Back to Journals » Drug Design, Development and Therapy » Volume 11
Anticancer effect of dentatin and dentatin-hydroxypropyl-β-cyclodextrin complex on human colon cancer (HT-29) cell line
Authors AL-Abboodi AS, Rasedee A , Abdul AB, Taufiq-Yap YH, Alkaby WA, Ghaji MS, Waziri PM, Al-Qubaisi MS
Received 28 July 2017
Accepted for publication 7 September 2017
Published 23 November 2017 Volume 2017:11 Pages 3309—3319
DOI https://doi.org/10.2147/DDDT.S147626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Ashwaq Shakir AL-Abboodi,1,2 Abdullah Rasedee,3 Ahmad Bustamam Abdul,1,4 Yun Hin Taufiq-Yap,5 Wafaa Abd Alwahed Alkaby,6 Mostafa Saddam Ghaji,7 Peter M Waziri,1,8 Mothanna Sadiq Al-Qubaisi1
1MAKNA-UPM, Cancer Research Laboratory, Institute of Bioscience, University Putra Malaysia, Serdang, Malaysia; 2Basic Science Branch, Faculty of Dentistry, University of Al-Qadisiyah, Al Diwaniyah, Iraq; 3Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, University Putra Malaysia, Serdang, Malaysia; 4Department of Biomedical Science, Faculty of Medicine and Health Science, University Putra Malaysia, Serdang, Malaysia; 5Department of Chemistry, Faculty of Science, University Putra Malaysia, Serdang, Malaysia; 6Department of Biomedical, Faculty of Biotechnology, University of AL-Qadisiyah, Al Diwaniyah, Iraq; 7Department of Anatomy and Histology, Faculty of Veterinary Medicine, University of Basrah, Basrah, Iraq; 8Department of Biochemistry, Kaduna State University, Main Campus, Kaduna, Nigeria
Introduction: Dentatin (DEN) (5-methoxy-2, 2-dimethyl-10-(1, 1-dimethyl-2propenyl) dipyran-2-one), a natural compound present in the roots of Clausena excavata Burm f, possesses pro-apoptotic and antiproliferative effects in various cancer cells. Because of its hydrophobicity, it is believed that its complexation with hydroxy-β-cyclodextrin (HPβCD) will make it a potent inhibitor of cancer cell growth. In the current work, the molecular mechanisms of apoptosis induced by DEN and DEN-HPβCD complex were demonstrated in human colon HT-29 cancer cells.
Materials and methods: After the human colon HT-29 cancer cells were treated with DEN and DEN-HPβCD complex, their effects on the expression of apoptotic-regulated gene markers in mitochondria-mediated apoptotic and death receptor pathways were detected by Western blot analysis and reverse transcription polymerase chain reaction. These markers included caspases-9, 3, and 8, cytochrome c, poly (ADP-ribose) polymerase, p53, p21, cyclin A as well as the Bcl-2 family of proteins.
Results: At 3, 6, 12, and 24 µg/mL exposure, DEN and DEN-HPβCD complex significantly affected apoptosis in HT-29 cells through the down-regulation of Bcl-2 and cyclin A in turn, and up-regulation of Bax, p53, p21, cytochrome c at both protein and mRNA levels. DEN and DEN-HPβCD complex also decreased cleaved poly (ADP-ribose) polymerase and induced caspases-3, -8, and -9.
Conclusion: Results of this study indicate that the apoptotic pathway caused by DEN and DEN-HPβCD complex are mediated by the regulation of caspases and Bcl-2 families in human colon HT-29 cancer cells. The results also suggest that DEN-HPβCD complex may have chemotherapeutic benefits for colon cancer patients.
