Back to Journals » Drug Design, Development and Therapy » Volume 12
Antifungal activity of Cardiospermum halicacabum L. (Sapindaceae) against Trichophyton rubrum occurs through molecular interaction with fungal Hsp90
Authors Gaziano R , Campione E , Iacovelli F , Marino D, Pica F , Di Francesco P, Aquaro S, Menichini F, Falconi M , Bianchi L
Received 31 October 2017
Accepted for publication 16 April 2018
Published 12 July 2018 Volume 2018:12 Pages 2185—2193
DOI https://doi.org/10.2147/DDDT.S155610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Roberta Gaziano,1,* Elena Campione,2,* Federico Iacovelli,3 Daniele Marino,1 Francesca Pica,1 Paolo Di Francesco,1 Stefano Aquaro,4 Francesco Menichini,4 Mattia Falconi,3 Luca Bianchi2
1Microbiology Section, Department of Experimental Medicine and Surgery, University of Rome ‘Tor Vergata’, Rome, Italy; 2Department of Systems Medicine, University of Rome ‘Tor Vergata’, Rome, Italy; 3Department of Biology, University of Rome ‘Tor Vergata’, Rome, Italy; 4Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Rende, Italy
*These authors contributed equally to this work
Introduction: Dermatophytosis is a superficial fungal infection limited to the stratum corneum of the epidermis, or to the hair and nails, and constitutes an important public health problem because of its high prevalence and associated morbidity. Dermatophyte fungi, especially 2 species, Trichophyton rubrum and Trichophyton mentagrophytes, are the predominant pathogens. Topical antifungal drugs, mainly azoles or allyamines, are currently used for the treatment of dermatophytoses, although in some cases, such as in nail and hair involvement, systemic treatment is required. However, therapeutic efficacy of current antifungal agents can be limited by their side effects, costs, and the emergence of drug resistance among fungi. Plant extracts represent a potential source of active antimicrobial agents, due to the presence of a variety of chemical bioactive compounds. In the present work, we evaluated in silico and in vitro the antifungal activity of an extract of the medicinal plant Cardiospermum halicacabum against T. rubrum suggesting a potential interaction with Hsp90 as playing an important role in both pathogenicity and drug susceptibility of T. rubrum.
Methods: We investigated in vitro the effect of different concentrations of C. halicacabum (from 500 to 31.25 µg) against a clinical isolate of T. rubrum. Furthermore, using a computational assessment, the interaction between different C. halicacabum active compounds and the fungal Hsp90 was also investigated.
Results: Our results indicate a clear-cut antifungal activity of the total plant extract at the highest concentrations (500 and 250 µg). Among all tested C. halicacabum compounds, the luteolin and rutin molecules have been identified in silico as the most important potential inhibitors of Hsp90. Based on these data, luteolin and rutin were also individually assessed for their antifungal activity. Results demonstrate that both substances display an antifungal effect, even if lower than that of the total plant extract.
Conclusion: Our data indicate a strong fungistatic effect of C. halicacabum against T. rubrum, suggesting its potential therapeutic efficacy in the treatment of dermatophytoses. Additionally, C. halicacabum compounds, and particularly luteolin and rutin, are all possible Hsp90 interactors, explaining their fungistatic activity.
