blotting, and real-time polymerase chain reaction assay.
Results: As4S4 significantly inhibited the migration and invasion of gastric cancer cell lines. The expression of E-cadherin and KLF4 was upregulated, while the expressions of β-catenin, VEGF, and Sp1 were downregulated following treatment with As4S4. Moreover, the protease activities of MMP-2 and MMP-9 were suppressed by As4S4 in MGC803 cells. Meanwhile, As4S4 effectively suppressed the abilities of tumor growth and invasion in the xenograft tumor model. We found that As4S4 upregulated the expression of E-cadherin and downregulated the expression of β-catenin, Sp1, VEGF, and CD34 in mouse tumor tissues, consistent with the results in vitro.
Conclusion: As4S4 inhibited the migration and invasion of gastric cancer cells by blocking tumor cell adhesion, decreasing the ability of tumor cells to destroy the basement membrane, and therefore suppressing their angiogenesis.
Keywords: As4S4, xenograft, realgar, E-cadherin, MMPs, VEGF
Introduction
Epidemiological investigations have shown that gastric cancer (GC) is the fourth most common malignant cancer in the world.1 Based on the data of GLOBOCAN 2012, approximately 1 million new cases of GC were diagnosed in 2012, and GC is the third main cause of cancer-related death (723,073 cases, 8.8% of total cancer-related death) after lung and liver cancers.2 It is well known that metastasis and invasion are basic properties of many malignant cancer cells and the main cause of cancer-related mortality.3 Although radical resection obviously prolongs the overall survival of patients diagnosed at early stages, GC still carries a poor prognosis with a high metastasis and recurrence rate. Therefore, identifying a compound with the ability to suppress GC metastasis is of great importance.
Arsenic sulfide (As4S4), the active ingredient of the traditional Chinese medicine realgar, has been used for several centuries in Oriental medicine. According to the 2010 Pharmacopoeia of the People’s Republic of China,4 As4S4 is widely used in combination with other traditional medicines for both external and internal treatment, such as for problems of skin disease, fever, infection, inflammation, and convulsion.5,6 Recently, because of the remarkable success of arsenic trioxide (As2O3), another important member of the arsenic compounds family, in the treatment of acute promyelocytic leukemia,7–9 more and more scholars have been focusing on the medicinal value of As4S4 in the field of cancer treatment due to its advantages of oral administration, relative safety, and ample resources compared with As2O3. Recent studies show that As4S4 has antitumor activities in several cancers in vitro and in vivo, especially for hematological malignancies.10,11 The antitumor mechanism of As4S4 remains poorly understood, but many studies have shown that it potently inhibits cell proliferation while inducing apoptosis.11–13
The process of tumor development, invasion, and metastasis is complex and evolutionary, consisting of multiple steps and involving numerous events at the cellular and molecular levels. The tumor cells need to separate from each other, suspend themselves in the circulation, penetrate through the vasculature, establish angiogenesis, and perform the epithelial–mesenchymal transition (EMT). All these processes are closely related.14,15 Recent studies have shown that each stage of this process is important for effective tumor invasion and metastasis.14,16
In our previous studies, we investigated the antitumor effect of As4S4 in a number of tumor cell lines, such as HepG2 cells (hepatocellular carcinoma), A375 cells (malignant melanoma), 8898 cells (pancreatic carcinoma), MKN45 cells (GC), and NB4 and MR2 cells (acute promyelocytic leukemia).11,17 Further explorations on GC have found that the antitumor effect of As4S4 is dependent on the induction of cell apoptosis, which may be associated with p53-dependent pathway.18 In the present study, we investigated the chemotherapeutic effects and the underlying molecular mechanism of As4S4 on the invasion and migration of GC cells both in vitro and in vivo. We found that As4S4 inhibited GC cell migration and invasion through increasing the ability of tumor cell adhesion, decreasing the ability of tumor cells to destroy the basement membrane, and suppressing angiogenesis.