Keywords: natural products, HPβCD, apoptosis, pro-apoptotic proteins, anti-apoptotic proteins
Introduction
Colorectal cancer is the third most frequent cancer, representing ~10% of all cancer cases. In 2012, >1.4 million colon cancer cases1 with 700,000 deaths2 were recorded worldwide. In the USA and Europe, colon cancers are more common in men than women. Colon cancer can be inherited, with >85% of colon cancer patients having family history of the disease.2
Dentatin (DEN), a very hydrophobic compound, is naturally occurring in Clausena excavata.3–6 DEN was shown to have inhibitory effects on breast,6 prostate7 and liver cancer cells.8 In many human cancer cells, DEN induced apoptosis by up-regulating pro-apoptotic proteins, for example, Bax and Bak6 and apoptotic protease-activating factor 1, activating caspases,7 and down-regulating antiapoptotic, for example, Bcl-Xl, poly (ADP-ribose) polymerase (PARP) and increasing leakage of cytochrome c from the mitochondria. Furthermore, DEN-mediated apoptosis was also shown to be associated with increase in tumor necrosis factor-related apoptosis-inducing ligand in cancer cells.7–10
In a previous study, we demonstrated that DEN has pro-apoptotic properties.11 DEN was also shown to have antimammary gland cancer effects in rats.12 The anticancer effects of DEN are quite selective, it being innocuous to normal cells.7–9 DEN in a complex with hydroxy-β-cyclodextrin (HPβCD) (DEN-HPβCD) also caused growth inhibition of MDA-MB-231, LNCaP, and HGT cell lines.11 The cancer inhibitory effect of DEN-HPβCD occurs through the induction of apoptosis, particularly by overexpressing the Bax proteins.9 In cancer cells, DEN directly acts on the mitochondria to release cytochrome c.6,7 The incorporation of lipophilic compounds into the HPβCD cavity does not only improve their water solubility while in complexation but also enhances therapeutic effects.13 Although it was shown that DEN either free or incorporated in HPβCD induces cancer cell death via apoptosis, the molecular mechanisms associated with their anticancer activities is not clear. In our current work, we investigated the anticancer molecular mechanism of DEN and DEN-HPβCD on the HT-29 cancer cells.
Materials and methods
Materials
The HPβCD (purity ≥98%) used in this investigation was procured from Sigma Aldrich (Taufkirchen, Germany). All the chemical materials and reagents used were analytical grade and ultrapure water was used during all the experimental steps.
Cell culture
Human colon cancer (HT-29) cells obtained from American Type Culture Collection (Manassas, VA, USA) were maintained in DMEM. The media were supplemented with 10% fetal bovine serum, 1% amphotericin B and 1% penicillin–streptomycin. The cells were maintained in a humidified incubator maintained at 37°C and under 5% CO2 and examined frequently under an inverted microscope (Micros, Salzburg, Austria).
Preparation of the inclusion complex
DEN in HPβCD solution at 1:1 molar ratio was prepared by dissolving 0.3264 g DEN in 5 mL chloroform and mixing with 1.4 g HPβCD in 20 mL of ultrapure water. The mixture was stirred at room temperature for 72 hours and filtered using 0.45 μm filter paper. The solution was frozen at −80°C and subsequently freeze-dried for 24 hours at −55°C. Freeze-drying method was employed to convert the complex from the liquid to solid state.
Western blotting analysis
The HT-29 cells were centrifuged at 1,000× g for 10 minutes and the cell pellet collected and lysed with lysis buffer (50 mM Tris–HCl pH 8.0, 120 mM NaCl, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride). Protein 40 μg was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels and electrophoresed. After electrophoresis, the protein was transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad; Hercules, CA, USA) and the membrane blocked with 5% non-fat dry milk in tris-buffered saline-Tween buffer 7 (0.12 M Tris-base, 1.5 M NaCl, 0.1% Tween 20) at room temperature for 2 hours. The membrane was then incubated with primary mouse antibody either against β-actin (1:2,000), caspase-3 (1:2,000), cytochrome c (1:2,000), Bax (1:2,000), Bcl-2 (1:2,000), p12 (1:2,000), p53 (1:2,000) or PARP (1:2,000) (Genomax Technologies Sdn Bhd., Selangor, Malaysia) overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody (goat anti-mouse 1:1,000) for 1 hour at room temperature. Protein-antibody complexes were detected by chemiluminescence (ECL System) (WesternBrightTM, Advansta, Menlo Park, CA, USA) before autoradiography (ChemiDoc MP imaging System/Bio-Rad, Kuala Lumpur, Malaysia).