Keywords: Trichophyton rubrum, dermatophytoses, Cardiospermum halicacabum, antifungal activity, Hsp90, molecular modeling, molecular docking, rutin, luteolin
Introduction
Dermatophytosis are superficial mycotic infections limited to the stratum corneum of the epidermis, or to the hair and nails, and represent some of the most common dermatologic diseases seen worldwide. Among dermatophytosis, onychomycosis is a chronic fungal infection of the fingernails or toenails that causes discoloration, thickening, and separation from the nail bed. Onychomycosis occurs in 10% of the general population but is more common in older adults; the prevalence is 20% in those older than 60 years and 50% in those older than 70 years. The increased prevalence in older adults is related to peripheral vascular disease, immunologic disorders, and diabetes mellitus. Onychomycosis is widely believed to be only a cosmetic problem, but it can be uncomfortable and can lead to cellulitis in older adults and foot ulcers in patients with diabetes.1,2 Fungal nail infections can be caused by many different types of fungi, most often dermatophytes, yeasts (Candida), and nondermatophytes. Among dermatophytes, Trichophyton rubrum and Trichophyton mentagrophytes are the most common agents of onychomycosis.3 Onychomycosis is more difficult to treat than most dermatophytosis because of the inherent slow growth of the nail. The treatment depends on the clinical type of the onychomycosis, the number of affected nails, and the severity of nail involvement.4 Topical treatments with amorolfine 5%, ciclopirox 8% nail lacquer, and tioconazole 28% solution, which need to be applied for prolonged periods of time and require strong adherence by the patients, are limited to superficial onychomycosis, and generally are unable to cure onychomycosis because of insufficient nail plate penetration. A systemic treatment with azole and allylamine is always required in proximal subungual onychomycosis and in distal lateral subungual onychomycosis involving the lunula region.3,5 The use of topical agents in combination with systemic therapy is also shown to be more effective in severe toenail onychomycosis and increases the cure rate.6 However, treatment for this illness is long term, and recurrences are frequently detected. Furthermore, treatment is limited by the high cost of conventional antifungal drugs; their adverse effects included headache, gastrointestinal problems, rash, and the emergence of drug resistance among fungal pathogens.
Therefore, there is a continuous and urgent need to discover novel antifungal agents and to identify new potential molecular drug targets for the treatment of fungal infections. Recently, scientific interest in medicinal plants has arisen as a new area due to the increased efficiency of plant-derived drugs for the treatment of various diseases. In this scenario, Cardiospermum halicacabum, belonging to the Sapindaceae family, is an herbaceous climber widely distributed in tropical and subtropical regions. It is seen all through the plains of Africa, America, Bangladesh, India, Malacca, and Pakistan. Several chemical constituents have been isolated from its, ie, β-arachidic acid, apigenin, apigenin-7-O-glucuronide, chrysoeriol-7-O-glucuronide, rutin, and luteolin-7-O-glucuronide.7–9 Various fatty acids were also isolated from seed oil.10 The plant-based herbal products like gel, cream, shampoo, spray, medical drops, and pills are commercially available and are helpful in dry itchy skin and scalp and in the treatment of chronic skin diseases, including psoriasis.8,11 The plant has been used in Ayurveda and folk medicine for a long time in the treatment of rheumatism, lumbago, cough, hyperthermia, nervous diseases (epilepsy and anxiety disorders), as a demulcent in orchitis, and in dropsy.12–15 Various pharmacological actions of C. halicacabum have been investigated in animal models. Particularly, Cardiospermum reduces cyclophosphamide (CTX)-induced toxicity and oxidative stress in mice and also regulates iNOS and COX-2 gene expression in LPS-stimulated macrophages.16 Furthermore, remarkable antipyretic and antiulcer activities of Cardiospermum have been shown in rats.17 The plant also shows antihyperglycemic activity against streptozotocin-induced diabetes in rats and antidiarrheal potential in mice.18 The ethanol extract of the plant suppresses the production of TNF-α and nitric oxide in human peripheral blood mononuclear cells.19 Additionally, the anti-inflammatory, analgesic, and vasodepressant activities of this plant have also been described.20 The leaf extract of C. halicacabum also possess appreciable antibacterial activity, antifungal activity against Candida albicans, antimalarial activity, and antifilarial activity; so this plant could potentially be used for the treatment of infectious diseases.21–23
Evaluation of the interconnection among drug resistance, stress response, and the signaling pathways activated in this processes has revealed Hsp90 as a novel potential cellular target for antifungal drugs.24–26 Hsp90 is an adenosine triphosphate (ATP)-dependent eukaryotic molecular chaperone. Its involvement in the pathogenicity and resistance of C. albicans, Aspergillus fumigatus, and T. rubrum to azole and echinocandin antifungals are well established.27–29 Recently, some natural products such as geldanamycin (a benzoquinone ansamycin antibiotic binding to the ATP-binding pocket of Hsp90) and resorcyclic acid lactone produced by certain fungal species have been studied as inhibitors of Hsp90.30 The recent application of some Hsp90 inhibitors as anticancer therapy has raised considerable interest in this protein as a target for new antifungal therapies.31 Within this context, the aim of the present study was to investigate the effect of C. halicacabum on the in vitro growth of T. rubrum. Furthermore, as suggested by molecular docking analysis, 2 of the most promising C. halicacabum active compounds have been tested, confirming their remarkable role in the inhibition of fungal growth.