Materials and methods
Cells, animals, and reagents
The human GC cell lines AGS and MGC803 were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, People’s Republic of China). No ethics statement was required from the institutional review board for the use of these cell lines. AGS cells were cultured in DMEM/F12 1:1 medium (Hyclone, Logan, UT, USA), and MGC803 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C under an atmosphere of 95% air and 5% CO2. All medium was supplemented with 10% fetal bovine serum (Thermo Fisher Scientific). BALB/C-nu/nu mice (4 weeks old, male, weighing 17–20 g) were purchased from Shanghai Laboratory Animal Research Center (Shanghai, People’s Republic of China) and maintained on standard chow and water. All experiments were performed in accordance with the guidelines of the laboratory animal handling protocols of Shanghai Jiao Tong University School of Medicine (Shanghai, People’s Republic of China). This study was approved by the ethics committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, People’s Republic of China). Highly purified realgar (Figure S1) supplied by the Shanghai Institute of Hematology (Shanghai, People’s Republic of China) was prepared from mined natural realgar and the preparation of As4S4 solution was performed as previously described.18 In brief, the high-purity realgar was dissolved in Dulbecco’s phosphate-buffered saline, and the content of As in the Dulbecco’s phosphate-buffered saline solution was determined by inductively coupled plasma atomic emission spectrometry at the Instrumental Analysis Center of Shanghai Jiao Tong University (Shanghai, People’s Republic of China). The stock solution of As4S4 was 277.2496 μM and then was diluted to work solution with complete culture medium (for cell culture) or normal saline (for animal experiment). The anti-E-cadherin, anti-β-catenin, and anti-Sp1 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA), anti-vascular endothelial growth factor (VEGF) and anti-KLF4 from Abcam (Cambridge, MA, USA), anti-CD34 from Sigma-Aldrich Co. (St Louis, MO, USA), and anti-β-actin antibody from Proteintech Group, Inc (Wuhan, People’s Republic of China).
Wound-healing assay
Wound-healing assay was performed as previously described.19 AGS and MGC803 cells were plated in six-well plates at a density of 5×105 cells/well and serum starved for 12 hours, respectively. Then, wounds were scratched with pipette tips and washed with phosphate-buffered saline to remove the suspended cells. The media containing 1% serum and different concentrations (0, 0.25, 0.5, and 1 μM) of As4S4 were used for cell culture. The assay was performed for 24 or 48 hours at 37°C and 5% CO2. Wounds were photographed with a DMI3000 B inverted microscope (Leica Microsystems, Wetzlar, Germany) at 0 hours, 24 hours, and 48 hours, and the migration rates of each group were calculated in accordance with the following formula, migration rate (%) = (wound area at 0 h − wound area at 24 h or 48 h)/wound area at 0 h × 100%.
Transwell invasion assay
For cell invasion analysis, MGC803 cells were cultured in serum-free RPMI 1640 medium overnight. In the bottom of the Transwell chamber (Corning Incorporated, Corning, NY, USA), 500 μL of RPMI 1640 medium with 10% fetal bovine serum were added, and the upper part of each chamber was seeded with 5×104 MGC803 cells with 200 μL RPMI 1640 medium which contained 1% serum and different concentrations (0, 0.5, and 1 μM) of As4S4. The assay was performed for 12 and 24 hours at 37°C and 5% CO2. Migrated cells were fixed with 4% paraformaldehyde for 20 minutes at room temperature and then analyzed by crystal violet staining, followed by observation under a DMI3000 B inverted microscope. The invasion rates of each group (12 h or 24 h) were calculated in accordance with the following formula, invasion rate (%) =the number of the migrated cells at 0.5 or 1 μM/the number of the migrated cells at 0 μM × 100%.