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA extraction
The extraction was done using the Aurum Total RNA Mini kit (Bio-Rad). After treatments, the cells were transferred to a 2 mL microcentrifuge tube and centrifuged at 20,000× g for 2 minutes. The supernatant was discarded and 350 μL lysis solution added to each tube followed by 350 μL 70% ethanol, and mixed thoroughly to obtain the homogenized lysate. The RNA binding column was inserted into a 2 mL wash tube and 700 μL homogenized lysate pipetted into the RNA binding column that was then centrifuged at 20,000× g for 30 seconds. Then, 700 μL low stringency wash solution was added to the RNA binding column and the column centrifuged at 20,000× g for 30 seconds. The ribonuclease-free deoxyribonuclease 1 was then added with mixing followed by 700 μL high stringency wash solution. After centrifugation at 20,000× g for 30 seconds, the wash solution was discarded and replaced with 700 μL of fresh low stringency wash solution. The column was recentrifuged at 20,000× g for 1 minute before discarding the wash solution. Then, the RNA was left for 1 minute before centrifuging at 20,000× g for 2 minutes to obtain the RNA. Nanodrop Bio Spectrometer was employed to quantify the RNA. All centrifugations were performed in the Eppendorf 5424 microcentrifuge (Eppendorf, Hamburg, Germany).
cDNA synthesis
First-strand complementary DNA (cDNA) was synthesized from 600 ng RNA using the iScript cDNA Synthesis kit (Bio-Rad). The cDNA synthesis was performed in the Mastercycler Gradient (Eppendorf AG, Eppendorf, Hamburg, Germany). The total reaction volume (cDNA 20 μL reaction) comprised of: 5× iScript reaction mix (4 μL), iScript reverse transcriptase (1 μL), nuclease-free water (13 μL) and RNA template (2 μL). The reaction mixture was incubated for 5 minutes at 25°C, then for 30 minutes at 42°C. The reaction mixture was finally heated at 85°C for 5 minutes and the synthesized cDNA stored at −20°C.
The cDNA PCR amplification
cDNA PCR amplification was carried out using a thermal cycler (C1000 Touch, Bio-Rad), and a My Taq Mix kit (Bioline, Taunton, MA, USA) was used for amplification. All the primers sequences used in RT-PCR were analyzed and components of the PCR reaction are described in Tables 1 and 2.
  | Table 1 Primer sequences of primers used in RT-PCR |
  | Table 2 The components of PCR reaction |
PCR condition
β-actin, p53 and Bax
The PCR cycling conditions for β-actin, p53, Bax were; amplication at 95°C for 1 minute, denaturation for 30 cycles at 95°C for 15 seconds, annealing at 60°C for 15 seconds, and primer extension at 72°C for 10 seconds, and with a final extension at 72°C for 7 minutes.
Caspases-9 and -8
The PCR cycling conditions for caspases-9 and -8 were; amplification at 95°C for 1 minute, denaturation for 30 cycles at 95°C for 15 seconds, annealing at 56.6°C for 15 seconds, primer extension at 72°C for 10 seconds, and final extension at 72°C for 7 minutes.
Cyclin A
The PCR cycling conditions for cyclin A were; amplication at 95°C for 1 minute, denaturation for 30 cycles at 95°C for 15 seconds, annealing at 52°C for 15 seconds, and primer extension at 72°C for 10 seconds, and final extension at 72°C for 7 minutes.
Agarose gel electrophoresis preparation
Agarose gel has been used to separate RNA. Agarose gel electrophoresis (1.5%) was prepared by dispensing 1.5 g agarose powder in a 200 mL conical flask containing 100 mL running buffer, which was prepared by mixing 2 mL stock TAE (50×) (a buffer solution containing a mixture of Tris base, acetic acid and EDTA) with 98 mL distilled water. The agarose gel was placed in the microwave to melt. Then, 1 μL 10 mg/mL ethidium bromide was added to the melted agarose gel and the gel poured into the casting tray to solidify. TAE buffer was poured into the chamber to completely submerge the gel.
RNA samples preparation
Each sample was mixed with loading buffer and 1 μL loading dye was mixed with 5 μL sample. The samples were loaded into the wells and the gel electrophoresed for 60 minutes at 110 Volts. The bands were then visualized under ultraviolet light (Bio-Rad gel documentation system/Bio-Rad).