Materials and methods
Plant material
The seeds of C. halicacabum were collected in Modica (Sicily, Italy) and authenticated by Dr NG Passalacqua, Natural History Museum of Calabria and Botanic Garden, University of Calabria, Italy. A voucher specimen (CLU 22013) was deposited at the Herbarium of University of Calabria.
Extraction procedure
The seeds (431 g) of C. halicacabum were exhaustively extracted by maceration with 1,500 mL of methanol (4×72 hours). The resulting solutions were evaporated under reduced pressure to give the extract (yield of 1.42%). The extract of C. halicacabum was analyzed for its total phenol and flavonoid content as previously described.9
Materials
A stock solution of C. halicacabum was prepared by dissolving 1 g of the plant extract in 1 mL of methanol. Further dilutions with sterile PBS were made to a final concentration of 10 mg/mL. Luteolin-7-O-glucuronide, rutin, and amphotericin B (Fungizone) were purchased from Sigma Aldrich (St Louis, MO, USA). Luteolin and rutin were dissolved in methanol to a concentration of 10 mg/mL and then diluted with sterile PBS to the desired concentrations ranging from 500 to 62.5 μg. Amphotericin B was dissolved in sterile water and diluted with physiological solution to appropriate concentration of 250 μg/mL.
Determination of antifungal activity
The T. rubrum strain used in this study was obtained from a clinical sample according to routine hospital laboratory procedures. The fungal strain was grown on Sabouraud dextrose agar (Difco, Detroit, MI, USA) supplemented with chloramphenicol at 30°C for 5–7 days. The antifungal activity in vitro of Cardiospermum extract and of its compounds luteolin and rutin was investigated by using a disc diffusion assay (Kirby–Bauer method). The antifungal drug amphotericin B was used as positive control. Briefly, a sterile swab dipped into the fungal suspension, adjusted to 2.0 McFarland standard turbidity, was spread uniformly over the entire surface of Sabouraud dextrose agar plate. Sterile filter paper discs were placed onto the surface of the agar plate and individually impregnated with 50 μL of twofold serial dilutions of Cardiospermum extract, ranging from 500 to 31.25 μg/disc, or the flavonoid compounds luteolin and rutin, ranging from 500 to 62.5 μg/disc, or amphotericin B ranging from 12 to 1.5 μg/disc. Plates were then incubated at 30°C for 48 hours and checked for clear zones of inhibition. PBS with 1% of methanol was used as negative control.