Gelatin zymography assay
The activity of MMP-2 and MMP-9 released from MGC803 cells was measured by gelatin zymography assays according to the methods reported by Yang et al20 with some modification. Firstly, MGC803 cells were incubated with As4S4 (0, 0.25, 0.5, 1, 2, or 4 μM) in serum-free media for 24 hours. The supernatants were collected by centrifugation for 10 minutes at 4°C at 1,000 rpm, then standardized according to the protein contents and loaded on 10% sodium dodecyl sulfate (SDS) polyacrylamide gel containing 1 mg/mL gelatin. Gels were run at 100 V for 150 minutes at 4°C. After electrophoresis, the gels were washed with 2.5% Triton X-100 for 30 minutes at 37°C to remove SDS and incubated in a reaction buffer (40 mM Tris-HCl, pH 8.0; 10 mM CaCl2; and 0.01% NaN3) at 37°C for 36 hours. Finally, the gels were stained for 30 minutes with 0.25% Coomassie blue R-250 followed by destaining with 10% glacial acetic acid and 40% methanol for 7 hours. The protease activity was detected using Image J version 1.47 software.
Protein extraction and Western blotting analysis
Total protein of MGC803 cells or tumor tissues was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai, People’s Republic of China). Analysis of protein concentrations and Western blotting were performed as previously described,18 with minor modifications. Briefly, equal protein content was subjected to 8% or 12% SDS polyacrylamide gel electrophoresis based on the molecular weight of protein needed and transferred onto polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA). After blocking the nonspecific binding sites with 5% nonfat milk, the membranes were incubated with the specific primary antibody at 4°C overnight and washed three times for 10 minutes each time in Tris-buffered saline and Tween 20. Then, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rat secondary antibody for 1 hour at 37°C. Protein bands were detected with an enhanced chemiluminescence system using the Immobilon Western Chemilum HRP Substrate kit (EMD Millipore) and were semiquantified using Image J software.
Real-time polymerase chain reaction
Total RNA from the MGC803 cells or tumor tissues was extracted using TRIzol (Takara Bio Inc., Otsu, Shiga, Japan). After RNA was quantified, cDNA was synthesized using the Prime Script RT-PCR Kit (Takara Bio Inc.) according to the manufacturer’s instruction. The primer sequences of targeted genes used in the polymerase chain reaction are listed in Table 1. Reactions were performed with the ABI PRISM 7500 System (Thermo Fisher Scientific). The analysis of the relative concentration for targeted genes was performed using the comparative cycle threshold (CT) (2−ΔΔCT) method.
  | Table 1 Primer sequences for real-time polymerase chain reaction |
In vivo xenograft tumor model
Xenograft tumor models were established by implanting MGC803 cells subcutaneously as previously described.18 At day 7 after tumor inoculation, mice implanted with cancer cells were randomly distributed into four groups consisting of seven mice each. The blank control group received 20 mL/kg normal saline (NS) once-a-day by intraperitoneal injection for 3 weeks; the positive control group received cyclophosphamide (CTX, 25 mg/kg in 0.4 mL); the low-dose group received low dose of As4S4 (1 mg/kg in 0.4 mL); and the high-dose group received relatively higher dose of As4S4 (2 mg/kg in 0.4 mL). The methods and times of administration of the drug-treated groups were in accordance with the blank control group. The weights of the mice and tumor sizes were measured every other day. The mice were sacrificed after treatment for 3 weeks and tumors were harvested and processed for Western blotting and immunochemistry assay.
Immunohistochemistry assay
The tumor specimens of the animals were fixed in 10% formaldehyde for 24 hours, embedded in paraffin, and sectioned into 3 μm-thick slices. Immunohistochemistry staining was performed as previously described.18 Slides were incubated with primary antibodies including E-cadherin (1:200), β-catenin (1:200), VEGF (1:150), and CD34 (1:150) overnight at 4°C, respectively. Finally, tissue sections were incubated with HRP-labeled goat anti-mouse or anti-rabbit IgG antibody for 1 hour at room temperature, followed by visualization with DAB (BOSTER, Wuhan, People’s Republic of China). The stained slides were observed and photographs obtained using a light microscope (DMI3000 B).
Statistical analysis
Experiments were repeated independently three times and experimental data were expressed as mean ± standard deviation. Data were analyzed using one-way ANOVA followed by the least significant difference test or the Games–Howell procedure. Statistical analysis was performed with SPSS software (v 13.0; SPSS Inc., Chicago, IL, USA) to evaluate the differences between groups. P<0.05 was deemed a statistically significant difference.
