Back to Journals » Drug Design, Development and Therapy » Volume 14
Arsenic Disulfide Promoted Hypomethylation by Increasing DNA Methyltransferases Expression in Myelodysplastic Syndrome
Authors Zhou QB , Liu ZT, Wang HZ, Guo XQ, Xu YG, Hu XM
Received 25 November 2019
Accepted for publication 25 March 2020
Published 30 April 2020 Volume 2020:14 Pages 1641—1650
DOI https://doi.org/10.2147/DDDT.S239158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Qing-Bing Zhou,1 Zheng-Tang Liu,1 Hong-Zhi Wang,2 Xiao-Qing Guo,2 Yong-Gang Xu,2 Xiao-Mei Hu2
1China Academy of Chinese Medical Sciences, Institute of Geriatric Medicine, Xiyuan Hospital, Beijing, People’s Republic of China; 2Department of Hematology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China
Correspondence: Xiao-Mei Hu
Department of Hematology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Xiyuan Playground No. 1, Haidian District, Beijing 100091, People’s Republic of China
Tel +86 186 1035 0593
Email [email protected]
Background: Previous studies have shown that DNA methylation plays a significant role in myelodysplastic syndrome (MDS). In addition to hypermethylation, aberrant hypomethylation can result in the transcriptional activation of oncogenes in cancer, including MDS. Therefore, drugs targeting DNA hypomethylation are needed for the treatment of MDS. This study aimed to investigate whether As2S2 promoted hypomethylation by increasing DNA methyltransferases (DNMTs) expression in MDS.
Patients and Methods: Ten bone marrow samples from MDS patients and 3 healthy donors were obtained for the examination of the DNA methylation with a Human Methylation 850K BeadChip. The mRNA expressions for the DNMTs in the ten MDS patients and 3 controls were compared by Q-PCR. Then, the MDS cell line SKM-1 was treated with As2S2. After 2 days of treatment, Human Methylation 850K BeadChip was applied to analyze the changes of gene methylation status in the cells. Q-PCR and Western blot were taken to test the changes of mRNA and protein expressions for DNMTs in SKM-1 cells after treatment.
Results: Five hundred ninety-two abnormally hypomethylated genes were found in MDS patients compared to those in controls by Human Methylation 850K. The mRNA expressions of DNMTs (DNMT1, DNMT3a and DNMT3b) in MDS patients were significantly lower than those in healthy individuals. The IC50 value of As2S2 for SKM-1 cells was 4.97 μmol/L.Treatment with As2S2 at 2 μmoL/L resulted in significant alterations in the methylation levels at 1718 sites in SKM-1 cells compared to those in the controls. Hypermethylation was observed in 1625 sites (94.58%), corresponding to 975 genes, compared to those in the controls. Finally, the expression levels of DNMTs (DNMT1, DNMT3a, and DNMT3b) significantly increased in SKM-1 cells treated with As2S2 at 2 μmoL/L and 4 μmoL/L.
Conclusion: These data show a potential clinical application of As2S2 as an innovative hypermethylation agent in MDS.
Keywords: arsenic disulfide, myelodysplastic syndrome, hypermethylation, SKM-1 cell line
Plain Language Summary
DNA aberrant hypomethylation plays an important role in the development of myelodysplastic syndrome (MDS). For example, Papaggeli PC demonstrated that the oncogenes c-myc and c-fos were often aberrantly hypomethylated in MDS. The aberrant hypomethylation of SALL4 was often observed in patients with higher risk of MDS. De-Hong Wua reported that MDS patients with the hypomethylation of let-7a-3 is associated with poor prognosis. So targeting the aberrant hypomethylation could be very important for the MDS treatment. However, there is no such drug that can promote the aberrant hypomethylation at present. In this study, 592 hypomethylated genes were found in MDS patients when compared with those in healthy people and the expression of DNA methyltransferases (DNMTs) was lower than those in healthy donors. More important, data indicated that arsenic disulfide (As2S2) could promote the hypomethylation in MDS cell line through increasing the expression of DNMTs. Our data show a potential clinical application of As2S2 as an innovative hypermethylation agent in the treatment of MDS.
