Back to Journals » Drug Design, Development and Therapy » Volume 13
Antileishmanial and antitrypanosomal activity of symmetrical dibenzyl-substituted α,β-unsaturated carbonyl-based compounds
Authors Alkhaldi AAM, de Koning HP , Bukhari SNA
Received 10 February 2019
Accepted for publication 4 March 2019
Published 24 April 2019 Volume 2019:13 Pages 1179—1185
DOI https://doi.org/10.2147/DDDT.S204733
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Abdulsalam AM Alkhaldi,1 Harry P de Koning,2 Syed Nasir Abbas Bukhari3
1Biology Department, College of Science, Jouf University, Sakaka, Aljouf 2014, Saudi Arabia; 2Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8TA, UK; 3Department of Pharmaceutical Chemistry, College of Pharmacy, Jouf University, Sakaka, Aljouf 2014, Saudi Arabia
Background: Human African Trypanosomiasis (HAT) and leishmaniasis are two of the most neglected challenging tropical diseases, caused by the kinetoplastid parasites Trypanosoma and Leishmania species, respectively. For both of these complex disease spectra, treatment options are limited and threatened by drug resistance, justifying urgent new drug discovery efforts.
Purpose: In the present study we investigated the antitrypanosomal and antileishmanial activity of a series of 21 symmetrical α,β-unsaturated carbonyl-based compounds, each featuring two 3-methoxybenzene attached to a central cyclohexanone, tetrahydro-4-pyranone scaffold or 4-piperidone ring. Structure-activity relationships were explored with respect to substitution on positions 3, 4 and 6 of the terminal 3-methoxybenzyl groups, and seven types of central ring.
Results: Compounds 3a, 3o, 3s and 3t, showed broad anti-kinetoplastid activity against all species and strains tested.
Conclusion: Compound 3o featuring N-methyl-4-piperidone was found to be the most potent analog and therefore can serve as a potential lead for the development of new drug candidates for trypanosomiasis and leishmaniasis.
Keywords: protozoan parasites, sleeping sickness, -α,β-unsaturated carbonyl-based compounds, cyclohexanone, piperidine
Introduction
Leishmaniasis and trypanosomiasis are arthropod-borne diseases of humans and animals caused by infection with protozoan hemoflagellates of the genus Leishmania and Trypanosoma, respectively. The two genera are incorporated into the family Trypanosomatidae, order Kinetoplastida. The most recent information released by the World Health Organization (WHO) shows that the extraordinary efforts at control, implemented at the peak of the epidemic in the early 2000s is reducing the number of new cases of HAT.1 However, HAT has been close to eradication half a century before now, and the subsequent discontinuation of control programs allowed the disease to re-emerge to epidemic levels throughout Africa, which had then to be combatted with the same drugs that were introduced in the 1930s and before.2,3 Combination of nifurtimox and eflornithine (NECT) is presently suggested for the treatment of the late stage HAT because NECT treatment is simpler, safer, shorter and less expensive than single-agent eflornithine (DFMO).4 Moreover, animal trypanosomiasis is a very important disease of livestock causing billions of dollars in economic damage and spreading from Africa to areas from South America to East Asia.5
Leishmaniasis is caused by approximately 20 Leishmania species, which are transmitted by phlebotomine sandflies.6,7 According to the World Health Organization (WHO) around 1 million new cases, which cause around 30,000 deaths, occur every year around the world, mostly in the tropics or sub-tropics. The disease is still spreading geographically and is far from being controlled, due in large part to ecological and climate changes, and affects predominantly poor populations but, increasingly also eastern and southern Europe.8,9 Among individual infectious diseases as the ninth largest disease burden, leishmaniasis should not be overlooked in deliberations of tropical disease urgencies.10,11
In recent years new α,β-unsaturated carbonyl-based compounds have been extensively investigated as multifunctional drug candidates. Previously we synthesized and evaluated several series of such compounds for anti-inflammatory,12 anticancer,13,14 immunomodulatory15,16 and anti-Alzheimer,17,18 identifying potent activities. Here, we have selected a series of such α,β-unsaturated carbonyl-based compounds for evaluation of their effects against a number of Leishmania and Trypanosoma strains including the well-characterized multi-drug resistant Trypanosoma brucei clonal line B48, which displays very high levels resistance to the two major classes of trypanocides, the diamidines and the melaminophenyl arsenicals, due to the loss of HAPT1 and TbAT1/P2 drug transporters.19
Materials and methods
Materials
Synthetic α,β-unsaturated carbonyl-based compounds with cyclohexanone, 4-piperidone or tetrahydro-4-pyranone moiety were synthesized (Scheme 1), characterized and reported by us previously.20 Stock solutions were prepared for each tested compound and diluted with complete medium for antiparasite testing, ensuring that the final DMSO concentration did not exceed 1% in the final conditions. Eflornithine (Sigma-Aldrich Ltd., Gillingham, UK) and pentamidine (Sigma-Aldrich Ltd., Gillingham, UK), the most widely used drugs for the treatment of HAT were selected as positive control.