Results
As4S4 suppressed the migration of GC cells
We had reported that As4S4 inhibited the proliferation of GC cells at concentrations between 0.31 μM and 10 μM.18 Based on the results of MTT assay, concentrations of As4S4 ranging from 0.25 to 1 μM which did not significantly inhibit cell viability or induce cell death were used for further examination. The wound-healing assay was performed to investigate the effects of As4S4 on GC cell migration. MGC803 and AGS cells were treated with As4S4 (0, 0.25, 0.5, or 1 μM) for 24 or 48 hours, individually. As shown in Figure 1A, the wound-healing ability in MGC803 cells decreased gradually in a dose-dependent manner with raised concentrations of As4S4. The migration rates of MGC803 cells after treatment with As4S4 at 0, 0.25, 0.5, and 1 μM for 24 hours were 50.17%, 45.35%, 22.81%, and 16.69%, respectively (Figure 1B). Meanwhile, the percentages at 48 hours were 86.54%, 74.7%, 50%, and 44.96%, respectively (Figure 1C). Then, the same effects were also discovered in AGS cells, with percentages of 57.9%, 50.22%, 23.73%, and 18.44% after treatment with As4S4 for 24 hours, respectively (Figure 1D and E).
As4S4 suppressed the invasion of GC cells
The effects of As4S4 on invasion of MGC803 cells were investigated using Transwell assays. MGC803 cells were exposed to different concentrations of As4S4 (0, 0.5, and 1 μM) for 12 and 24 hours, individually. After treatment with As4S4 at 0.5 μM and 1 μM for 12 hours, the average invasion rates of MGC803 were 71.61% (P<0.01) and 35.3% (P<0.001) of the cell invasion at 0 μM, respectively. With the treatment time of As4S4 extended to 24 hours, the percentages were 33.8% and 19.1%, respectively (P<0.001) (Figure 2). These findings show that As4S4 significantly suppressed the invasion of MGC803 cells in a dose- and time-dependent manner.
As4S4 inhibited the activity of MMP-2 and MMP-9 in MGC803 cells
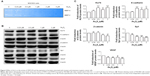
To explore whether As4S4-induced GC cell migration and invasion were associated with the activity of MMP-2 and MMP-9, we investigated the effect of As4S4 on the activity of MMP-2 and MMP-9 of MGC803 cells treated with As4S4 (0, 0.25, 0.5, 1, 2, and 4 μM) for 24 hours by gelatin zymographic analysis. In the presence of As4S4, the level of MMP-2 and MMP-9 activity was significantly decreased. As shown in Figure 3A, the level of MMP-2 and MMP-9 activity was decreased in the presence of As4S4 from 0.25 to 1 μM. With the concentration of As4S4 increased to 2 μM and 4 μM, the inhibitory effects on the activity of MMP-2 and MMP-9 appeared to be more obvious. This was likely due to the reduction of the secretion of MMP-2 and MMP-9 which was induced by the cell cytotoxicity of As4S4.
As4S4 regulated the expression of metastasis-related proteins of MGC803 cells
E-cadherin is a kind of transmembrane glycoprotein which plays a critical role in EMT and the metastatic process. Meanwhile, β-catenin is one of the critical members of the complex adhesive connection of E-cadherin/catenin. Western blotting results showed that with the increase of the concentration of As4S4, the expression of E-cadherin protein in MGC803 cells was upregulated, while the expression of β-catenin was downregulated (Figure 3B and C). KLF4 is a zinc finger transcription factor which has a transcriptional activation and suppression dual function in different cells.21 KLF4 has been found to play a role as a tumor suppressor gene in human GC.22 One study showed that KLF4 could bind to the promoter of MMP-2 and E-cadherin directly to regulate their expression.23 In this study, we found the expression of KLF4 protein was upregulated by As4S4 when the expression of E-cadherin was elevated (Figure 3B and C).
VEGF is one of the most effective factors that can induce angiogenesis.24,25 There is substantial evidence suggesting that the expression level of VEGF is closely associated with tumor metastasis and prognosis.26,27 In addition, the upstream regulatory element Sp1 could bind to the promoter site of VEGF to regulate the transcription of VEGF and further manipulate tumor angiogenesis and metastasis. As shown in Figure 3B, we found that there was an approximately 70% reduction in the expression of VEGF after treatment with As4S4, while the expression of Sp1 was suppressed.