Introduction
Abnormal DNA methylation plays an important role in nearly all kinds of cancer.1,2 Aberrant hypomethylation and hypermethylation events are common in acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS).2–6 DNA hypermethylation has caused great interest because of its direct impact on tumour suppressor genes. Hypermethylation in the promoter of cancer-related genes causes the reversible silencing of tumor suppressor genes.7 The targeted treatment of DNA hypermethylation has become a research goal, and the approval of azacitidine (AZA) and decitabine (DAC) for treatment of MDS represented the most significant progress in the last dozen years.8 However, studies have shown that many patients do not acquire response after demethylation therapy and other patients eventually relapsed who initially respond to DAC or AZA treatment.9 Thus, there is an obvious need to develop novel drugs for DNA methylation-targeted therapy.
Cancer progression is also associated with DNA hypomethylation, which also affects the expression of cancer-related genes and drives the leukaemogenic process in MDS and AML.10 DNA hypomethylation is involved in the development of cancer because it leads to transcriptional activation of oncogenes. Papaggeli PC demonstrated that proto-oncogenes c-myc and c-fos were often aberrantly hypomethylated in MDS and AML.11 A study showed that the frequency of SALL4 hypomethylation significantly increased in patients with a higher risk of MDS.12 De-Hong Wua reported that in MDS patients, the hypomethylation of let-7a-3 is associated with poor prognosis.13 Therefore, drugs targeting DNA hypomethylation are needed for the treatment of patients with MDS. However, there are no such drugs that can improve the aberrant hypomethylation in MDS.
In the present study, we found that many abnormally hypomethylated genes existed in MDS patients and that As2S2 could upregulate the hypomethylation by increasing DNMTs expression in MDS cell line SKM-1.
Patients and Methods
Patients and Samples
A total of ten MDS patients were included for the methylation checking in the study. Table S1 shows the details regarding the MDS patients, who were diagnosed according to 2008 WHO classification system.14 Bone marrow cells were obtained from MDS patients and 3 healthy donors. The healthy donors were used as controls for the checking (Table S2). All patients and healthy individuals provided informed consent, and the study was approved by the medical ethics committee of Xiyuan hospital (2018XLA005-2). The sample collection was conducted in accordance with declaration of Helsinki.
Reagents and Cell Line
As2S2 (Sigma-Aldrich, Missouri, America) was dissolved in 1 M NaOH, and the PH value was adjusted to 7.35–7.45 with the use of HCL to make a stock solution. The MDS cell line SKM-1,15 established from a patient with myelomonocytic leukaemia derived from myelodysplastic syndrome, was provided by Professor Su-ning Chen in the First Affiliated Hospital of Soochow University, Institute of Hematology of Jiangsu Province. The use of the gifted cell line was approved by the medical ethics committee of Soochow University. SKM-1 cells were cultured in RPMI-1640 supplemented with 10% inactivated FBS (Gibco, California, USA).
WST-8 Cell Viability Assay and Drug Treatment
Cell proliferation was measured using water-soluble WST-8 during a spectrophotometric assay (EnoGene, Nanjing, China). The cells were seeded at a density of 1× 104 cells per well in flat-bottomed 96 well plates. Serially diluted concentrations of As2S2 were added to the wells. After 48 h of incubation, 10 µL of WST-8 reagent was added. The absorbance of the samples was measured using a microplate reader (BioTek, Vermont, America) at 450 nm. The experiments were repeated twice. The 50% inhibitory concentration value (IC50) was calculated and the drug treatment concentrations were determined. In this study, SKM-1 cells were seeded in 6 well plates and treated with As2S2 at 0 μmoL/L, 1 μmoL/L, and 2 μmoL/L for 48h, respectively; then, the cells were collected for subsequent experiments.