Results
Western blot analysis
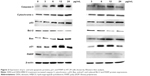
Caspase-3
When the HT-29 cell line was exposed to 3 μg/mL of DEN and DEN-HPβCD for 24 hours, caspase-3 protein expression increased by 1.86-fold and 1.13-fold compared with that in untreated cells, respectively. At 6 μg/mL, the expression was markedly increased by 2.73- and 1.76-fold while the highest increase was obtained when the treatments were given at 12 μg/mL, at 3.66- and 2.66-fold, respectively. With 24 μg/mL treatment concentration, the increase in the caspase-3 protein expression was slightly lower than at 12 μg/mL (Figure 1). The results show that caspase-3 protein expression was induced by DEN and DEN- HPβCD in a dose-dependent manner, with free DEN producing greater effect.
Cytochrome c
Figure 2 shows expression of cytochrome c protein in colon cancer HT-29 cells treated for 24 hours with DEN and DEN-HPβCD complex. There were gradual increases in the cytochrome c expression with increase in dose. The greatest effect was seen with 24 μg/mL of DEN and DEN-HPβCD complex treatments that increased the cytochrome c protein expression by 1.79- and 1.29-fold higher than the controls, respectively (Figure 3).
p53
The expressions of p53 protein in DEN- and DEN-HPβCD-treated HT-29 cells are shown in Figure 4. The HT-29 cells showed marked increase in p53 protein expression in a dose-dependent manner. The highest p53 protein expression was with 24 μg/mL DEN and DEN-HPβCD complex treatments, reaching ~238% and 213% that of the control cells, respectively.
Bcl-2
Bcl-2 protein expression in HT-29 cells decreased gradually with increase in DEN and DEN-HPβCD complex concentrations reaching the lowest value at treatment concentrations of 12 and 24 μg/mL (Figure 5).
Bax
Pro-apoptotic Bax protein expression in HT-29 cells treated with DEN and DEN-HPβCD complex is shown in Figure 2. The expressions of the protein increased with increase in treatment concentration. The highest expression was at 12 μg/mL of DEN and DEN-HPβCD complex, reaching ~180% and 170% of the control values, respectively (Figure 6).
p21
Treatment with 3 μg/mL DEN and DEN-HPβCD did not cause significant (p>0.05) up-regulation in p21 protein expression of HT-29 cells (Figure 7). Shape increases in p21 expression began with 6 μg/mL DEN and DEN-HPβCD complex treatment by 2.4- and 2.7-fold, respectively (Figure 7). The p21 expressions peaked with 12 μg/mL DEN and DEN-HPβCD complex treatment, and the expression decreased with treatment concentration of 24 μg/mL.
Poly (ADP-ribose) polymerase
Expression of PARP protein decreased, compared with control, with DEN and DEN-HPβCD complex treatments at all concentrations showing both compounds suppressed PARP expressions (Figure 8).
Reverse transcription-polymerase chain reaction
RT-PCR was used to determine the effect of DEN and DEN-HPβCD complex on certain apoptosis- and cell cycle-associated genes in HT-29 cells. RT-PCR analysis of mRNA expression for samples was estimated by the thickness of bands.
Caspase-3
Figure 9 shows the gene expression caspase-3 in HT-29 cells treated with DEN and DEN-HPβCD complex. The expression of caspase-3 gene in the HT-29 was up-regulated as a result of treatments. The highest expression in the DEN-treated cells was with treatment concentration of 12 μg/mL (Figure 9). DEN produced greater effect on HT-29 caspase-3 gene expression than DEN-HPβCD complex.
Caspase-9
Caspase-9 gene expression increased in the DEN- and DEN-HPβCD complex-treated HT-29 cells (Figure 10). The results show that caspase-9 decreased when the DEN concentration exceeded 6 μg/mL. Clear increase in caspase-9 expression in DEN-HPβCD-treated cells began at 12 μg/mL treatment dose.
Caspase-8
Caspase-8 gene expressions in HT-29 cells treated with DEN and DEN-HPβCD complex are shown in Figure 11. The highest gene expressions were at 6 and 12 μg/mL of both compounds. The caspase-8 gene expression decreased when the treatment concentration increased to 24 μg/mL, for both compounds.
Bax
Bax gene expression increased slightly after 24 hours exposure to 3 μg/mL of DEN and DEN-HPβCD compared with control (Figure 12). The expression level continued to increase gradually with increase in treatment concentrations. The Bax gene expression was greatest at treatment dose of 12 μg/mL.
p53
Figure 13 shows the p53 gene expressions in HT-29 cells after treatment with DEN and DEN-HPβCD. The expression levels of p53 gene in the HT-29 cells increased with increase in treatment concentrations.