Molecular docking simulation methods
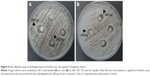
Protein–ligand molecular docking has been used to evaluate binding mode and energy between T. rubrum Hsp90 and 13 C. halicacabum active compounds. The ATP natural cofactor has been included in the analysis. The docking simulations have been executed using the AutoDock Vina 1.1.2 program through the AutoDock/Vina PyMOL plugin (http://wwwuser.gwdg.de/~dseelig/adplugin.html) (The PyMOL Molecular Graphics System Version 1.5.0.4, Schrödinger, LLC, New York, NY, USA).32,33 The 13 compounds and ATP structural files (SDF file) have been obtained from the PubChem compound database (https://pubchem.ncbi.nlm.nih.gov), converted into mol2 files, and filled with hydrogens using the Open Babel program.34 In the absence of T. rubrum Hsp90 X-ray structure, the crystal structure of Hsp90 from Saccharomyces cerevisiae, deposited in the Protein Data Bank (PDB, http://www.rcsb.org/pdb) with PDB ID code 2CG9 whose sequence shares around 76% of identity and 95% of query coverage with T. rubrum Hsp90-like protein (Uniprot sequence ID: F2SVB4) (http://www.uniprot.org/uniprot/F2SVB4), has been used as a template to build the T. rubrum Hsp90 model structure as reported in Figure 1.35 The model has been generated using the SWISS-MODEL protein modeling tool (http://www.swissmodel.expasy.org) and, after a structural check, has been used as a receptor for the molecular docking simulations of ATP, the natural Hsp90 cofactor, and C. halicacabum compounds in the ATP-binding site.36 The side chains belonging to the Hsp90 ATP-binding site (Glu32, Asn36, Asp78, Phe83, Asn91, Ser98, Gln118, Val121, Phe123, Arg375) have been considered rotatable to simulate the receptor flexibility during the docking procedure. The dimensions of the chosen docking box in Å were as follows: X =22.50; Y =26.25; Z =22.50. The AutoDock/Vina program selects, for each docking simulation, 10 ligand poses representing the cluster centroids of all the evaluated solutions. Each docking simulation run takes about 40′ (elapsed real time) on a dedicated Intel i7-4700 CPU workstation.
  | Figure 1 Structural model of T. rubrum Hsp90 dimeric structure generated using as a template the crystal structure of Hsp90 from Saccharomyces cerevisiae (PDB ID: 2CG9). |
Results
Antifungal activity of C. halicacabum against T. rubrum
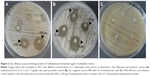
T. rubrum is the most important causative agent of dermatophytoses. The antifungal activity of C. halicacabum against T. rubrum has never been studied. For the first time, this study aims to primarily evaluate the effect of the medicinal plant C. halicacabum on T. rubrum growth in vitro. Our experimental results (Figure 2) show a considerable inhibitory effect on fungal growth in the areas treated with 500 or 250 μg of C. halicacabum extract, whereas concentrations <250 μg (125, 62.5, and 31.25 μg) apparently did not affect the growth of the fungus. It is interesting to note that the inhibition zone diameter of the total extract at 500 μg/disc overlaps approximately with that of the standard antifungal amphotericin B at the highest concentration used (12 μg/disc), suggesting that this natural extract has a significant potential antifungal efficacy, as a function of the dose employed in the in vitro experiments.
Interactions of C. halicacabum active compounds in T. rubrum Hsp90 ATP-binding site
Previous works have been demonstrated that Cardiospermum inhibited the growth of bacterial as well as fungal strains, but the mechanisms underlying the antifungal activity still as yet incompletely understood. Recently, Hsps have been implicated in several fungal processes, including pathogenicity.29 Herein, we investigated by a molecular docking approach the interactions between a total of 13 compounds of Cardiospermum and T. rubrum Hsp90 ATP-binding site. As reported in Table 1, a group of 6 molecules, showing energies between −10 and −11 kcal/mol (kaempferol, apigenin, cholecalciferol, all-trans-retinoic acid, quercetin, and chrysoeriol), may be considered as potential competitive inhibitors of the ATP natural ligand characterized by an interaction energy of −10.2 kcal/mol. Another set of compounds (calycosin-7-O-β-D-glucopyranoside, 1-hentriacontanol, pentadecanoic acid, 3,4-dihydroxybenzoic acid, and 3,4-dihydroxybenzaldehyde) show energies between −9.3 and −6.2 kcal/mol indicating a lower affinity, though still sufficiently high for the calycosin molecule, in comparison with the preceding group. Among all tested compounds, the rutin and luteolin molecules have been identified as the most important potential inhibitors of Hsp90. In fact, their structures, as observed with the ATP molecule, completely fill the Hsp90 ATP-binding site in the N terminal domain of the T. rubrum model structure, establishing a large number of interactions (Figures 3 and 4). In particular, these molecules are fully stabilized by several hydrophobic contacts and hydrogen bonds established with the ATP-binding site residues. Similar interaction energies have been obtained for the rutin and luteolin compounds (−12.1 and −11.9 kcal/mol, respectively), showing values about 2.0 kcal/mol lower than that obtained with the redocking of the ATP molecule (Table 1). These results strongly indicate that C. halicacabum compounds are all possible Hsp90 interactors, explaining its high fungistatic effect, although rutin and luteolin may be considered as the most efficient competitive inhibitors of the primary chaperon Hsp90.