Human Methylation 850K BeadChip Analysis
Genomic DNA was extracted from bone marrows and SKM-1 cells using a NucleoSpin Tissue kit (Macherey-Nagel, Germany). Illumina Inc. provided the 850K DNA methylation array, which is a highly reproducible device for DNA methylation detection.16 In our study, the methylation status in DNA samples were analysed by the Illumina Human Methylation 850K array. Briefly, an EZ DNA methylation kit (Zymo Research, CA, USA) was used and the bisulfite conversion of 1μg DNA of each sample was performed. Then, bisulfite-treated DNA was hybridized on Methylation 850 BeadChip, following the Infinium HD Methylation protocol. SQ fluorescent scanner was used. Fully methylated DNA produced a ratio that approaches 1, whereas if methylation was completely absent, then the ratio would approach 0. The differentially methylated genes were analysed by GO analysis, which is an important bioinformatics tool for screening related functions.17,18
Reverse Transcription PCR
Cells from bone marrow and SKM-1 were collected and total RNA was extracted with the use of an Ultrapure RNA Kit (CW bio, Beijing, China). Then, reverse transcription was performed and fluorogenic quantitative PCR was performed in 25 µL reaction volumes containing Ultra SYBR Mixture, forward and reverse primers (Sangon, Shanghai, China), a cDNA template and water. GAPDH was chosen as the housekeeping gene. All of the primers used are listed in Table S3. The amplification conditions were 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min using a Line Gene 9600 Plus (Bioer Technology, Hangzhou, China). PCR products were confirmed by melting curve analysis. Relative changes for the target genes were determined after normalization to the expression of GAPDH.
Western Blotting
Protein was extracted with the use of Protein Extraction Kit (Gene pool, China) according to manufacturers’ instructions. Then, the protein concentration was determined by a BCA protein assay. Next, protein samples were loaded to SDS-PAGE and transferred to a PVDF membrane. After blocking in 5.0% non-fat milk for 1 h at room temperature, the specific primary antibodies were incubated with the PVDF membrane at 4°C overnight (Abcam, Cambridge, USA). Then, secondary antibodies were added, enhanced chemiluminescence (ECL) reagents (Thermo, USA) were used to detect the antigen-antibody binding. Quantity One v.4.6.2 was utilized for the quantification of the total gray area of each protein band.
Statistical Analysis
Statistical significance of the difference between the values of the methylation status for different samples that were measured with Human Methylation 850K was assessed using Bayesian and linear regression. CpG sites with both p-values <0.05 and a minimum change of ±0.1 in the β-values were considered significant. The expressions of mRNA and proteins among different groups were analysed using one-way ANOVA with Dunnett’s test. A value of P< 0.05 was considered significant.
Results
Many Abnormally Hypomethylated Genes Existed in MDS Patients
No differences were observed in gender and age between the MDS patients and healthy donors (P=0.729 and 0.865). Then, we analysed data from >853,000 CpG sites with an Illumina Methylation EPIC BeadChip in BM samples from ten MDS patients and 3 healthy individuals. The Bayesian and linear regression analysis showed there were 2421 sites that were significantly differentially methylated in MDS patients compared to those in healthy individuals. Among these sites, 1118 were hypomethylated and 1303 were hypermethylated in MDS patients, which corresponded to 592 hypomethylated and 654 hypermethylated genes, respectively, compared to those in healthy individuals (data not shown). Heatmap and Volcano plot between ten MDS patients and 3 healthy individuals are shown in Figure 1A and B. According to Go analysis, these aberrant hypomethylated genes took part in many cancer-related functions and pathways, including the apoptotic process, cell proliferation, the Wnt receptor signalling pathway, and the glutamate receptor signalling pathway (Figure 1C).
The Lower Expressions of DNMTs in MDS Patients
We analyzed mRNA expressions of DNMTs (DNMT1, DNMT3a and DNMT3b) in the ten untreated MDS patients by real-time fluorescent quantitative PCR. The expressions of these 3 genes in MDS patients were significantly lower than those in healthy donors (p-value<0.05) (Figure 2).