Cell culture
Trypanosoma brucei bloodstream forms (BSF) in-vitro
The selected compounds were tested on bloodstream forms of two strains of Trypanosoma brucei: a standard drug-sensitive wild-type strain of Trypanosoma brucei brucei (Lister s427-WT) and B48, which was adapted from the clone TbAT1-KO by deletion of the TbAT1 drug transporter,21 itself derived from s427WT by removal of the TbAT1 gene,21 by prolonged exposure to pentamidine in vitro.19 Thus, these cells have neither TbAT1/P2 transporter nor the high affinity-pentamidine transporter (HAPT1) genes.19,22 Both strains were cultured in HMI-9 medium (pH 7.4) supplemented with 10% heat-inactivated Fetal Calf Serum (FCS, BioSera) and 14 µl/L of 13.4 M β-mercaptoethanol (Sigma), as described by Hirumi and Hirumi.23 In a flow cabinet through filtration medium was sterilized. T. b. brucei cultures were incubated at 37 ºC and 5% CO2 and passaged in vented flasks three times a week.
Leishmania major and leishmania mexicana promastigotes
Leishmania major strain Friedlin (LmjF) and Leishmania mexicana (strain MNYC/BZ/62/M379) were cultured in essential medium (HOMEM) and 10% FCS, pH 7.4, in plastic flasks at 25 °C. The cultures were passed to fresh medium three times per week, exactly as described previously.24
Alamar blue assay
Resazurin (Alamar Blue) is commonly used as a metabolic function indicator. It is a non-fluorescent, blue dye that is mixed with cell cultures containing various drug concentrations, to determine the sensitivity of African trypanosomes or Leishmania cultures to the test compounds in vitro.25,26 Living cells metabolize the resazurin to red and fluorescent resorufin. Preparation of Alamar Blue reagent involves the dissolution of 12.5 milligrams of Resazurin sodium salt (Sigma) in 100 mL of phosphate-buffered saline (PBS) of pH 7.4, which is then filter-sterilized and stored in the dark at 4 ºC. For T. b. brucei bloodstream forms the assays were performed as described27 in 96-well plates with 1 × 105 cell/well in the presence of 23 doubling dilutions of test compound, and one well for each dilution series receiving growth medium only, for 48 h at 37 °C/5% CO2. The Alamar blue solution was added for a further 24 h incubation before fluorescence was measured in a FLUOstar Optima plate reader (BMG Labtech, Durham, NC, USA), λex 540 nm, λem 590 nm. EC50 values were calculated by non-linear regression to a sigmoidal curve with variable slope using Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). For Leishmania promastigotes the procedure was slightly adapted24 and the final density was 1 × 106 cell/well with the initial incubation for 72 h followed by incubation with Alamar blue for 48 h as total of 120 h. All assays were repeated at least 3 times independently and each were positively controlled with pentamidine and eflornithine.
Results and discussion
In the present study, the effect of synthetic α,β-unsaturated carbonyl-based compounds on the growth of promastigotes of L. major, L. mexicana and different strains of Trypanosoma brucei brucei was evaluated in vitro. Structures of all tested compounds are shown in Table 1 while the antitrypanosomal and leishmanicidal activities of compounds are shown in Table 2.