  | Table 1 Docked compounds’ structures and energies |
  | Figure 3 Schematic view of the best molecular docking complex between Trichophyton rubrum Hsp90 and ATP (left panel), rutin (central panel), and luteolin (right panel). |
  | Figure 4 Best molecular docking complex between Trichophyton rubrum Hsp90 and ATP (left panel), rutin (central panel), and luteolin (right panel). |
Luteolin and rutin exert a direct antifungal activity against T. rubrum
Based on the data obtained by molecular docking study, the in vitro antifungal activity of the 2 Cardiospermum bioactive flavonoids, luteolin and rutin, has been also experimentally investigated. The results presented in Figure 5A show that luteolin-7-O-glucoside at the highest concentration of 500 μg exhibited a significant antifungal effect which, however, was considerably lower than that of the total extract of C. halicacabum (Figure 2A), but higher than that of rutin (Figure 5B). The inhibition zone diameters induced by luteolin and rutin, at the highest tested concentrations, overlap with those of amphotericin B at 3 and 1.5 μg, respectively (Figure 2B).
Discussion
Dermatophytes are fungal pathogens that commonly cause superficial infections of keratinized structures such as skin, nails, and hair in immunocompetent individuals. The prevalence rate of superficial mycotic infection worldwide has been found to be 20%–25%. However, in rare cases, dermatophytosis may be invasive, such as in Majocchi’s granuloma, a dermatophyte infection of dermal and subcutaneous tissue. Patients with deep dermatophytosis may have a history of uncontrolled superficial fungal infections such as tinea unguium and tinea cruris. Depending on the amplitude and the site of infection, dermatophytoses can be difficult to cure and systemic treatment with azole, allylamine, and griseofulvin is always required. Particularly, terbinafine, the orally available allylamine antifungal agent, is considered to be a first-line drug for the treatment of dermatophytosis owing to its good mycological and pharmacokinetic profile.37 Sertaconazole is a newer topical imidazole antifungal drug used in Europe for the treatment of superficial skin mycoses such as dermatophytosis.38
However, to date, despite the advent of new antifungal agents for topical and systemic treatment of superficial mycotic infections, an increase in the number of fungi that are resistant to the antifungal drugs currently in clinical use has been reported. Moreover, the toxic side effects of conventional antifungal therapies make dermatophytosis an infection increasingly difficult to treat. Furthermore, fungi are eukaryotic organisms like mammals, and so the number of antifungal drugs targeting structures that are unique to fungi is extremely limited. Thus, the development of innovative antifungal strategies to overcome drug resistance and toxicity of the actually available antifungal drugs is undoubtedly a current medical need for the effective treatment of mycotic infections.