Effects of As2S2 on the Proliferation of SKM-1 Cells
The chemical structure of As2S2 is shown in Figure 3A. The inhibition of proliferation was observed in SKM-1 cell line after treatment with As2S2 at concentrations ranging from 0 to 16 μM for 48 h in a dose-dependent manner compared to that in controls (Figure 3B). The IC50 of As2S2 for SKM-1 cells was 4.97 μmol/L.
As2S2 Improved the Hypomethylation in SKM-1 Cells
We conducted an analysis of the changes in the DNA methylation status in SKM-1 cells after treatment with AS2S2 using an Infinium Human Methylation 850K BeadChip. SKM-1 cells were divided into 3 groups and were treated with 0 (control), 1 (low-dose) or 2 μmol/L (high-dose) of AS2S2 for 48 h.
There were 9 samples that underwent methylation analysis. The control group contained A1, A2 and A3; B1, B2, B3 and C1, C2, C3 represent low-dose group and high-dose group, respectively. The analysis of the mean methylation of cytosines showed that the methylation level in the high-dose group was higher than that in other groups (Figure 4A). The red represents hypermethylated sites, and the green represents hypomethylated sites in Figure 4B and C. The distribution of differentially methylated sites on the chromosomes between low-dose group and control group revealed that 1 μmol/L AS2S2 treatment had little effect on DNA methylation in SKM-1 cells (Figure 4B). However, methylation status at a large number of sites changed after 2 μmol/L AS2S2 treatment compared to those in controls (Figure 4C).
Furthermore, as shown in Tables 1 and S4, the methylation of 1718 sites was significantly changed by treatment with AS2S2 at 2 μmol/L for 48 h, which corresponded to 1032 genes. Among the 1032 genes, 975 genes (94.47%) were hypermethylated following the treatment compared to that in the controls and part of these hypermethylated genes induced by AS2S2 at 2 μmol/L were listed in Table S5. In the low-dose group, only 12 sites (9 genes) were differentially methylated after 1 μmol/L-AS2S2 treatment compared to those in control group (Tables 1, S4). Table 2 shows the distribution of the differentially methylated sites and the positions of genes in the high-dose group compared to those in control group.
 |
Table 1 Number of Differently Methylated Sites Among 3 Groups |
 |
Table 2 Distribution of Differently Methylated Sites in the Positions of Genes in High-Dose Comparing with Those in Control Group |
As2S2 Decreased the mRNA Expression of the Hypermethylated Genes in SKM-1 Cells
To address the question of whether AS2S2 treatment changed mRNA expression levels of the hypermethylated genes in high-dose group compared to those in controls, FGF1 and IGF1 were chosen to undergo RT-PCR analysis, based on the results from the Human Methylation 850K. Treatment with 2 μmoL AS2S2 for 48 h resulted in a significant decrease in FGF1 and IGF1 mRNA expression compared to the mRNA expression in control cells, while 1 μmoL AS2S2 treatment for 48 h did not change the expression compared to that in controls (Figure 5).
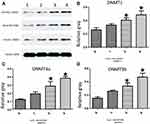
As2S2 Increased the Expressions of DNMT1, DNMT3a, and DNMT3b in As2S2-Treated SKM-1 Cells
We next focused on mRNA and protein expressions of DNMTs following As2S2 treatment. SKM-1 cells were treated with 0, 1, 2 and 4 μmol/L of As2S2 for 48 h. As shown in Figures 6 and 7, compared to controls, 2 and 4 μmol/L As2S2 treatments significantly increased expressions of DNMT1, DNMT3a, and DNMT3b, whereas 1 μmol/L As2S2 treatment had no effect on expressions of DNMTs.
Discussion
The present work is the first to address the effects of AS2S2 on DNA methylation in SKM-1 cells, to the best of our knowledge, demonstrating that treatment with AS2S2 could increase the level of DNA methylation by increasing DNMTs expression in MDS cells.