As4S4 inhibited GC xenograft growth and regulated the expression of metastasis-related proteins
To investigate the effect of As4S4 on tumor growth and metastasis in vivo, we developed an animal model of GC, and the tumor formation was monitored. Meanwhile, the expression of associated proteins was measured by Western blotting and immunohistochemistry assay. After treatment with As4S4 for 3 weeks, the growth rate of groups treated with As4S4 was slower than that in the blank control group (P<0.05). Tumor growth inhibition in the positive control group (cyclophosphamide, 25 mg/kg), low-dose group (As4S4, 1 mg/kg), and high-dose group (As4S4, 2 mg/kg) was 52.81%, 38.01%, and 26.79%, respectively.18 As shown in Figure 4A and B, the expression of E-cadherin in xenografts was significantly upregulated after treatment with As4S4, while the expression of VEGF and Sp1 was downregulated. We also examined the effect of As4S4 on the expression of E-cadherin, β-catenin, MMP-2, MMP-9, Sp1, VEGF, and CD34 in tumor tissues at the RNA level (Figure 4C). Immunohistochemistry data (Figure 4D) showed that As4S4 treatment dramatically increased the expression of E-cadherin and reduced the expression of VEGF, consistent with the results of Western blotting. In addition, the protein expression of β-catenin and CD34 was decreased by As4S4 treatment, consistent with both in vitro and in vivo data.
Discussion
As4S4, a traditional Chinese medicine, has been used clinically for thousands of years as an important alternative remedy for a wide variety of diseases.5 Recently, an increasing number of researches aim to develop As4S4 as an anticancer agent.6,10,17,28 The molecular mechanism of its antitumor effects in leukemic cells is related to the ability to induce apoptosis11,13,29–31 and the redistribution of PML-RARα protein.11 Although we previously demonstrated the anticancer activities of As4S4 in GC,16,17 the therapeutic efficacy against tumor invasion and metastasis and the underlying mechanism remained unclear. Thus, we examined the inhibitory effects and related molecular mechanisms of As4S4 on GC invasion and metastasis.
In the present study, we have demonstrated that As4S4 has the ability to inhibit GC invasion and migration (Figures 1 and 2). As4S4 also inhibited the enzymatic activities of MMP-2 and MMP-9 in MGC803 cells (Figure 3A). In addition, using xenograft as a model, we found that As4S4 suppressed the ability of tumor growth and invasion effectively (Figure 4). Furthermore, our study also indicates that the inhibitory effects might involve a variety of mechanisms which consist of increasing tumor cell adhesion, decreasing the ability of tumor cells to destroy the basement membrane, and blocking angiogenesis. This is the first report related to the inhibitory effects and the molecular mechanisms of As4S4 on GC invasion and metastasis.
Several previous studies have shown that MMPs, as important enzymes degrading the extracellular matrix, played important roles in tissue repair, angiogenesis, apoptosis, tumor invasion, and metastasis.32–34 As important members of the MMP family, increases in activity and expression of MMP-2 and MMP-9 have been frequently observed in many human cancers with invasive and metastatic capability.35,36 In our study, the enzymatic activities and expressions of MMP-2 and MMP-9 in GC were confirmed by gelatin zymography assay and real-time polymerase chain reaction analysis. We found that As4S4 apparently reduced the activities of MMP-2/9 in MGC803 cells (Figure 3A) as well as the expressions of MMP-2/9 in tumor tissues (Figure 4D). Moreover, compared with the control group, the suppression effect of As4S4 was more significant with the dosage increased. Moreover, the Transwell assay findings indicated that As4S4 inhibited the movement of MGC803 cells through the polycarbonate membrane filter in a dose- and time-dependent manner (Figure 4), and that is the biological phenomenon associated with the inhibition of expressions and activities of MMP-2/9.