Recently, there is also an increasing interest in plant-derived products for their potential application in the treatment of mycotic infections. Particularly, C. halicacabum is a climbing plant which has been used in Chinese medicine for a long time owing to its natural anti-inflammatory and antioxidant properties. In the past years, a significant antibacterial and antifungal activity in vitro of C. halicacabum has also been described. Shareef et al39 reported that ethanol extract proved to have a strong inhibition activity against S. cerevisiae and Aspergillus niger, whereas in case of C. albicans ethanol, aqueous, and oil extracts recorded only a moderate inhibition activity. Seed oil was also active against animal pathogens such as Micosporillum gypsiccus and T. mentagrophyte. By contrast, ethanol, aqueous and oil extracts did not influence the in vitro growth of Trichophyton longifusis, Trichophyton tonsurans, and Microsporum canis.39 Moreover, an important antimicrobial effect of ethanolic extract of C. halicacabum against C. albicans and multidrug resistant human pathogenes, such as Staphylococcus aureus and Escherichia coli, was also described by Jeyadevi et al.21
Among dermatophytes, T. rubrum is the main fungus that commonly causes deep and invasive infections, especially in immunocompromised patients, including those undergoing solid organ or bone marrow transplantation, those with diabetes mellitus, hematological malignancy, human immunodeficiency disorder, and those using long-term steroid therapy. On the basis of these evidences, in the present study we investigated the effect of C. halicacabum against T. rubrum growth. The results show that C. halicacabum total extract is able to exert an efficient dose-dependent inhibitory activity in vitro on T. rubrum.
The molecular mechanism by which C. halicacabum exerts its antimicrobial activity is still unclear. The antibacterial activity of phenol- and flavonoid-rich plant extracts could be attributed to their ability to form complexes with the bacterial cell wall.40
On the other hand, it is known that Hsp90 is conserved from bacteria to eukaryotic cell, and recent works indicate that in fungi Hsps are implicated in several processes, including pathogenicity, phase transition in dimorphic fungi, and antifungal drug resistance.41 Cowen et al25 demonstrated that in vitro treatment with geldanamycin, an inhibitor of Hsp90 which interferes with the ATP-binding domain, reduced the emergence of resistance to azoles and echinocandins in human pathogenes such as C. albicans and A. fumigatus.28 Furthermore, chemical inhibition of Hsp90 resulted in increased susceptibility of T. rubrum to itraconazole and micafungin, and decreased its growth in human nails in vitro.29 These evidences prompted us to also investigate, using molecular docking approaches, whether C. halicacabum compounds may directly interfere with the ATP-binding site of T. rubrum Hsp90. Interestingly, among a total of 13 plant compounds assessed, the flavonoids luteolin and rutin have been identified as the most important potential inhibitors of Hsp90, although all tested plant compounds have been shown to have affinity for Hsp90. These data are supported by our in vitro experiments which show a direct antifungal activity exerted individually by luteolin and rutin against T. rubrum, even though their antifungal efficacy was lower than that of the total plant extract. Overall, these results suggest that each single bioactive compound presumably interacts directly with the ATP-binding pocket of Hsp90, and all together can work in agonist and synergistic way, potentiating the antifungal action of the total plant extract. Furthermore, the Hsp90 interaction may be an important molecular mechanism underlying the antifungal property of C. halicacabum, but it would not be the only 1 orchestrating fungal morphogenesis and virulence. The antifungal efficacy of C. halicacabum may be due to multiple molecular interactions among its bioactive compounds and different target sites in a single or multiple intracellular pathways that are critical for the fungal growth. On the basis of these evidences, we propose that the antibacterial activity exerted by flavonoids is due not only to their interaction with the cell wall of bacteria, but also to their ability to directly interfere with the Hsp90 function.