 | Table 1 Structures of synthetic α,β-unsaturated carbonyl based compounds (cyclohexanone, tetrahydro-4-pyranone scaffold and 4-piperidone derivatives) |
 | Table 2 Effect of α,β-unsaturated carbonyl based compounds on Trypanosoma brucei brucei bloodstream forms and Leishmania mexicana and Leishmania major strains |
Antitrypanosomal activity
In vitro trypanocidal properties of synthetic α,β-unsaturated carbonyl-based compounds were assessed for two different types of Trypanosoma brucei brucei where one was WT and second was the highly resistant T. b. brucei clonal line B48 (Table 2). In this assay, reference drugs eflornithine and pentamidine were used as positive controls. Pentamidine was found very potent with EC50=0.005 µM for wild type and 0.37µM for clonal line B48 (P<0.001), while EC50=19.47 and 22.6 uM were determined for eflornithine-treated WT and B48 (P>0.05) parasites, respectively. Among the tested compounds, twelve derivatives inhibited both strains, with EC50 values within 2-fold different for the two strains, as compared to a 74-fold (increase) difference for pentamidine, showing that this class of compounds is not cross-resistant with diamidines and melaminophenyl arsenicals (Bridges et al, 2007). The highest inhibitory activity was exhibited by 3o (EC50=1.7 µM) for clonal line B48 and second most strong inhibitor was synthetic compound 3a with an EC50 value of 2.8 µM, closely followed by 3s showing inhibition with an EC50 of 3.55 µM.
An interesting structure-activity relationship was observed for synthetic α,β-unsaturated carbonyl-based derivatives. Based on their chemical structure all 21 compounds can be divided into seven main groups, with three compounds in each group. Seven groups are suggested on the basis of R1 substitution in the central ring that linked the two substituted 3-methoxybenzene moieties. In each of the 7 groups the aromatic ring substitutions R1ʹ, R3ʹ, R4ʹ were kept the same [(i) H/H/Cl; (ii) H/OCH3/Cl; (iii) OCH3/OCH3/Br], allowing direct deduction of the role of the central ring modifications for each set of aromatic ring substitutions.
It is immediately clear that the R1 position substitution in the central linker played a vital part in the biological properties of these types of compounds, as we have previously reported for anti-inflammatory activities.28 However, in the case of anti-inflammatory and anticancer activities20 different substitutions correlated with highest potency.
All three compounds with a tetrahydro-4-pyranone linker, 3s, 3t and 3u, exhibited good activity, meaning that oxygen at the R1 position was favorable for activity against both strains of T. b. brucei. Within this group, 3s displayed the highest activity, indicating that addition of Rʹ3 and Rʹ4 methoxy groups were not favored with this central ring. Among the other six linkers, the one with N-CH3 at R1 (3m-3o) exhibited the strongest trypanocidal activity and 3o was the most active compound in the entire series (EC50(WT) =1.83±0.06). However, for the 1-methyl-4-piperidones the activity dramatically improved with the sequential addition of methoxy groups at Rʹ3 and Rʹ4, which appears to indicate that these compounds may have a different target than the tetrahydro-4-pyranones, where the methoxy groups had a deleterious effect. This notion is strengthened by the understanding that the tetrahydropyran oxygen is a strong H-bond acceptor, whereas the ring nitrogen of 1-methylpiperidone is unable to engage in similar H-bonding; similar reasoning can be applied to the methoxy substitution. Thus, the potential interactions of the most active compounds in these groups, 3s and 3o, respectively, with a cellular target would be very different, yet their trypanocidal activities are similar.
Indeed, the favorability of the methoxy substitutions appeared to differ depending on the identity of R1, probably because this influenced the 3D structure through the orientation/rotation of the aromatic rings relative to the central structure and each other (Figure 1). These observations mean that further extension of the SAR in this series should focus on one central group, optimizing the substituents on the aromatic rings, especially as the importance of the 2ʹ methoxy and the 1ʹ halogen for antitrypanosomal activity cannot be assessed from the current as they are present in all derivatives. However, the present data (1) reveal which central rings are optimal, (2) provide a limited SAR starting point for these scaffolds and (3) establish that even with the very limited exploration of this chemical space several trypanocides with EC50 values in the range 1–5 µM could be identified. This activity is obviously much superior to eflornithine (Table 2) and close to that of the standard veterinary trypanocide diminazene aceturate as well.21 Moreover, the α,β-unsaturated carbonyl-based compounds were not cross-resistant with diamidines and arsenical trypanocides as they displayed the same activities against the multi-drug resistant clone B48.