DNA methylation belongs to epigenetics and plays an important role in tumourigenesis through regulating gene expression. Previous many studies have shown that abnormal DNA methylation is a key event in MDS.19 Hypomethylating agents targeting hypermethylation are already employed for the treatment of MDS. Besides hypermethylation, abnormal hypomethylation has also been observed in cancer. In fact, early studies showed that hypomethylation was the dominant change in cancer by measuring the global 5-methylcytosine content. Hypomethylation can lead to chromosomal instability and the transcriptional activation of oncogenes in cancer, including in MDS.10,20-23
In our study, we also found that many abnormally hypomethylated genes existed in MDS patients compared to those in healthy donors, which is consistent with previous reports.10 GO analysis showed that these abnormally hypomethylated genes took part in cancer-related functions and pathways, such as cell proliferation, the apoptotic process, and the Wnt receptor signalling pathway, which supported the importance of hypomethylation in MDS. To normalize the aberrant hypomethylation in MDS, drugs targeting hypomethylation will be required.
Arsenic disulfide (realgar) has been used therapeutically as part of traditional Chinese medicine for more than 2000 years.24 It is of great interest to understand the effects of As2S2 on DNA methylation in MDS. In this study, we performed a genome-wide methylation analysis in SKM-1 cells treated with As2S2 with an Illumina Human Methylation 850K Array. The data showed that treatment with As2S2 mainly induced DNA hypermethylation, and the DNA methylation microarray analyses presented here identified hundreds of hypermethylated genes. For example, As2S2 treatment induced GLUD1 hypermethylation at CpG islands. Previous studies have found that GLUD1 is often upregulated in many cancers and the inhibition of GLUD1 results in a reduction in cancer cell proliferation and growth.25,26 Another gene HSPA5 was hypermethylated after As2S2 treatment, which is a member of the molecular chaperone family.27 HSPA5 promotes cell survival and has been found to be upregulated in many kinds of cancer cells.28,29 Targeting HSPA5 may be a good option for the treatment of cancer. Taken together, these observations suggest that As2S2 treatment causes hypermethylation of cancer-related genes, which might be the main mechanism of action of As2S2 in the treatment of MDS. Furthermore, this mechanism is different from that of the DNA methylation inhibitors decitabine and azacitidine, which have the ability to demethylate aberrantly hypermethylated genes, causing hypomethylation.30
It is well known that DNA methylation is an epigenetic modification that can play an important role in the control of gene expression, and the presence of methylated CpG islands in promoter regions typically suppresses expression, while hypomethylation leads to over-expression.31 Consistent with multiple previous reports, our results showed that the mRNA expression of the hypermethylated genes FGF1 and IGF1R decreased with As2S2 treatment compared to those in the controls. FGF1 is a 155 amino acid non-glycosylated polypeptide that functions as a proliferation, differentiation, and survival factor in a wide variety of cell types.32 A significant decrease in the expression of the hypermethylated FGF1 gene was observed in our study. FGF1 is a well-known angiogenic growth factor that is essential for tumour growth and may serve as a potential therapeutic target for cancer treatment.33 IGF1R, as a driver oncogene, is overexpressed in cancers such as breast, thyroid, prostate, and ovarian cancers, colorectal cancer cells that regulates cancer cell proliferation by modulating apoptotic signalling.34 IGF1R promotes cancer spreading and metastasis and is considered an attractive target in the treatment of cancer.35,36 In our study, treatment with As2S2 at 2 μmol/L induced a significant decrease in the mRNA expression of the hypermethylated gene IGF1R compared to that in the controls. These observations, along with the fact that aberrant gene-specific hypomethylation is common in MDS, provide a rationale for the use of As2S2 in the treatment of MDS.
DNA methylation, or the covalent addition of a methyl group from S-adenosylmethionine (SAM) to cytosine by DNMTs, is an essential epigenetic modification of the genome in mammalian cells.37,38 In our study, the mRNA expression of DNMTs significantly decreased in MDS patients. However, As2S2 treatment significantly increased the expression of DNMTs at the mRNA and protein levels in SKM-1 cells. The results show that As2S2 upregulated the level of DNA methylation by increasing DNA methyltransferases expression in SKM-1 cell line, and As2S2 may be an innovative hypermethylation agent.