Conclusion
Our results demonstrate that the whole-plant extract of C. halicacabum could possibly be a great source of bioactive agents capable of inhibiting the fungal growth by interfering with Hsp90, which can be considered a potential target for novel antifungal strategies. Overall, these evidences suggest that C. halicacabum extract, alone or in combination therapy with lower doses of conventional antimycotic drugs to reduce their side effects, can represent a new potential therapeutic strategy for the treatment of infections caused by fungi which show resistance to the current antifungal therapies. However, all studies investigating the antifungal activity of C. halicacabum have been carried out in vitro. Further investigations in experimental animal models of mycotic infections are necessary to assess in vivo the efficacy and safety of this natural compound for future potential translation of research into clinical practice.
Acknowledgments
We thank Dr Claudia Monari and Dr Gabriella Moroni for their assistance in writing the manuscript and Graziano Bonelli for his excellent technical help. We also thank Dr Rosa Tundis, Dr Marco Bonesi, and Dr Luigi Losi for providing the C. halicacabum plant extract.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Roujeau JC, Sigurgeirsson B, Korting HC, Kerl H, Paul C. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209(4):301–307. | ||
Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29(6):1202–1207. | ||
Westerberg DP, Voyac MJ. Onychomycosis: current trends in diagnosis and treatment. Am Fam Physician. 2013;88(11):762–770. | ||
Iorizzo M, Piraccini BM, Rech G, Tosti A. Treatment of onychomycosis with oral antifungal agents. Expert Opin Drug Deliv. 2005;2(3):435–440. | ||
Campione E, Paternò EJ, Costanza G, et al. Tazarotene as alternative topical treatment for onychomycosis. Drug Des Devel Ther. 2015;9:879–886. | ||
Ameen M, Lear JT, Madan V, Mohd Mustapa MF, Richardson M. British Association of Dermatologists’ guidelines for the management of onychomycosis. British J Dermatol. 2014;171(5):937–958. | ||
Raza SA, Hussain S, Riaz H, Mahmood S. Review of beneficial and remedial aspects of Cardiospermum halicacabum L. Afr J Pharmacy Pharmacol. 2013;7(48):3026–3033. | ||
Subramanyam R, Newmaster SG, Paliyath G, Newmaster CB. Exploring ethnobiological classifications for novel alternative medicine: a case study of Cardiospermum halicacabum L. (Modakathon, Balloon Vine) as a traditional herb for treating rheumatoid arthritis. Ethnobotany. 2007;19(1):1–16. | ||
Menichini F, Losi L, Bonesi M, Pugliese A, Loizzo MR, Tundis R. Chemical profiling and in vitro biological effects of Cardiospermum halicacabum L. (Sapindaceae) aerial parts and seeds for applications in neurodegenerative disorders. J Enzyme Inhib Med Chem. 2014;29(5):677–685. | ||
Mikolajczak KL, Smith CR, Tjarks LW. Cyanolipids of Cardiospermum halicacabum L. and other sapindaceous seed oils. Lipids. 1970;5(10):812–817. | ||
Jong MC, Ermuth U, Augustin M. Plant-based ointments versus usual care in the management of chronic skin diseases: a comparative analysis on outcome and safety. Complement Ther Med. 2013;21(5):453–459. | ||
Council of Scientific and Industrial Research. The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products, Raw Materials. Vol. 3. New Delhi: Council of Scientific and Industrial Research; 1992:269–271. | ||
Kumar R, Murugananthan G, Nandakumar K, Talwar S. Isolation of anxiolytic principle from ethanolic root extract of Cardiospermum halicacabum. Phytomedicine. 2011;18(2–3):219–223. | ||
Jayasilan D. Anticonvulsant activity in herbal extract. Inter J Sci Res. 2017;6(6):123–124. | ||
Neuwinger HD. African Traditional Medicine: A Dictionary of Plant Use and Applications. Stuttgart, Germany: Medpharm GmbH Scientific Publishers; 2000. | ||