It is well established that the invasion and metastasis of tumor involves multiple biological processes, including cell proliferation, motility, and adhesion; degradation of the basement membrane; neovascular invasion; metastatic deposit; and other aspects.14,37 Studies38,39 have shown that primary tumor invasion and metastasis often started with the decreased tumor cell adhesion which leads to the spread of tumor cells from their original locations. E-cadherin is a transmembrane glycoprotein that plays a critical role in EMT and the metastatic process. It has been reported that E-cadherin is widely expressed on the surface of normal epithelium cells and is considered to be a tumor suppressor gene in many malignancies. Its expression is closely correlated to the prognosis.40–42 In this study, we showed by Western blotting analysis that As4S4 treatment significantly increased the expression of E-cadherin in MGC803 cells (Figure 3B). Meanwhile, we found that the increased E-cadherin protein level was correlated with the upregulation of KLF4 and the downregulation of β-catenin in MGC803 cells (Figure 3B).
In this study, we also evaluated the expressions of VEGF and Sp1 by Western blotting. Our data showed that As4S4 significantly downregulated the level of VEGF and Sp1 in MGC803 cells (Figure 3B), and this inhibitory effect was dose-dependent. These results indicate that the inhibitory effect of As4S4 may be related to the suppression of blood vessel formation.
By establishing the xenograft model of GC, we also assessed the inhibitory effects of As4S4 in GC in vivo. We found that As4S4 inhibited the growth of GC. The tumors in the blank control group (NS) showed a more aggressive phenotype and more prominent vascular circulation compared with the As4S4-treated groups. As4S4 upregulated the expression of E-cadherin and KLF4 but downregulated the expression of β-catenin, MMP-2/9, VEGF, SP1, and CD34 in the tumor tissues of xenograft mice (Figure 4). These findings were certainly consistent with the observations in vitro and further indicate that As4S4 may be a potential candidate as an active agent for inhibiting GC metastasis. In order to further observe the effects of As4S4 on tumor invasion and metastasis in vivo, we plan to establish models of human gastric carcinoma in nude mice by orthotopic transplantation in our future studies. Such models would allow us to monitor the tumor growth and distant metastasis by using the whole-body fluorescence imaging technique.
Conclusion
Our results demonstrate that As4S4 can effectively inhibit the migration and invasion of GC cells. This inhibitory effect may be a result of As4S4 effecting multiple mechanisms, including increasing the ability of tumor cell adhesion, decreasing the ability of tumor cells to destroy the basement membrane, and reducing angiogenesis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81274142, 30300139), the Natural Science Foundation of Science and Technology Commission of Shanghai Municipality (11ZR1423400), and the Key Project of Shanghai Municipal Education Commission (07zz43).
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
GLOBOCAN 2012: Estimated Cancer Incidence, Mortality And Prevalence Worldwide in 2012: World [webpage on the Internet]. Lyon: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed January 29, 2015. | ||
Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–450. | ||
Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. 2010 ed. Li Y, Zhao YY, Yu HP, Song HL, Chen JQ, editors. Beijing: China Medical Science Press; 2010:316. | ||
Liu J, Lu Y, Wu Q, Goyer RA, Waalkes MP. Mineral arsenicals in traditional medicines: orpiment, realgar, and arsenolite. J Pharmacol Exp Ther. 2008;326:363–368. | ||
Wu J, Shao Y, Liu J, Chen G, Ho PC. The medicinal use of realgar (As4S4) and its recent development as an anticancer agent. J Ethnopharmacol. 2011;135:595–602. | ||
Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88(3):1052–1061. | ||
Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. | ||
Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. | ||
Lu DP, Qiu JY, Jiang B, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99(9):3136–3143. | ||
Chen S, Fang Y, Ma L, Liu S, Li X. Realgar-induced apoptosis and differentiation in all-trans retinoic acid (ATRA)-sensitive NB4 and ATRA-resistant MR2 cells. Int J Oncol. 2012;40(4):1089–1096. | ||