Conclusion
In summary, our study suggests that many hypomethylated genes existed in MDS patients due to the low expression of DNMTs. As2S2 promotes DNA methylation in SKM-1 cells by increasing the expression of DNMTs. Furthermore, As2S2 treatment can lead to the hypermethylation of cancer-related genes, which may be the main mechanism of action in the treatment of MDS. Whether the in vitro effects of As2S2 translate into better response and survival rates in patients with MDS needs to be examined in clinical trials in the future.
Acknowledgments
The authors thank China Academy of Chinese Medicine Scientific Foundation (ZZ13-YQ-010), Beijing Natural Scientific Foundation (7174344) and the National Natural Scientific Foundation of China (81603490, 81774140) for funding. Professor Ma gave us much help in this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Pan YB, Liu GH, Zhou FL, Su BJ, Li YR. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med. 2018;18(1):1–14. doi:10.1007/s10238-017-0467-0
2. Nebbioso A, Tambaro FP, Dell’Aversana C, Altucci L. Cancer epigenetics: moving forward. PLoS Genet. 2018;14(6):e1007362. doi:10.1371/journal.pgen.1007362
3. Qian J, Zhu Z-H, Lin J, et al. Hypomethylation of PRAME promoter is associated with poor prognosis in myelodysplastic syndrome. Br J Haematol. 2011;154(1):153–155. doi:10.1111/j.1365-2141.2011.08585.x
4. Bollati V, Baccarelli A, Hou L, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67(3):876–880. doi:10.1158/0008-5472.CAN-06-2995
5. Chen Q, Lin J, Yao DM, et al. Aberrant hypomethylation of DDX43 promoter in myelodysplastic syndrome. Br J Haematol. 2012;158(2):283–296. doi:10.1111/j.1365-2141.2012.09138.x
6. Nakayama M, Wada M, Harada T, et al. Hypomethylation status of CpG sites at the promoter region and overexpression of the human MDR1 gene in acute myeloid leukemias. Blood. 1998;92(11):4296–4307. doi:10.1182/blood.V92.11.4296
7. Yamashita K, Hosoda K, Nishizawa N, Katoh H, Watanabe M. Epigenetic biomarkers of promoter DNA methylation in the new era of cancer treatment. Cancer Sci. 2018;109(12):3695–3706. doi:10.1111/cas.13812
8. Flotho C, Sommer S, Lübbert M. DNA-hypomethylating agents as epigenetic therapy before and after allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes and juvenile myelomonocytic leukemia. Semin Cancer Biol. 2018;51:68–79. doi:10.1016/j.semcancer.2017.10.011
9. Montalban-Bravo G, Garcia-Manero G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am J Hematol. 2018;93(1):129–147. doi:10.1002/ajh.24930
10. Jelinek J, Liang S, Neumann F, et al. Cancer drivers affected by aberrant DNA methylation in MDS and AML. Blood. 2011;118(21):1716. doi:10.1182/blood.V118.21.1716.1716
11. Kortsaris AC, Matsouka PT. Aberrant methylation of c-myc and c-fos protooncogenes and p53 tumor suppressor gene in myelodysplastic syndromes and acute non-lymphocytic leukemia. J BUON. 2003;8(4):341–350.