Pratheeshkumar P, Kuttan G. Cardiospermum halicacabum inhibits cyclophosphamide induced immunosupression and oxidative stress in mice and also regulates iNOS and COX-2 gene expression in LPS stimulated macrophages. Asian Pac J Cancer Prev. 2010;11(5):1245–1252. | ||
Sheeba MS, Asha VV. Effect of Cardiospermum halicacabum on ethanol induced gastric ulcers in rats. J Ethnopharmacol. 2006;106(1):105–110. | ||
Rao NV, Prakash KC, Kumar SM. Pharmacological investigation of Cardiospermum halicacabum Linn in different animal models of diarrhoea. Indian J Pharmacol. 2006;38(5):346–349. | ||
Venkatesh BKC, Krishnakumari S. Cardiospermum halicacabum suppresses the production of TNF-α and nitric oxide by human peripheral blood mononuclear cells. African J Biomed Res. 2006;9(2):95–99. | ||
Gopalakrishnan C, Dhannanjayan R, Kameshwaran L. Studies on the pharmacological actions of Cardiospermum halicacabum. Indian J Phisiol Pharmacol. 1976;20(4):203–208. | ||
Jeyadevi R, Sivasudha T, Ilavarasi A, Thajuddin N. Chemical constituents and antimicrobial activity of Indian green leafy vegetable Cardiospermum halicacabum. Indian J Microbiol. 2013;53(2):208–213. | ||
Waako PJ, Gumede B, Smith P, Folb PI. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J Ethnopharmacol. 2005;99(1):137–143. | ||
Khunkitti W, Fujimaki Y, Aoki Y. In vitro antifilarial activity of extracts of the medicinal plant Cardiospermum halicacabum against Brugia pahangi. J Helminthol. 2000;74(3):241–246. | ||
Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell. 2008;7(5):747–764. | ||
Cowen LE, Singha SD, Kohlerb JR, et al. Harnessing HSP90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A. 2009;106(8):2818–2823. | ||
Wirk B. Heat shock protein inhibitors for the treatment of fungal infections. Recent Pat Antiinfect Drug Discov. 2011;6(1):38–44. | ||
Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309(5744):2185–2189. | ||
Lamoth F, Juvvadi PR, Fortwendel JR, Steinbacha WJ. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot Cell. 2012;11(11):1324–1332. | ||
Tiago RJ, Peres NTA, Martins MP, et al. Heat shock protein90 (Hsp90) as a molecular target for the development of novel drugs against the dermatophyte Trichophyton rubrum. Front Microbiol. 2015;6:1241. | ||
Piper PW, Millson SH. Spot light on the microbes that produce heat Shock protein 90-targeting antibiotics. Open Biol. 2012;2(12):120–138. | ||
Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. | ||
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. | ||
Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 2010;24(5):417–422. | ||
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. | ||
Ali MM, Roe SM, Vaughan CK, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–1017. | ||
Biasini M, Bienert S, Waterhouse A, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42(Web Server issue):W252–W258. | ||
Newland JG, Abdel-Rahman SM. Update on terbinafine with a focus on dermatophytoses. Clin Cosmet Investig Dermatol. 2009;2:49–63. | ||
Croxtall JD, Plosker GL. Sertaconazole: a review of its use in the management of superficial mycoses in dermatology and gynaecology. Drugs. 2009;69(3):339–359. | ||
Shareef H, Rizwani GH, Mahmood S, Khursheed R, Zahid H. In vitro antimicrobial and phytochemical analysis of Cardiospermum halicacabum L. Pak J Bot. 2012;44(5):1677–1680. | ||
Rameshkumar A, Sivasudha T, Jeyadevi R, et al. In vitro antioxidant and antimicrobial activities of Merremia emarginata using thio glycolic acid-capped cadmium telluride quantum dots. Colloids Surf B Biointerfaces. 2013;101:74–82. | ||
Lamoth F, Juvvadi PR, Steinbach WJ. Heat shock protein 90 (Hsp90) in fungal growth and pathogenesis. Curr Fungal Infect Rep. 2014;8(4):296–301. | ||
Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera – a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. | ||
Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51(10):2778–2786. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.