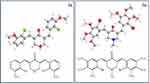 | Figure 1 Energy-minimized 3D structures of compounds 3s and 3o, produced using Chem3D Ultra 10.0 (CambridgeSoft). |
Antileishmanial activity
The extracellular insect-infective promastigote form of Leishmania is addressed in many drug screening assays against Leishmania which can be performed readily, thus being additional suitable for automation even though the Leishmania parasite exists in the human host in its intra-macrophage amastigote form.29 In the current study, all synthetic α,β-unsaturated carbonyl-based compounds were tested on promastigotes of L. major and L. mexicana (Table 2). Eight synthetic compounds showed inhibition, with EC50 values in the range 3 to 41.5 µM for L. major and very similar activities against L. mexicana. The most active compound was again 3o (EC50 3.02±0.10 µM), which performed superior to the positive control pentamidine (EC50 4.34±0.17 µM). The activity was generally below that displayed for T. b. brucei but in general the compounds most active against the trypanosome strains were also the most active against both Leishmania species (mexicana & major). In fact, we found very strong correlation between the trypanocidal and antileishmanial activity of these compounds, as 3a, 3b, 3o, 3p, 3s, 3t and 3u displayed activities against two trypanosoma strains as well as two distinct Leishmania species, and some compounds like 3c, 3f, 3h, 3i, 3j, 3m and 3q have no effect against any of the parasites. For antileishmanial activity, again tetrahydro-pyran-4-one backbone was found best inhibitor among all other linkers as all three compounds bearing this backbone were found to have various levels of activity - yet the best activity was by 3o, with a 1-methyl 4-piperidone backbone.
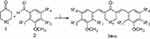 | Scheme 1 Synthesis scheme of α,β-unsaturated carbonyl-based compounds. Reagents and conditions: (i) NaOH, EtOH, room temperature. |
Conclusion
In this study some synthetic α,β-unsaturated carbonyl-based compounds with three types of substitution pattern and seven types of central linker have been identified with potential applications against trypanosomiasis and leishmaniasis. Total twenty-one derivatives were evaluated and twelve of them exhibited good trypanocidal and/or antileishmanial activities. The most promising results were obtained for compound 3o that showed the best inhibition of Trypanosoma brucei brucei wild type (WT) as well as T. b. brucei clonal line B48 and strong activity against Leishmania promastigotes, exceeding the efficacy of the positive control. Further studies should be conducted to further explore the SAR and better understand the mechanism of action of these molecules. Thus, these series of new α,β-unsaturated carbonyl-based could be considered as promising hit compounds against leishmaniasis and trypanosomiasis requiring further hit optimization and safety evaluations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barrett MP. The elimination of human African trypanosomiasis is in sight: report from the third WHO stakeholders meeting on elimination of gambiense human African trypanosomiasis. PLoS Negl Trop Dis. 2018;12(12):e0006925. doi:10.1371/journal.pntd.0006925
2. Stich A, Barrett MP, Krishna S. Waking up to sleeping sickness. Trends Parasitol. 2003;19(5):195–197.
3. Delespaux V, de Koning HP. Drugs and drug resistance in African trypanosomiasis. Drug Resist Updat. 2007;10(1–2):30–50. doi:10.1016/j.drup.2007.02.004
4. Kansiime F, Adibaku S, Wamboga C, et al. A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda. Parasit Vectors. 2018;11(1):105. doi:10.1186/s13071-018-2634-x
5. Giordani F, Morrison LJ, Rowan TG, De Koning HP, Barrett MP. The animal trypanosomiases and their chemotherapy: a review. Parasitology. 2016;143(14):1862–1889. doi:10.1017/S0031182016001268
6. Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–154. doi:10.2147/CLEP.S44267
7. Aluru S, Hide M, Michel G, Banuls AL, Marty P, Pomares C. Multilocus microsatellite typing of leishmania and clinical applications: a review. Parasite. 2015;22:16. doi:10.1051/parasite/2015016
8.