Yin T, Wu YL, Sun HP, et al. Combined effects of As4S4 and imatinib on chronic myeloid leukemia cells and BCR-ABL oncoprotein. Blood. 2004;104(13):4219–4225. | ||
Wang H, Liu S, Lu X, Zhao X, Chen S, Li X. Gene expression profile changes in NB4 cells induced by realgar. Chin Med J (Engl). 2003;116(7):1074–1077. | ||
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. | ||
Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459(5):465–475. | ||
Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112:3–25. | ||
Ding W, Zhang L, Kim S, et al. Arsenic sulfide as a potential anti-cancer drug. Mol Med Rep. 2015;11(2):968–974. | ||
Zhang L, Tian W, Kim S, et al. Arsenic sulfide, the main component of realgar, a traditional Chinese medicine, induces apoptosis of gastric cancer cells in vitro and in vivo. Drug Des Devel Ther. 2015;9:79–92. | ||
Pan X, Han H, Wang L, et al. Nitidine chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway. Cancer Lett. 2011;313(2):181–191. | ||
Yang W, Li Q, Pan Z. Sphingosine-1-phosphate promotes extravillous trophoblast cell invasion by activating MEK/ERK/MMP-2 signaling pathways via S1P/S1PR1 axis activation. PLoS One. 2014;9(9):e106725. | ||
Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. | ||
Katz JP, Perreault N, Goldstein BG, et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128(4):935–945. | ||
Tang W, Zhu Y, Gao J, et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer. 2014;110:450–458. | ||
Brown LF, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–269. | ||
Heidenreich R, Machein M, Nicolaus A, et al. Inhibition of solid tumor growth by gene transfer of VEGF receptor-1 mutants. Int J Cancer. 2004;111(3):348–357. | ||
Yu XM, Lo CY, Chan WF, Lam KY, Leung P, Luk JM. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res. 2005;11(22):8063–8069. | ||
Lee SH, Lee SJ, Jin SM, et al. Relationships between lymph node metastasis and expression of CD31, D2-40, and vascular endothelial growth factors A and C in papillary thyroid cancer. Clin Exp Otorhinolaryngol. 2012;5(3):150–155. | ||
Baláž P, Sedlák J. Arsenic in cancer treatment: challenges for application of realgar nanoparticles (a minireview). Toxins (Basel). 2010;2(6):1568–1581. | ||
Chen SY, Liu SX, Li XM. In vitro study on arsenic sulfide (realgar)-induced apoptosis of retinoic acid susceptible or resistant acute promyelocytic leukemia cell lines. Journal of Medical Colleges of PLA. 2002;17(1):43–47. | ||
Tian Y, Liu Y, He P, et al. Arsenic sulfide promotes apoptosis in retinoid acid resistant human acute promyelocytic leukemic NB4-R1 cells through downregulation of SET protein. PLoS One. 2014;9(1):e83184. | ||
Qi J, He P, Chen W, Wang H, Wang X, Zhang M. Comparative proteome study of apoptosis induced by As4S4 in retinoid acid resistant human acute promyelocytic leukemia NB4-R1 cells. Leuk Res. 2010;34(11):1506–1516. | ||
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. | ||
Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255–264. | ||
Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21(7):1104–1117. | ||
Basset P, Okada A, Chenard MP, et al. Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutic implications. Matrix Biol. 1997;15:535–541. | ||
Johnsen M, Lund LR, Rømer J, Almholt K, Danø K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–671. | ||
Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51(18 Suppl):5054s–5059s. | ||
Li F, Li C, Zhang H, et al. VI-14, a novel flavonoid derivative, inhibits migration and invasion of human breast cancer cells. Toxicol Appl Pharmacol. 2012;261:217–226. | ||
Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. | ||
Mohammadizadeh F, Ghasemibasir H, Rajabi P, Naimi A, Eftekhari A, Mesbah A. Correlation of E-cadherin expression and routine immunohistochemistry panel in breast invasive ductal carcinoma. Cancer Biomark. 2009;5:1–8. | ||
Han L, Ma Q, Li J, et al. High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PLoS One. 2011;6(11):e27074. | ||
Kapitanović S, Cacev T, Antica M, et al. Effect of indomethacin on E-cadherin and beta-catenin expression in HT-29 colon cancer cells. Exp Mol Pathol. 2006;80(1):91–96. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.