Cyclin A
After exposure of the HT-29 cells to DEN and DEN-HPβCD for 24 hours, the cyclin A gene decreased with increasing treatment concentrations (Figure 14). The untreated HT-29 cells showed the highest cyclin A gene expression. The DEN and DEN-HPβCD treatment downregulated HT-29 cell cyclin A gene.
β-actin
The β-actin expression in the HT-29 cells is shown in Figure 15. The concentrations of DEN and DEN-HPβCD complex did not seem to affect the expression of β-actin gene in the treated cells. The gene was equally expressed at all treatment concentrations.
Discussion
Cells exposed to stress respond by showing unique morphological and biochemical changes.14 Some cells die through apoptosis, autophagy, or necrosis as a result of stress.15 Whether the traumatized cells die depends on the extent of type of injury and cell genotype.16 Both apoptosis and autophagy are parts of the genetically encoded programmed cell suicidal death with specific features.17 Such inherent programs act as repair mechanisms to dispose of effete and damaged cells without affecting homeostasis, the immune system or tissue maintenance.14
Our study attributed the effect of DEN on the HT-29 cells to apoptosis and cell cycle arrest. DEN, either free or in complex with HPβCD caused the reduction in Bcl-2 while increasing Bax expression in a dose-dependent manner. This effect can be attributed to the potential of the drug to affect p53 expression. p53 is a multifunctional protein involved in activation of the transcription factors that regulate apoptotic genes expression.14 With increase in p53 in the HT-29 cells, DEN inhibits proliferation of the cancer cells which contributes to its antitumor effect. DEN also induced an increase in p21 protein expression in a dose-dependent manner. This also impacts on the apoptotic pathway through the induction of p53 gene and protein expression.
The G2 phase is highly dominated by 2 types of cyclin-dependent kinase; cdk1 and 2 enzymes whose action is inhibited by p21. DEN, free or as DEN-HPβCD also decreases cyclin A which is responsible for the regulation of cell cycle progression. The effects of DEN on the cycle regulators results in G2 phase arrest in the treated HT-29 cells and this effect increases with increase in treatment concentration.
The results also showed that both DEN and DEN-HPβCD complex increased cytochrome c expression in HT-29 cells. The effect of DEN on cytochrome c was greater by DEN than DEN-HPβCD. This may be mediated through the up-regulation of Bax and down-regulation of Bcl-2 factors regulating the mitochondrial membrane potential. The cytochrome c plays a majority role like caspase-3, -9, and -8 in inducing apoptosis of the HT-29 cells.18 The DEN and DEN-HPβCD complex at high doses had greater tendency to induce necrosis rather than apoptosis. This effect is postulated to be the cause of decrease in caspase activities with treatment of 24 μg/mL of the compounds.19
PARP acts as a safeguard against development of single-stranded DNA breaks when the cells are exposed to stressful stimuli.
When DNA damage occurs, PARP binds to the DNA to begin repair.20 This is the basis of cell survival in tissues. DEN and DEN-HPβCD caused the down-regulation of PARP in the HT-29 cells. This prevents damaged HT-29 cells from recovering. By down-regulation of PARP, DEN and DEN-HPβCD encourage damaged HT-29 cells to undergo apoptosis and this prevents the proliferation of the cancer cells.
Statistical analysis
All the experiments were conducted in triplicate, and the results are reported in terms of mean ± SD. Statistical analysis was accomplished by using SPSS throughout the experiments. The analysis of variance was carried out using the analysis of variance technique, and a value of p<0.05 was deemed to be of statistical significance.
Conclusion
The study shows that DEN and DEN-HPβCD complex are toxic to the HT-29 cells. The anti-HT-29 cell effects of DEN and DEN-HPβCD are through the induction of apoptosis and cell cycle arrest. The results show that DEN-HPβCD, in particular has potential to be developed into an anticancer drug carrier system.