12. Lin J, Qian J, Yao DM, et al. Aberrant hypomethylation of SALL4 gene in patients with myelodysplastic syndrome. Leuk Res. 2013;37(1):71–75. doi:10.1016/j.leukres.2012.10.014
13. Wu DH, Yao DM, Yang L, et al. Hypomethylation of let-7a-3 is associated with poor prognosis in myelodysplastic syndrome. Leuk Lymphoma. 2017;58(1):96–103. doi:10.1080/10428194.2016.1187273
14. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi:10.1182/blood-2009-03-209262
15. Nakagawa T, Matozaki S. The SKM-1 leukemic cell line established from a patient with progression to myelomonocytic leukemia in myelodysplastic syndrome (MDS)-contribution to better understanding of MDS. Leuk Lymphoma. 1995;17(3–4):335–339. doi:10.3109/10428199509056841
16. Moran S, Arribas C, Esteller M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics. 2016;8(3):389–399. doi:10.2217/epi.15.114
17. Berardini TZ. The gene ontology in 2010: extensions and refinements. Nucleic Acids Res. 2009;38:331–335.
18. Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi:10.1038/75556
19. Issa JP. The myelodysplastic syndrome as a prototypical epigenetic disease. Blood. 2013;121(19):3811–3817. doi:10.1182/blood-2013-02-451757
20. Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300(5618):455. doi:10.1126/science.1083557
21. Pogribny IP, Beland FA. DNA hypomethylation in the origin and pathogenesis of human diseases. Cell Mol Life Sci. 2009;66(14):2249–2261. doi:10.1007/s00018-009-0015-5
22. Wu H, Chen Y, Liang J, et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature. 2005;438(7070):981–987. doi:10.1038/nature04225
23. Van Tongelen A, Loriot A, De Smet C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017;396:130–137. doi:10.1016/j.canlet.2017.03.029
24. Emadi A, Gore SD. Arsenic trioxide - an old drug rediscovered. Blood Rev. 2010;24(4–5):191–199. doi:10.1016/j.blre.2010.04.001
25. Plaitakis A, Kalef-Ezra E, Kotzamani D, Zaganas I, Spanaki C. The glutamate dehydrogenase pathway and its roles in cell and tissue biology in health and disease. Biology. 2017;6(1):11.
26. Jin L, Li D, Alesi GN, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27(2):257–270. doi:10.1016/j.ccell.2014.12.006
27. Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–276. doi:10.1038/nrc3701
28. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi:10.1038/nrm3270
29. Arap MA, Lahdenranta J, Mintz PJ, et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6(3):275–284. doi:10.1016/j.ccr.2004.08.018
30. Heuser M, Yun H, Thol F. Epigenetics in myelodysplastic syndromes. Semin Cancer Biol. 2018;51:170–179. doi:10.1016/j.semcancer.2017.07.009
31. Baer C, Claus R, Frenzel LP, et al. Extensive promoter DNA hypermethylation and hypomethylation is associated with aberrant microRNA expression in chronic lymphocytic leukemia. Cancer Res. 2012;72(15):3775–3785. doi:10.1158/0008-5472.CAN-12-0803
32. Raju R, Palapetta SM, Sandhya VK, et al. A network map of FGF-1/FGFR signaling system. J Signal Transduct. 2014;2014:962962. doi:10.1155/2014/962962
33. Li J, Wei Z, Li H, et al. Clinicopathological significance of fibroblast growth factor 1 in non-small cell lung cancer. Hum Pathol. 2015;46:1821–1828. doi:10.1016/j.humpath.2015.07.022
34. Wang X, Zhu Q, Lin Y, et al. Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. Br J Cancer. 2017;117(9):1371–1382. doi:10.1038/bjc.2017.297
35. Pollak M. The insulin receptor/insulin-like growth factor receptor family as a therapeutic target in oncology. Clin Cancer Res. 2012;18(1):40–50. doi:10.1158/1078-0432.CCR-11-0998
36. Zorea J, Prasad M, Cohen L, et al. IGF1R upregulation confers resistance to isoform-specific inhibitors of PI3K in PIK3CA-driven ovarian cancer. Cell Death Dis. 2018;9:944. doi:10.1038/s41419-018-1025-8
37. Klein CB, Costa M. DNA methylation and gene expression: introduction and overview. Mutat Res. 1997;386(2):103–105. doi:10.1016/S1383-5742(96)00046-4
38. Robertson KD, Jones PA. DNA methylation: past, present and future directions. Carcinogenesis. 2000;21:461–467. doi:10.1093/carcin/21.3.461
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.