9. Martins-Melo FR, Lima Mda S, Ramos AN
10. Alcântara LM, Ferreira TCS, Gadelha FR, Miguel DC. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. Int J Parasitol Drugs Drug Resist. 2018;8(3):430–439. doi:10.1016/j.ijpddr.2018.09.006
11. Pedrique B, Strub-Wourgaft N, Some C, et al. The drug and vaccine landscape for neglected diseases (2000-11): a systematic assessment. Lancet Glob Health. 2013;1(6):e371–e379. doi:10.1016/S2214-109X(13)70078-0
12. Bukhari SN, Lauro G, Jantan I, Bifulco G, Amjad MW. Pharmacological evaluation and docking studies of alpha,beta-unsaturated carbonyl based synthetic compounds as inhibitors of secretory phospholipase A(2), cyclooxygenases, lipoxygenase and proinflammatory cytokines. Bioorg Med Chem. 2014;22(15):4151–4161. doi:10.1016/j.bmc.2014.05.052
13. Qin H-L, Shang Z-P, Jantan I, et al. Molecular docking studies and biological evaluation of chalcone based pyrazolines as tyrosinase inhibitors and potential anticancer agents. RSC Adv. 2015;5(57):46330–46338. doi:10.1039/C5RA02995C
14. Bukhari SNA, Jantan I, Unsal Tan O, Sher M, Naeem-ul-Hassan M, Qin H-L. Biological activity and molecular docking studies of curcumin-related α,β-unsaturated carbonyl-based synthetic compounds as anticancer agents and mushroom tyrosinase inhibitors. J Agric Food Chem. 2014;62(24):5538–5547. doi:10.1021/jf501145b
15. Jantan I, Bukhari SNA, Lajis NH, Abas F, Wai LK, Jasamai M. Effects of diarylpentanoid analogues of curcumin on chemiluminescence and chemotactic activities of phagocytes. J Pharm Pharmacol. 2012;64(3):404–412. doi:10.1111/j.2042-7158.2011.01423.x
16. Arshad L, Jantan I, Bukhari SNA, Haque MA. Immunosuppressive effects of natural α,β-unsaturated carbonyl-based compounds, and their analogs and derivatives, on immune cells: a review. Front Pharmacol. 2017;8:22. doi:10.3389/fphar.2017.00022
17. Bukhari SNA, Jantan I, Masand VH, et al. Synthesis of α, β-unsaturated carbonyl based compounds as acetylcholinesterase and butyrylcholinesterase inhibitors: characterization, molecular modeling, QSAR studies and effect against amyloid β-induced cytotoxicity. Eur J Med Chem. 2014;83:355–365. doi:10.1016/j.ejmech.2014.06.034
18. Zha G-F, Zhang C-P, Qin H-L, et al. Biological evaluation of synthetic α,β-unsaturated carbonyl based cyclohexanone derivatives as neuroprotective novel inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-β aggregation. Bioorg Med Chem. 2016;24(10):2352–2359. doi:10.1016/j.bmc.2016.04.015
19. Bridges DJ, Gould MK, Nerima B, Maser P, Burchmore RJ, de Koning HP. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol. 2007;71(4):1098–1108. doi:10.1124/mol.106.031351
20. Qin HL, Leng J, Zhang CP, et al. Synthesis of alpha,beta-unsaturated carbonyl-based compounds, oxime and oxime ether analogs as potential anticancer agents for overcoming cancer multidrug resistance by modulation of efflux pumps in tumor cells. J Med Chem. 2016;59(7):3549–3561. doi:10.1021/acs.jmedchem.6b00276
21. Matovu E, Stewart ML, Geiser F, et al. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell. 2003;2(5):1003–1008.
22. Munday JC, Eze AA, Baker N, et al. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J Antimicrob Chemother. 2014;69(3):651–663. doi:10.1093/jac/dkt442
23. Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75(6):985–989.
24. Alzahrani KJH, Ali JAM, Eze AA, et al. Functional and genetic evidence that nucleoside transport is highly conserved in leishmania species: implications for pyrimidine-based chemotherapy. Int J Parasitol Drugs Drug Resist. 2017;7(2):206–226. doi:10.1016/j.ijpddr.2017.04.003
25. Fumarola L, Spinelli R, Brandonisio O. In vitro assays for evaluation of drug activity against leishmania spp. Res Microbiol. 2004;155(4):224–230. doi:10.1016/j.resmic.2004.01.001
26. Raz B, Iten M, Grether-Buhler Y, Kaminsky R, Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997;68(2):139–147.
27. Rodenko B, van der Burg AM, Wanner MJ, et al. 2,N6-disubstituted adenosine analogs with antitrypanosomal and antimalarial activities. Antimicrob Agents Chemother. 2007;51(11):3796–3802. doi:10.1128/AAC.00425-07
28. Bukhari SNA, Lauro G, Jantan I, Bifulco G, Amjad MW. Pharmacological evaluation and docking studies of α,β-unsaturated carbonyl based synthetic compounds as inhibitors of secretory phospholipase A2, cyclooxygenases, lipoxygenase and proinflammatory cytokines. Bioorg Med Chem. 2014;22(15):4151–4161. doi:10.1016/j.bmc.2014.05.052
29. De Muylder G, Ang KKH, Chen S, Arkin MR, Engel JC, McKerrow JH. A screen against leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis. 2011;5(7):e1253. doi:10.1371/journal.pntd.0001370
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