Acknowledgments
This research was supported by science fund research grant (02-01-04-sf1210), Ministry of Science, Technology and Innovation, Malaysia. The author (ASA-A) is grateful to the University of Al-Qadisiyah, Ministry of Higher Education and Scientific Research, Iraq.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Grant WB. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermatoendocrinol. 2012;4(2):203–211. | ||
Ågesen TH, Sveen A, Merok MA, et al. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61(11):1560–1567. | ||
Arbab IA, Abdul AB, Abdelwahab SI. Clausena excavata Burm. f. (Rutaceae): A review of its traditional uses, pharmacological and phytochemical properties [Doctoral thesis]. Serdang, Malaysia: University Putra Malaysia; 2015. | ||
Cheng SS, Chang HT, Lin CY, et al. Insecticidal activities of leaf and twig essential oils from Clausena excavata against Aedes aegypti and Aedes albopictus larvae. Pest Manag Sci. 2009;65(3):339–343. | ||
Arbab IA, Abdul AB, Aspollah M, Abdelwahab SI, Ibrahim MY, Ali Z. A review of traditional uses, phytochemical and pharmacological aspects of selected members of Clausena genus (Rutaceae). J Med Plants Res. 2012;6(38):5107–5118. | ||
Arbab IA, Abdul AB, Sukari MA, et al. Dentatin isolated from Clausena excavata induces apoptosis in MCF-7 cells through the intrinsic pathway with involvement of NF-κB signalling and G0/G1 cell cycle arrest: a bioassay-guided approach. J Ethnopharmacol. 2013;145(1):343–354. | ||
Arbab IA, Looi CY, Abdul AB, et al. Dentatin induces apoptosis in prostate cancer cells via Bcl-2, Bcl-xL, Survivin downregulation, caspase-9,-3/7 activation, and NF-κB inhibition. Evid Based Complement Alternat Med. 2012;2012:856029. | ||
Andas AR, Abdul AB, Rahman HS, et al. Dentatin from clausena excavata induces apoptosis in HEPG2 cells via mitochondrial mediated signaling. Asian Pac J Cancer Prev. 2015;16(10):4311–4316. | ||
Ashwaq AS, Al-Qubaisi MS, Rasedee A, Abdul AB, Taufiq-Yap YH, Yeap SK. Inducing G2/M Cell Cycle Arrest and Apoptosis through Generation Reactive Oxygen Species (ROS)-Mediated Mitochondria Pathway in HT-29 Cells by Dentatin (DEN) and Dentatin Incorporated in Hydroxypropyl-β-Cyclodextrin (DEN-HPβCD). Int J Mol Sci. 2016;17(10):E1653. | ||
Kawaii S, Tomono Y, Ogawa K, et al. Antiproliferative effect of isopentenylated coumarins on several cancer cell lines. Anticancer Res. 2000;21(3B):1905–1911. | ||
Ashwaq A-AS, Rasedee A, Abdul AB, Taufiq-Yap YH, Al-Qubaisi MS, Eid EE. Characterization, drug release profile and cytotoxicity of Dentatin-Hydroxypropyl-β-Cyclodextrin complex. J Incl Phenom Macrocycl Chem. 2017;87(1–2):167–178. | ||
Arbab IA, Sani NA, Ibrahim MY, Abdalla B. Dentatin from Clausena excavata induces apoptosis and reduces the tumors size of La-7 induced mammary carcinogenesis in sprague dawley rats. Int J Adv Multidiscip Res. 2015;2:67–73. | ||
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–1035. | ||
Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. | ||
Tan ML, Ooi JP, Ismail N, Moad AI, Muhammad TS. Programmed cell death pathways and current antitumor targets. Pharm Res. 2009;26(7):1547–1560. | ||
Santos S, Silva AM, Matos M, Monteiro SM, Álvaro AR. Copper induced apoptosis in Caco-2 and Hep-G2 cells: expression of caspases 3, 8 and 9, AIF and p53. Comp Biochem Physiol C Toxicol Pharmacol. 2016;185:138–146. | ||
Bialik S, Zalckvar E, Ber Y, Rubinstein AD, Kimchi A. Systems biology analysis of programmed cell death. Trends Biochem Sci. 2010;35(10):556–564. | ||
Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–194. | ||
Ladilov Y, Appukuttan A. Role of soluble adenylyl cyclase in cell death and growth. Biochim Biophys Acta. 2014;1842(12 Pt B):2646–2655. | ||
Morales J, Li L, Fattah FJ, et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr. 2014;24(1):15–28. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.